Abstract
In this research work, thermal foaming of bottom ash and sodium silicate geopolymer is proposed as a production process for light weight bricks. The composition and temperatures were studied and optimized to get the most suitable intumescence properties for the lightweight construction applications. For this purpose, four different compositions (i.e., 10%, 20%, 30%, and 40% bottom ash (BA)) were cured at four different curing temperatures (CT) (i.e., 200, 400, 500, and 600 °C). Sodium silicate (SS) to sodium hydroxide (SH) ratio was kept constant in order to keep the activation capacity of the solution constant in all the samples so that the effect of composition and CT could be studied effectively. All samples were characterized by bulk density, foamability, compression test, XRD, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), water absorption index (WAI), and weight loss index (WLI). These characterizations finally led to the optimized parameters to get the most appropriate intumescence properties. It was found that bottom ash and sodium silicate geopolymer foams have good potential to produce lightweight aerated blocks.
1. Introduction
Due to global warming and energy crisis, efforts have been taken to obtain highly efficient construction materials. Aerated concretes are increasingly being adopted, especially in non-loadbearing walls, in order to condition the inside environment while using the least possible amount of energy.
Aerated concretes are mainly produced from Portland cements and raised by mixing with metal powders, like aluminium, and then reacting with acidic ashes, which create hydrogen gas. Unfortunately, such aerated concrete technology involves the use of Portland cement and autoclaves to create expansive reactions and consolidate the bricks. Both the raw materials and the technology are not environmentally friendly [1]. Researchers are therefore trying to obtain sustainable construction materials from waste materials like bottom ash without the need of expensive autoclaves.
With growing industrial energy demands, most countries are now fulfilling their energy requirements from coal-burning power plants [2]. The coal used in the power plants for combustion produces bottom ash (BA) which is used for landfills. Twenty percentage of the total coal ash produced is the BA. The particle size of BA is larger than that of fly ash and nearly equals the size of sand particles [3,4]. The coal ash fuses, agglomerates, and settles down to the bottom of the furnace, therefore it is called bottom ash [5].
In order to get aerated concretes from coal ashes, a mixture of different approaches have been undertaken.H2O2 and organic precursors have been combined and reacted [6]. CaCO3, which releases CO2while heat treating at higher temperatures (800–850 °C), creates foams [7,8]. Lu et al. have used sodium silicate as a foaming agent to produce fly ash-based foam by thermal treatment using sodium borate as a fluxing agent [9]. In our previous work, we developed a geopolymer foam by microwave process [10]. Sodium silicate (SS) is a commonly used industrial material which swells with the application of heat; it was also utilized in our present research as a foaming agent [11].
In this research work, we investigated a thermal foaming process at a lower temperature compared to other works, without using sodium borate or any other fluxing agents. Different compositions were tested, and all samples were characterized for their physical, microstructural, and mechanical properties.
2. Materials and Experimental Procedures
BA used in this research was taken from Batala Steel mills, Lahore, Pakistan. NaOH was purchased from the local market and SS was taken from a local industry (35 wt.% solid content and SiO2:Na2O/3:1). A 14M NaOH solution [10,12] was prepared by dissolving 200 g of NaOH pellets in357mL water. The SS/NaOH ratio was kept constant for all the compositions in order to keep the capacity of the activating agent constant so that the effect of the other parameters on the intumescence properties could be studied. Stainless steel open moulds were used for casting the slurry to allow the geopolymer to expand freely when cured at the specific temperature.
Prior to use, BA was dried at 120 °C in an oven for 48 h. Then, different predefined weight percentages of BA, SS, and NaOH solutions were mixed properly in a ball mill for 2 h to make a homogeneous slurry. The slurry was poured into the stainless steel moulds. The furnace was preheated to the set temperature, and then the moulds containing the slurry were placed in the furnace for rapid heating and left for 5 h to cure. After 5 h, these samples were allowed to cool and were then demoulded.
3. Sampling and Characterization
Different compositions (10%, 20%, 30%, and 40% BA) were used. The weight percentages of SS and NaOH were adjusted in each sample with a SS/NaOH ratio at 15in order to study the effect of BA content and curing temperature (CT) on the intumescence behaviour of thermal geopolymer foams (TGPFs). The samples were designated according to the percentage content of BA and the CT. The sample designations and compositions are represented in Table 1.

Table 1.
Sample designations and compositions.
The samples were manually cut to proper shape and dimensions with a hacksaw for the characterization.
For the bulk density and foamability, the samples were cut in orthogonal blocks, and their edges were measured to calculate the volume. The bulk density was calculated by the following formula:
The apparent density of TGPFs was calculated using a pycnometer, and the foamability was calculated using the following formula:
The water absorption index (WAI) is a measure of open porosity [13,14]. All the samples were weighed and then placed in water for 48 h. After 48 h, these samples were taken out of water and then weighed again. WAI values were calculated by the following formula:
where m1 is the weight of the sample before water absorption and m2 is the weight of the sample after water absorption.
The weight loss index (WLI) test has been used as a measure of resistance to dissolution in water and the degree of geopolymerization [15]. The samples were weighed and then placed in the water for 48 h. After 48 h in water, the samples were taken out of water and dried in an oven at 120 °C. After drying, these samples were weighed again. WLI was calculated by using the following formula:
where m1 is the weight of the sample before immersing in water and m2 is the weight of the sample after drying in the oven.
Samples were subjected to a compression test, FTIR analysis, SEM, and XRD. The FTIR analysis was carried out on FT/IR4100typeA spectrometer, Jasco, Tokyo, Japan at a scanning speed of 2mm/sec and a resolution of 4 cm−1. Compression tests were carried out on TIRAtest 2810 E6 Universal Testing Machine, Germany according to ASTM standards C-365. A load cell of 10kN and a strain rate of 1mm/min were employed in the test.
4. Results and Discussion
Upon heating to over 100 °C, the mixture of sodium silicate and ash swelled due to the endothermic process associated with the emission of water vapour. Vapour was trapped in the sticky viscous slurry, resulting in small bubbles forming in the slurry. The formed foam became almost rapidly rigid as the hydrated silicate condensed due to water loss by vaporization. Subsequently, it acquired greater mechanical strength with the development of geopolymerization reactions.
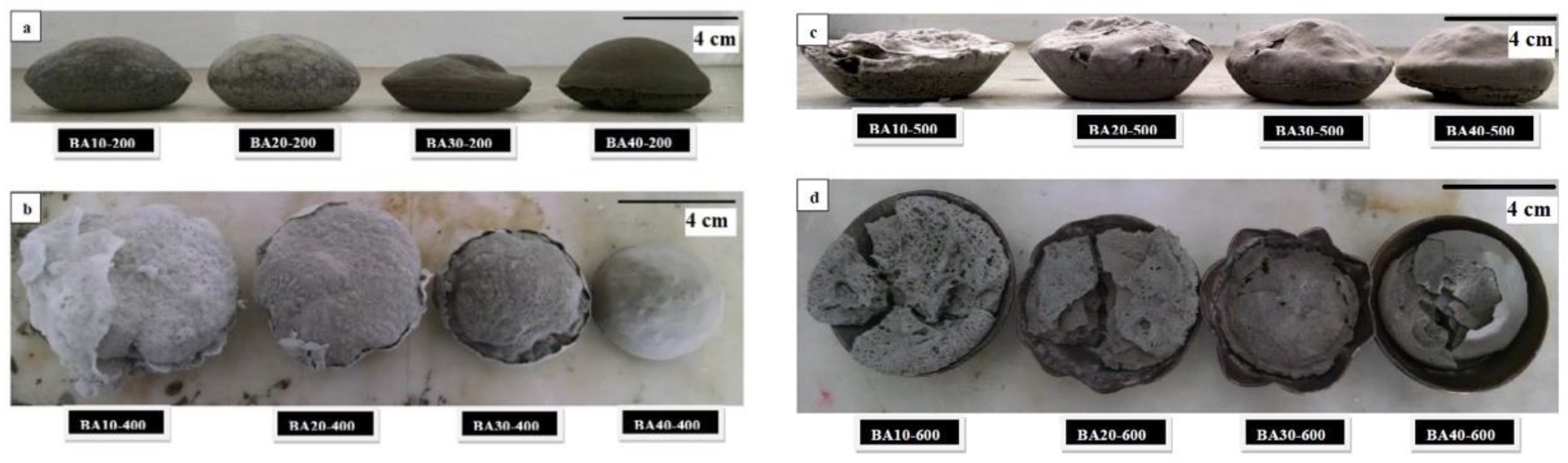
The aerated samples after demoulding are shown in Figure 1. It can be seen in the figure that all TGPFs have one common property, namely, the expansion of foams decreases with the increasing BA content or decreasing liquid/solid ratio. However, the samples cured up to 500 °C were easy to demould. Demoulding was easiest for samples cured at lower temperature (e.g., 200 °C) and more difficult for samples cured at 600 °C. Samples cured at 600 °C were broken after demoulding, as shown in Figure 1d. Due to the transition at a viscous sticking phase above 500 °C, these samples were strongly stuck onto the walls of the moulds and were difficult to demould. Above 500 °C, the system crossed its glass transition temperature [16], therefore due to viscous flow, densification and strengthening occurred.

Figure 1.
Thermal geopolymer foams (TGPFs) of different compositions cured at, (a) 200 oC; (b) 400 oC; (c) 500 oC; (d) 600 oC, right after demoulding.
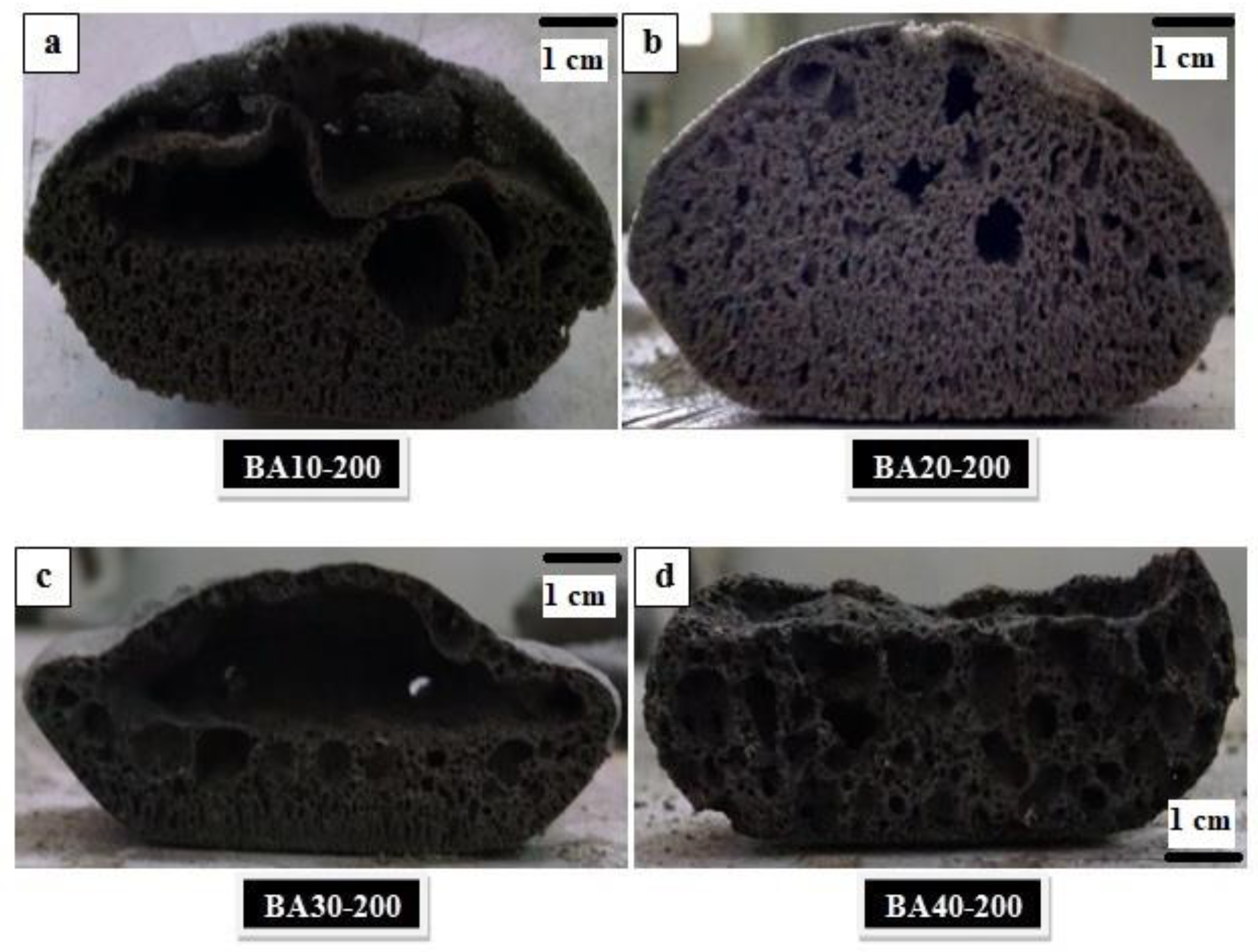
The cross-sections of samples cured at 200 °C are shown in Figure 2. From these cross-sections, it is clear that the expansion of TGPF was maximized with the high SS content and decreased with higher bottom ash content. A domed portion at the top can be seen which was cut from the samples, and only the bottom portions of the samples were used for the characterization of TGPFs. It can be seen from Figure 2a,b that the porosity was uniformly distributed in the BA10-200 and BA20-200 samples because these compositions contained higher percentages of foaming agent(SS), and the liquid/solid ratio was higher, which helped with uniform porosity. In the samples BA30-200 and BA40-200, the foamability was somewhat nonuniformly distributed, as shown in Figure 2c,d, because these compositions had lower percentages of the foaming agent and a lower liquid/solid ratio. These two samples showed pores of different sizes. Interconnected porosity can easily be seen in all these samples.
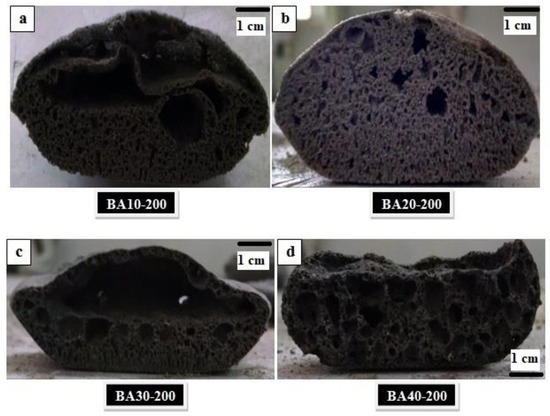
Figure 2.
Cross-sections of TGPFs containing, (a) 10%; (b) 20%; (c) 30%; (d) 40% BA cured at 200 °C.
4.1. SEM Analysis
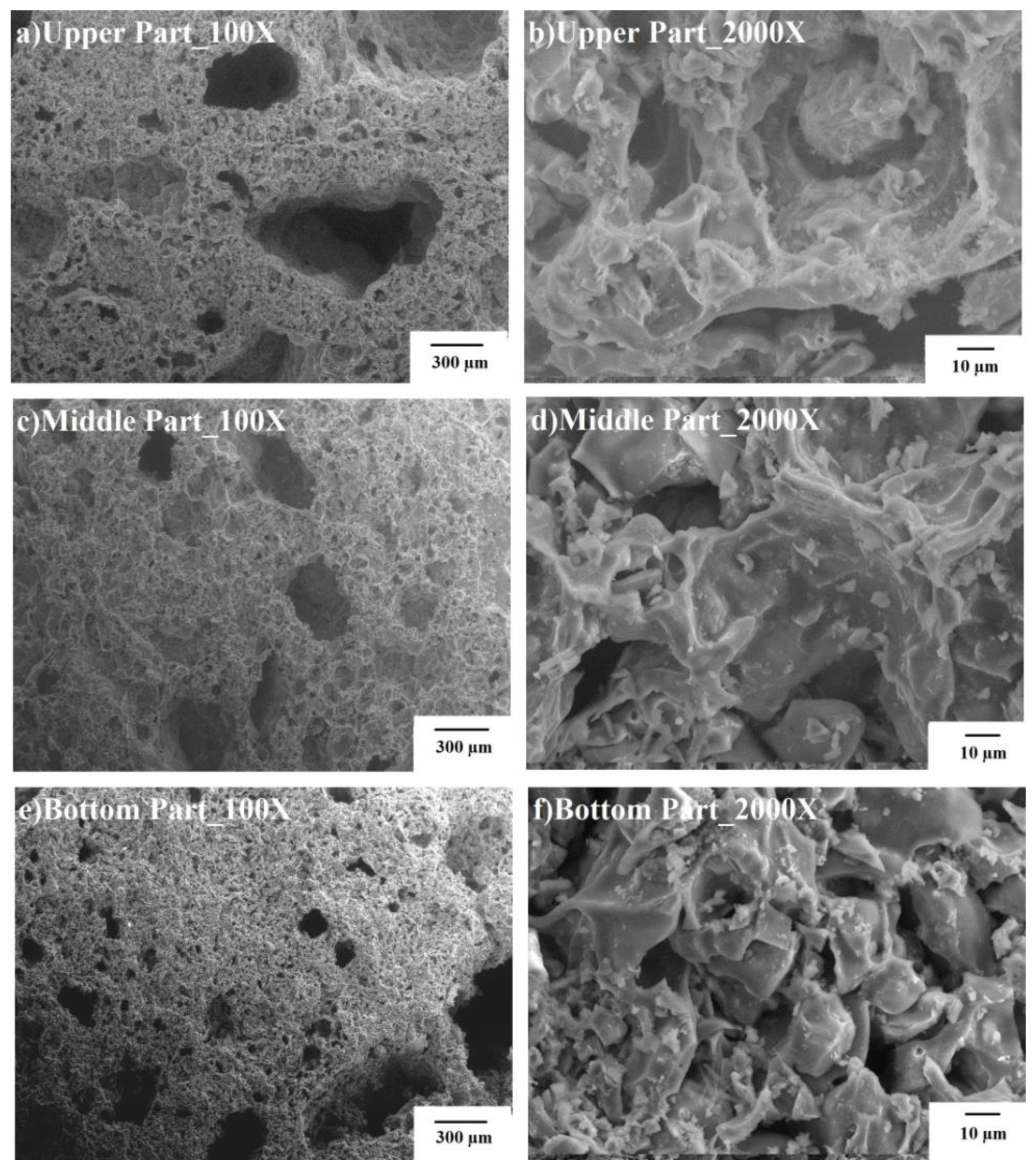
Figure 3 shows SEM micrographs of BA10-500, BA20-500, and BA40-500.This figure shows that as the BA content increased, the porosity of the TGPFs decreased because the solid content of the slurry was increasing or the liquid/solid ratio was decreasing. BA10-500 was heavily porous, and the pores ranged from 5 μm to 2 cm and were non-uniformly distributed. The morphology of the pores was irregularly shaped, as shown in Figure 3. From the higher magnification images, it is evident that there was a continuous structure, and no separate grain can be distinguished. Thus, BA10-500 was composed of a highly refined slurry which was higher in SS concentration, lightweight, and the least dense. BA20-500 was composed of uniform pores from 400 μm down to 10 μm. The morphology was more spheroidal, as can be seen in the micrograph of BA20-500.BA40-500 showed less porosity, and the sizes of the poreswere1mm down to 10 μm. This shows that the network seemed to be absent, which is the result of the activated bottom ash, as is visible in the micrographs of BA10-500 and BA20-500.
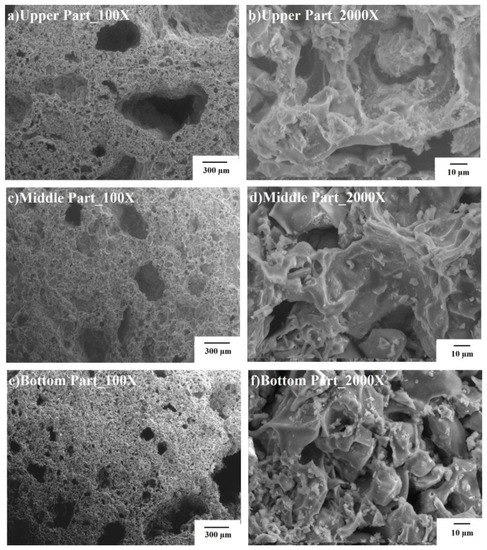
Figure 3.
SEM images for TGPFs.
4.2. XRD
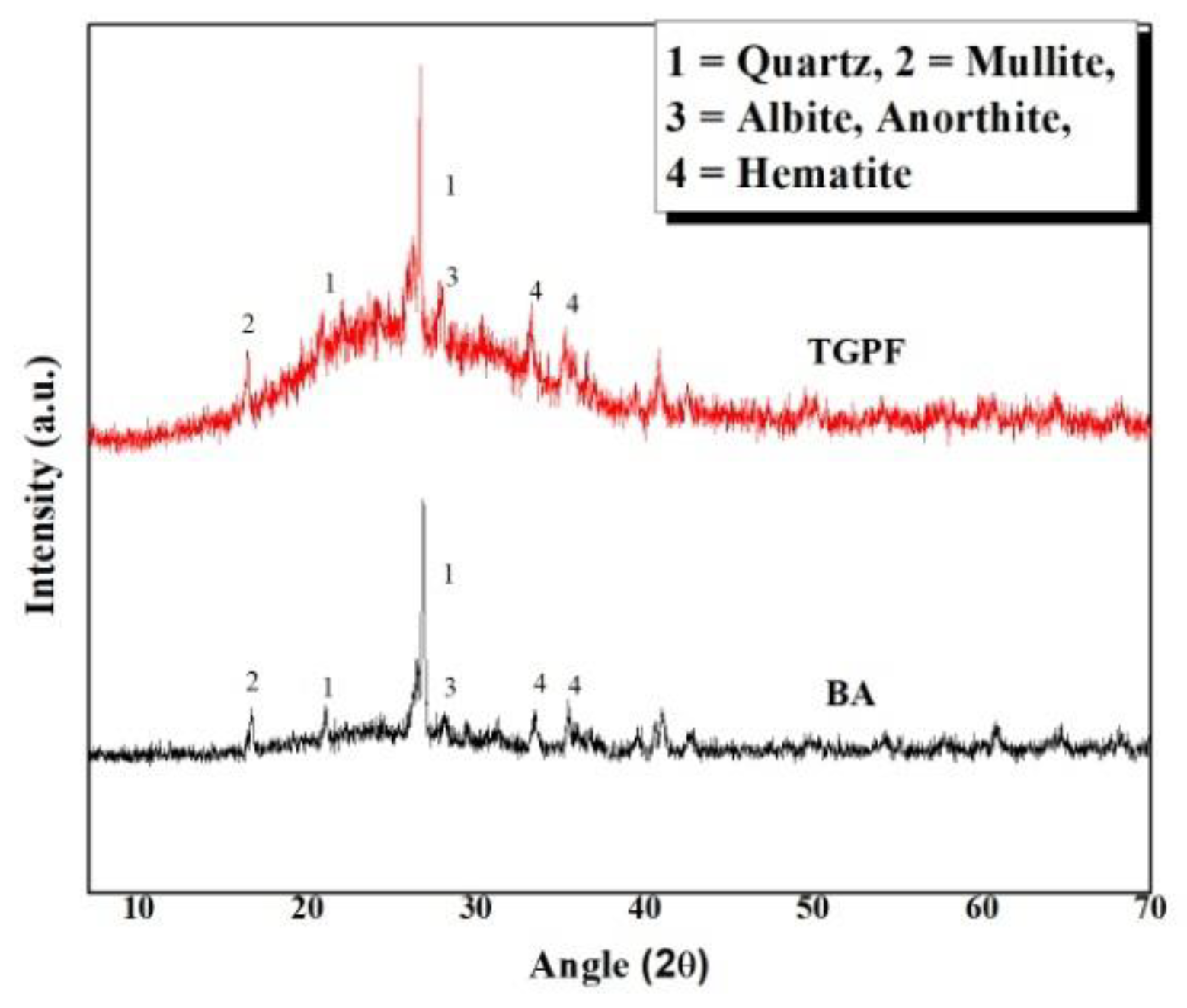
In Figure 4, a comparison of the XRD patterns of BA and TGPF is shown. This comparison was carried out to observe any phase changes between BA and TGPF. Only one pattern of TGPF is presented here because all of the TGPFs demonstrate a similar pattern.
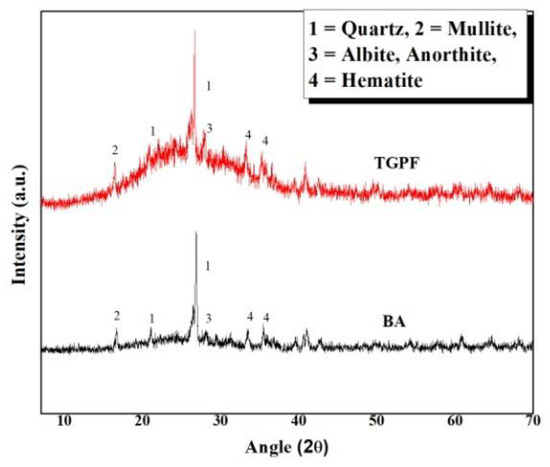
Figure 4.
XRD pattern of bottom ash (BA) and TGPF.
A hump can be seen in the pattern of the TGPF between 15°–40°(2θ) which is absent in the pattern of the BA. It is attributed to the glassy phase in the TGPF derived from the transformation of SS in a vitreous foam. The four phases from BA patterns (i.e., quartz, mullite, anorthite, and hematite) are also present in the TGPF. The anorthite peak of the TGPF is slightly higher than that of the BA, which indicates that geopolymerization has occurred with the formation of anorthite hydrate [16,17]. The peak of quartz in both the patterns is high, but it is slightly higher in TGPF than for BA.
4.3. FTIR Results
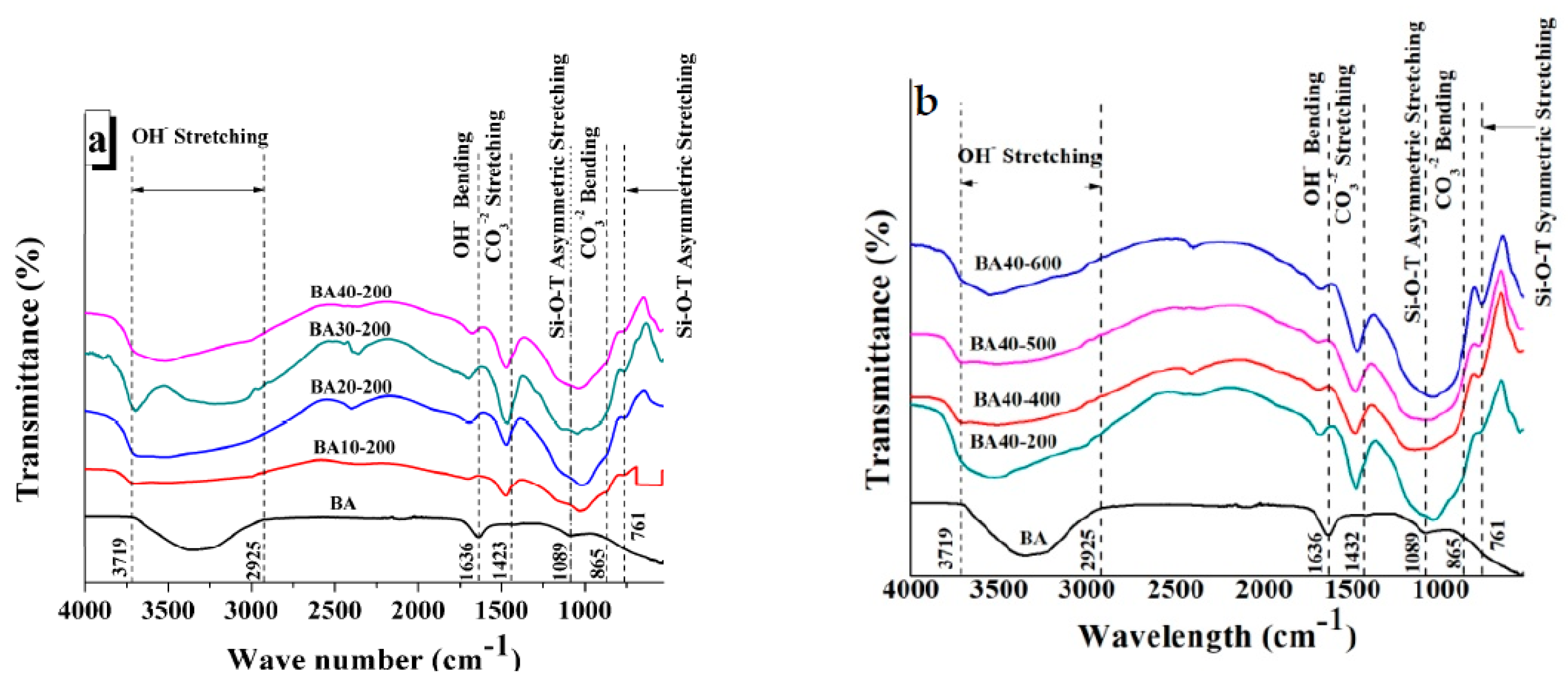
Figure 5a shows the FTIR transmittance of BA and TGPFs of different compositions cured at the same temperature (200 °C), and Figure 5b presents the FTIR analysis of BA and TGPFs with the same BA content (40%) but cured at different temperatures. Different peaks can be seen in Figure 5, which represent specific bond vibrations. Drop lines have been superimposed on the FTIR curves with reference to the bottom ash curve to show any shift of the same peaks in the FTIR curves of TGPFs. Between 3719–2925 cm−1, OH- stretching bonds can be observed. At 1636 cm−1, OH- bending vibrations can be observed. These peaks have shifted towards higher wavenumbers in the case of TGPFs as compared to bottom ash. The appearance of OH- bonds shows the presence of moisture which is absorbed from the atmosphere by TGPFs during handling. This confirms the hygroscopic nature of TGPFs. The CO3−2 stretching and bending bonds appear at 1423 cm−1 and 865 cm−1, respectively. The appearance of these bonds in the FTIR curves confirms the presence of sodium carbonate in TGPFs, which is formed by the reaction of the SS used as the foaming agent and the CO2 absorbed from the atmosphere. It can also be seen from Figure 5 that the carbonate stretching peaks shifted towards higher wavenumbers in the curves of TGPFs. All these results of the FTIR analysis are in accordance with results of Ehsan et al. [10].
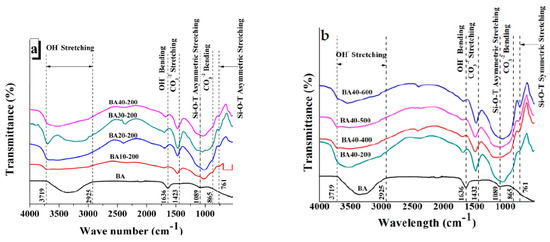
Figure 5.
FTIR analysis of BA and TGPFs of (a) different compositions cured at 200 °C and (b) 40% BA composition cured at different temperatures.
Peaks of Si-O-T asymmetric and symmetric stretching of BA appear at 1089 cm−1 and 761 cm−1, respectively. The asymmetric Si-O-T peaks of the TGPFs are more prominent as compared to BA. The Si-O-T asymmetric stretching peaks of TGPFs are deeper than BA, and these curves also shifted towards the lower wavenumbers, which indicates that geopolymerization occurred.
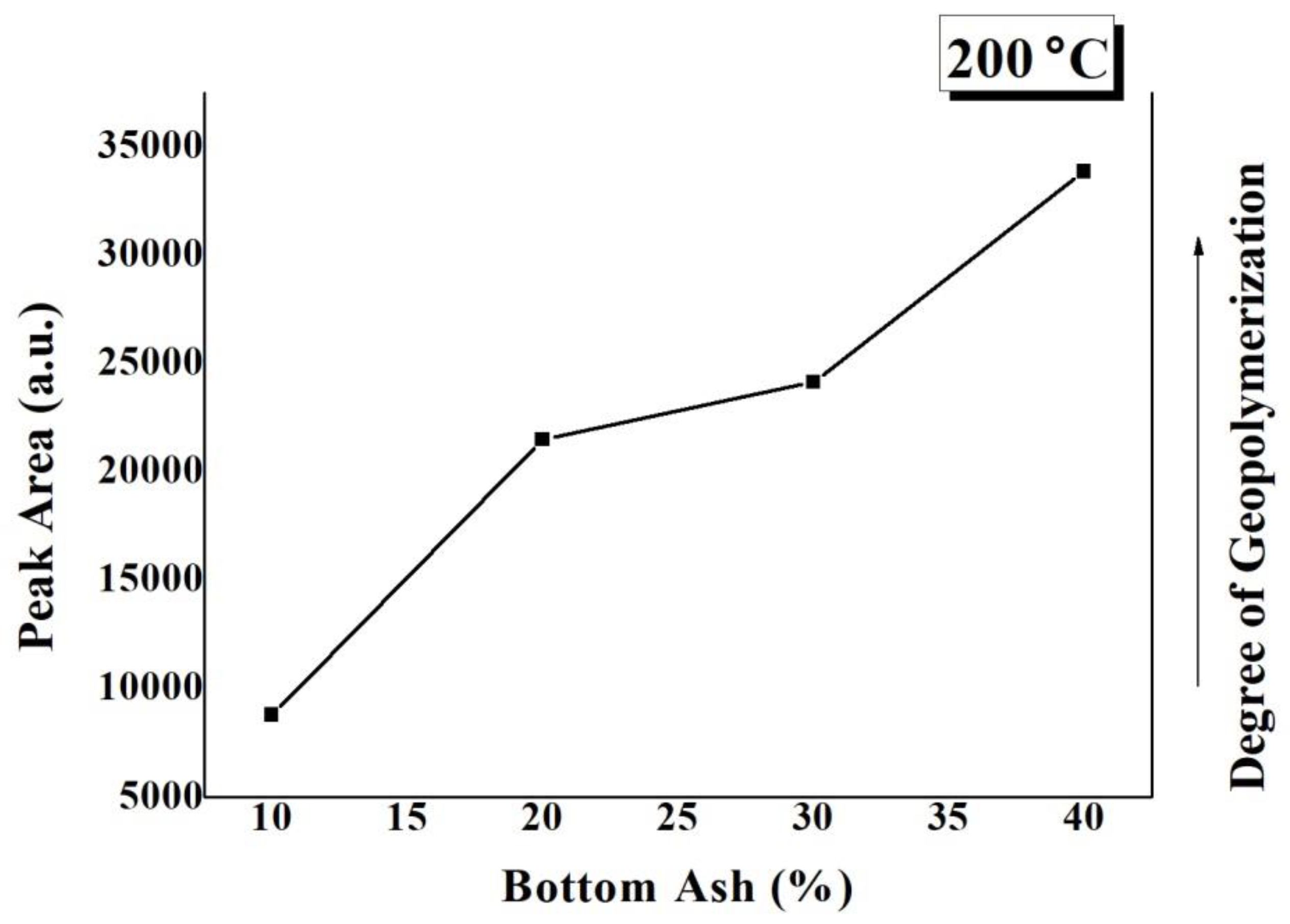
Figure 6 is a plot of calculated peak areas of the Si-O-T asymmetric stretching bonds appearing in the FTIR curves of TGPFs in Figure 5a versus BA content. The peak area of the asymmetric stretching bond is a measure of the degree of geopolymerization [10,18]. It can be seen from Figure 6 that as the BA content increases, the peak area of the asymmetric stretching bonds increases, and hence the degree of geopolymerization increases with the increasing BA content at a constant CT.
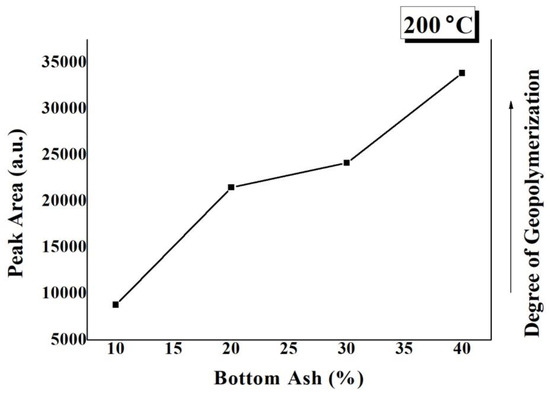
Figure 6.
Degree of geopolymerization dependence on bottom ash content.
It can be seen from Figure 5b that the peaks of Si-O-T asymmetric stretching bonds of BA40-200 and BA40-600 have been shifted more towards lower wavenumbers and are also deeper, as compared to the peaks of BA40-400 and BA40-500. This shows that the degree of geopolymerization is higher in BA40-200 and BA40-600 than in BA40-400 and BA40-500.
4.4. Bulk Density and Foamability
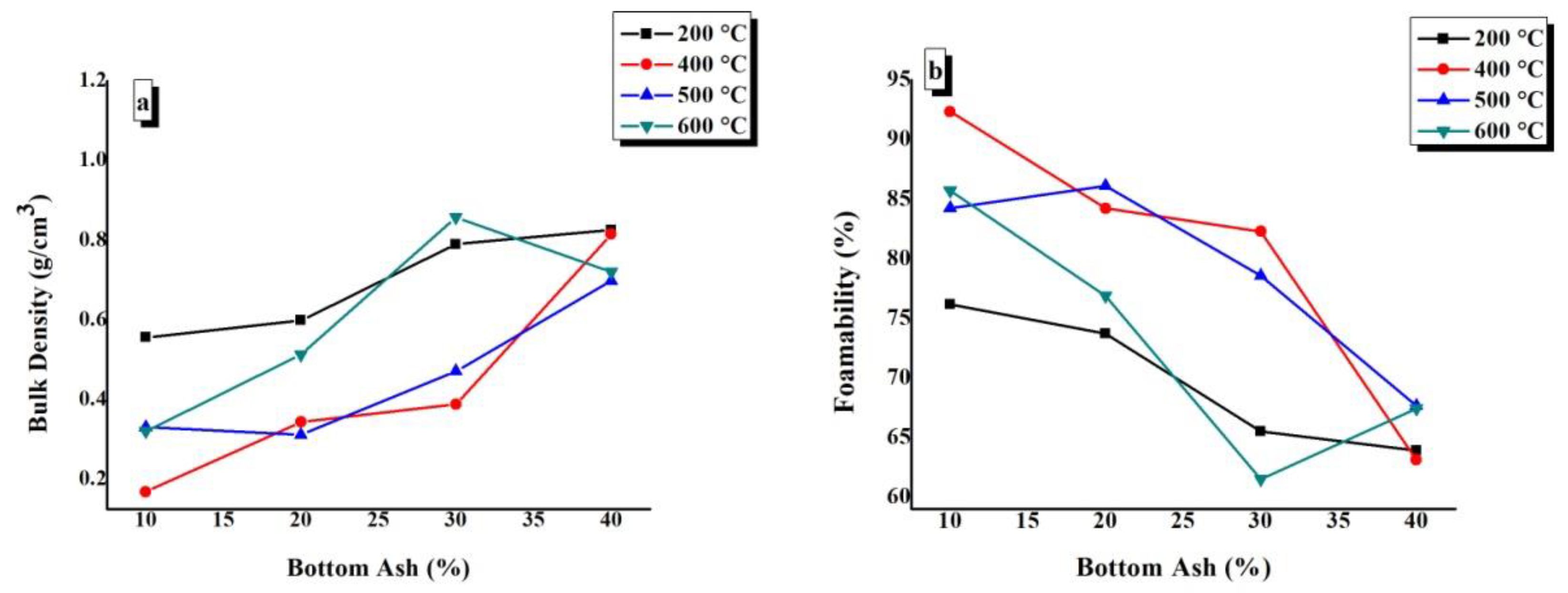
The calculated values of bulk density and foamability were plotted against BA content at various CTs, as shown in Figure 7. It can be seen from Figure 7a,b that there is a general trend of increasing bulk density and decreasing foamability with an increase in BA content at each CT. Figure 7a,b shows that the minimum bulk density and maximum foamability are presented by TGPFs cured at 400 °C and 500 °C, as can be seen from 400 °C and 500 °C curves.. There is an exception at 600 °C, where the bulk density decreases when we increase BA content from 30% to 40%. The higher density in samples treated at 600 °C is attributed to the viscous sintering which takes place above glass transition temperature of sodium silicate.
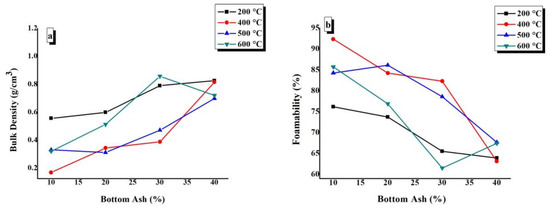
Figure 7.
Effect of bottom ash content on (a) bulk density and (b) foamability of TGPFs.
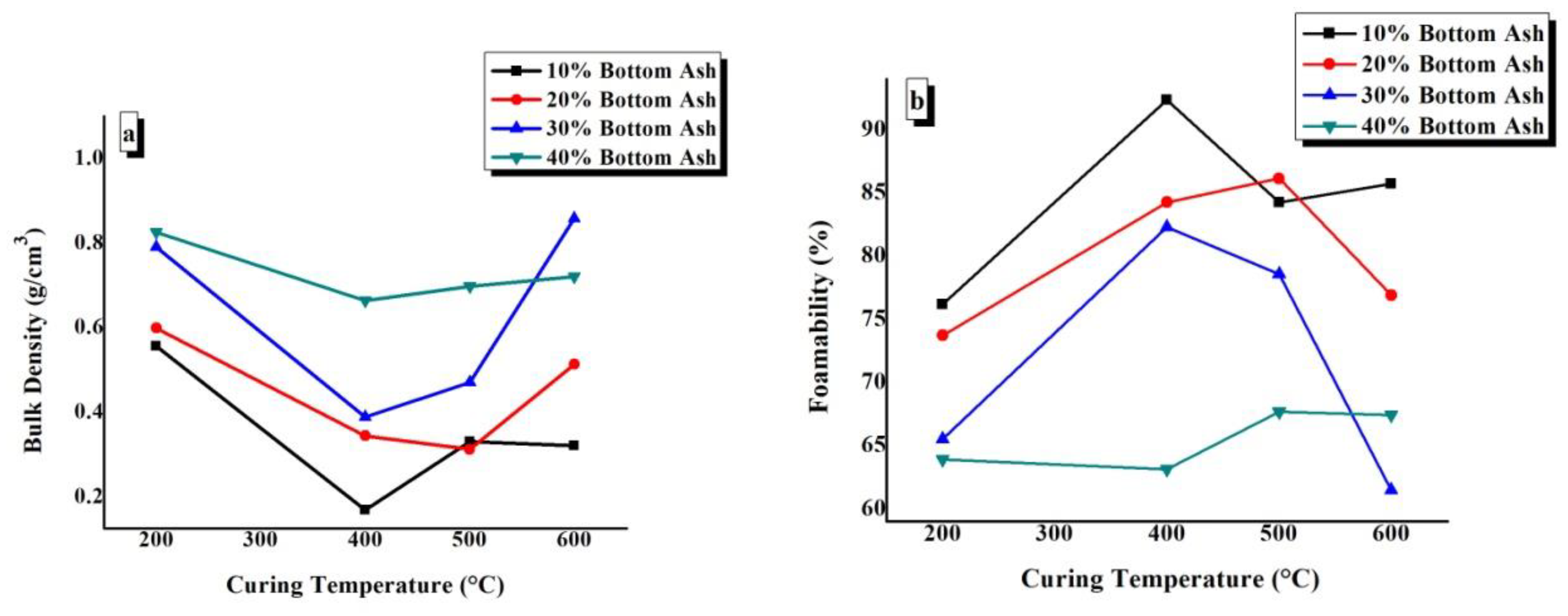
In Figure 8a,b, the bulk density and foamability values of TGPFs have been plotted against CT for all the compositions. Figure 8 shows that as we increase CT from 200 °C to 400 °C, bulk density decreases and foamability increases. Then with the increase in CT from 400 °C to 600 °C, there is an increase in bulk density and decrease in foamability in general. Figure 8a,b shows that 10% and 20% BA compositions demonstrated the minimum density and maximum foamability at all of the curing temperatures. The maximum bulk density can be seen for the 40% BA curve, as shown in Figure 8a, and the maximum foamability can be observed for the 10% BA composition, as shown in Figure 8b. The maximum bulk density of 0.858 g/ cm3was observed for BA30-600, and the maximum foamability of 92.34% was observed for BA10-400.
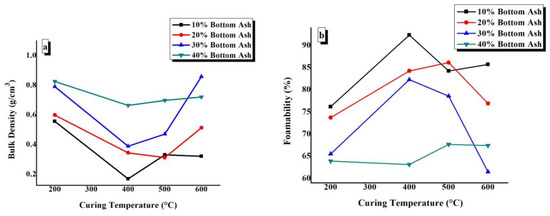
Figure 8.
Effect of curing temperature on (a) the bulk density and (b) the foamability of TGPFs.
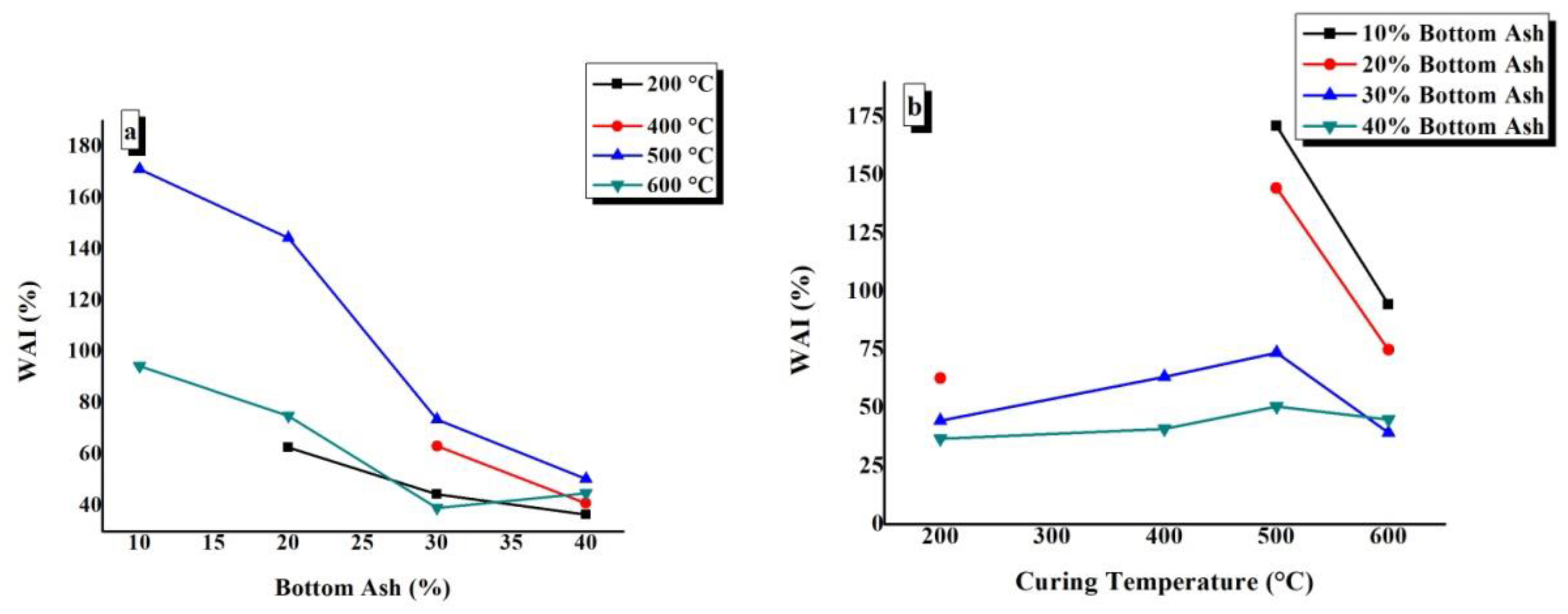
4.5. Water Absorption Index (WAI)
WAI values of TGPFs were plotted against BA and CT, as shown in Figure 9. The constant CT and BA content are indicated in the top right corner of the graphs. Figure 9a shows that the points of BA10-200, BA10-400, and BA20-400 are absent because these samples decomposed or dissolved completely after 48h in water due to the solubility of the SS. It can be seen from Figure 9a that WAI values decrease as the bottom ash content increases at all of the curing temperatures. With the increase in the bottom ash content, the foamability of the TGPFs decreased, and the WAI is directly related to the foamability of TGPFs, hence the WAI decreased with increased bottom ash content. At 600 °C, WAI increases as the bottom ash content increases from 30% to 40%. This behaviour is due to the irregular foaming presented by 40%BA composition when cured at 600 °C, as discussed earlier, and is in accordance with the decrease in bulk density and increase in foamability as the BA is increased from 30% to 40% at 600 °C curing temperature, as was mentioned earlier in 4.4. Figure 9a shows that the maximum WAI values are obtained at 500 °C CT for all the compositions, as can be seen from the 500 °C curve.
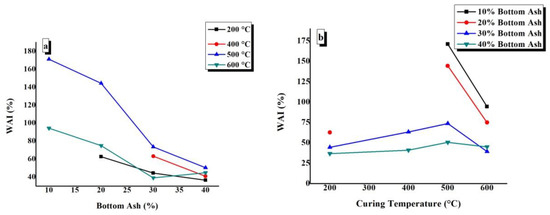
Figure 9.
Effect of (a) bottom ash content and (b) curing temperature on the water absorption index (WAI) of TGPFs.
It is clear from Figure 9b that the WAI values increase as the curing temperature increases from 200 °C to 500 °C for all of the compositions. As the temperature increases from 500 °C to 600 °C, there is a decrease in WAI values due to the viscous phase transition from 500 °C to 600 °C. Figure 9b shows that WAI values are at the maximum for the 10% BA composition, as can be seen from the curve of the 10% BA composition. The maximum WAI value of 171%can be observed for BA10-500 and the minimum WAI value of 36.68% can be observed for BA40-200.
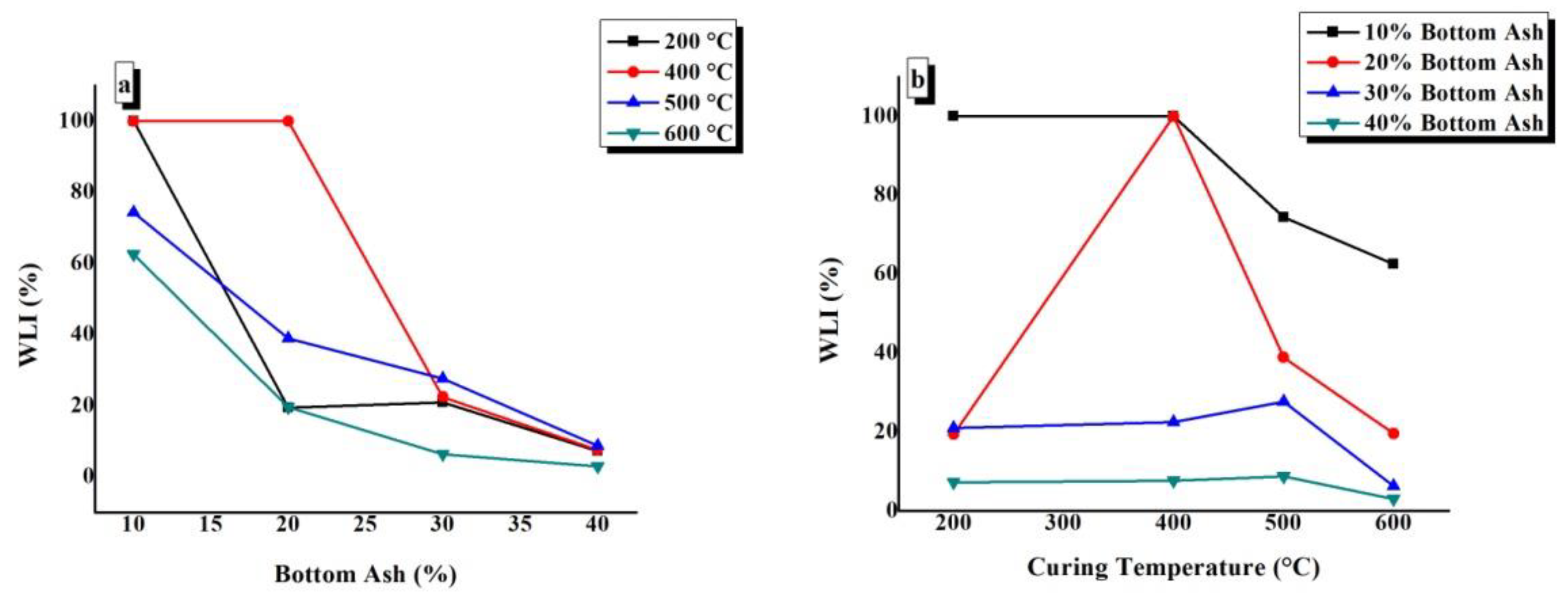
4.6. Weight Loss Index (WLI)
WLI values of TGPFs were plotted against BA content at different CTs, as shown in Figure 10a. It can be seen from the figure thatBA10-200, BA10-400, and BA20-400 have WLI values of 100% (i.e., these samples completely dissolved in water or disintegrated in the form of powder, as has been mentioned before in WAI test). Figure 10a shows that the WLI values of TGPFs decrease with the increase in the BA content. We can see from the figure that the highest values of WLI are for the TGPFs cured at 400 °C and 500 °C, which show the minimum degree of geopolymerization, which is in accordance with the FTIR analysis. The FTIR curves for 400 °C and 500 °C in Figure 5b show the minimum degree of geopolymerization, as discussed in 4.3. The WLI curve at 600 °C is the lowest in Figure 10a, which shows that the TGPFs cured at 600 °C have the minimum WLI values and are most stable in water.
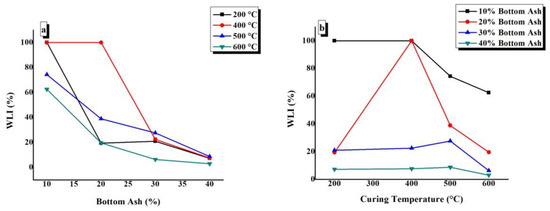
Figure 10.
Effect of (a) bottom ash content and (b) curing temperature on the WLI of TGPFs.
The WLI values of TGPFs were plotted against CT for different compositions, as shown in Figure 10b. It can be seen from the figure that WLI increases as CT increases from 200 °C to 500 °C and then it decreases again when CT is increased from 500 °C to 600 °C, which is due to the viscous phase transition from 500 °C to 600 °C. The WLI values are at the minimum for the 40% bottom ash content, as shown by the 40% BA curve in Figure 10b (i.e., the maximum degree of geopolymerization, which is in good agreement with FTIR analysis, as shown in Figure 6). Therefore, the degree of geopolymerization is inversely proportional to the WLI value. Figure 10b shows that when TGPFs with40% bottom ash are cured at different temperatures, there is a very small difference in the WLI values of the TGPFs. The BA40-400 sample shows the minimum WLI value of 3% (i.e., BA40-400 is the most stable sample in water).
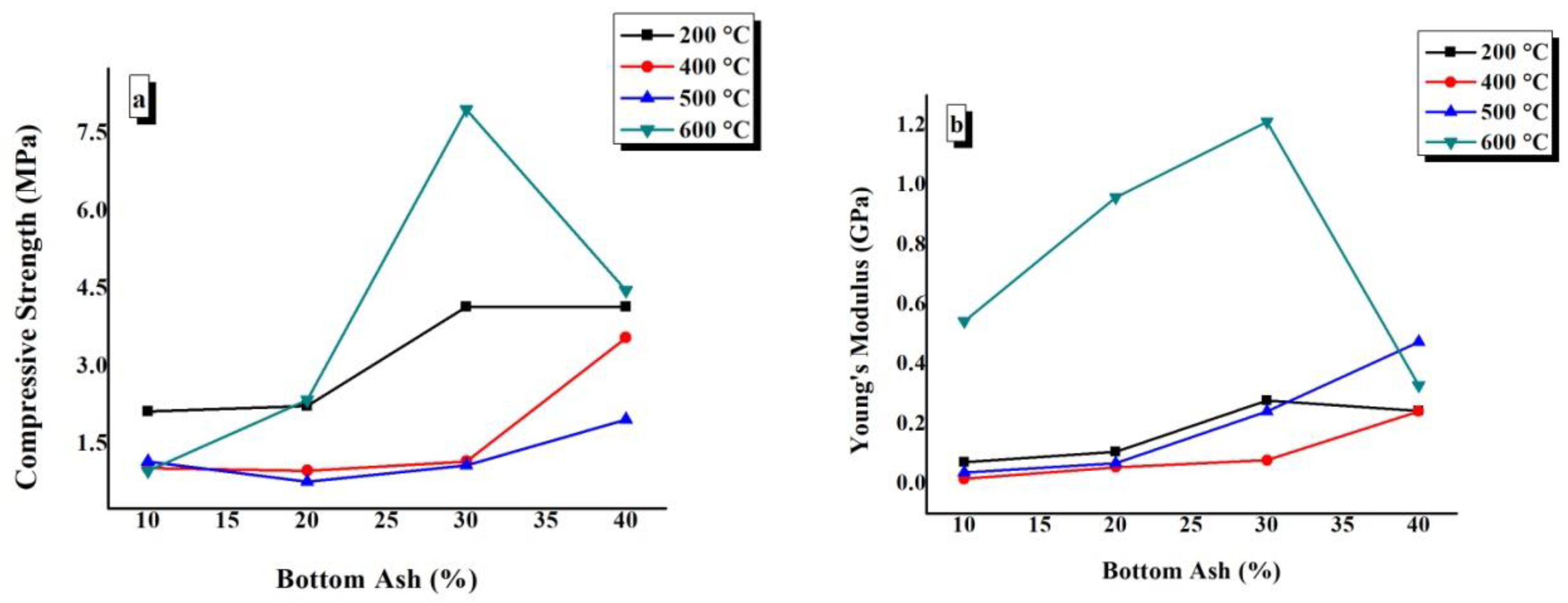
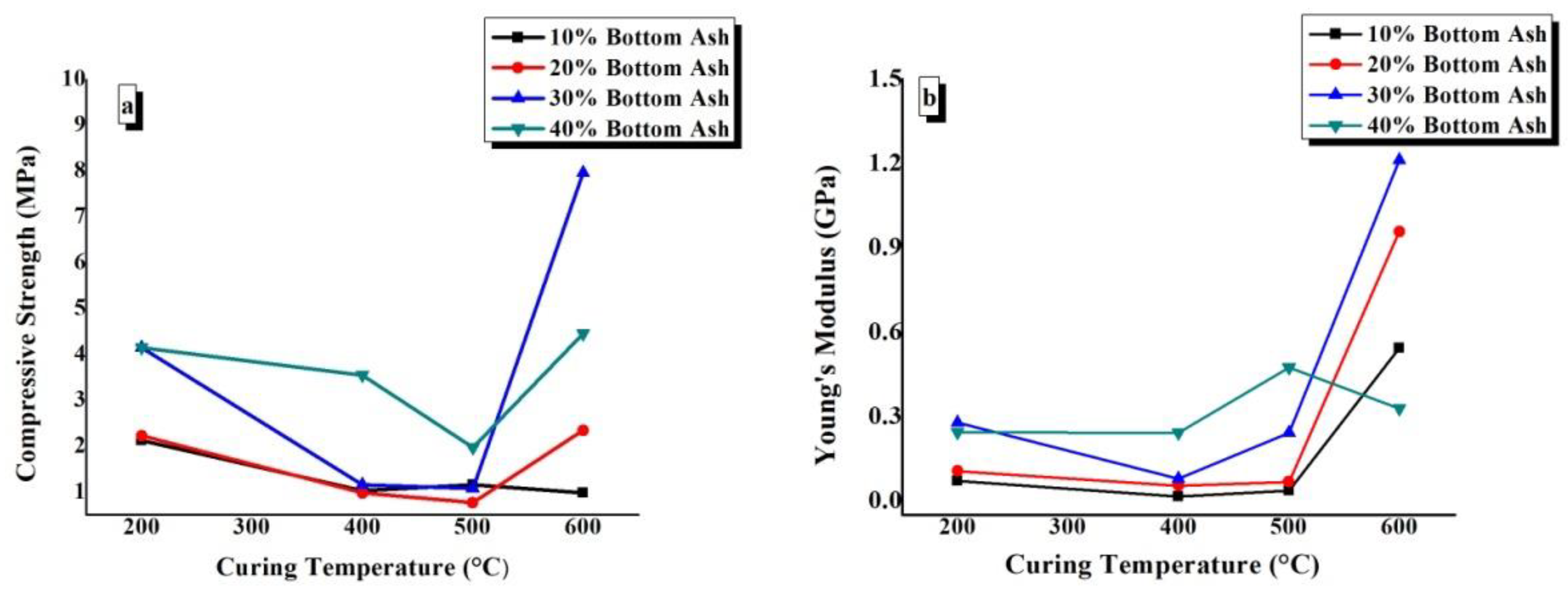
4.7. Compressive Strength and Young’s Modulus
The compressive strength and Young’s modulus values have been plotted against the BA content of TGPFs cured at a constant temperature in Figure 11. In Figure 12, compressive strength and Young’s modulus are plotted against curing temperature for all of the compositions.
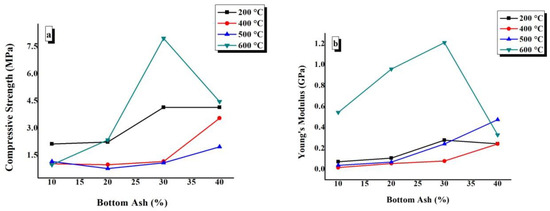
Figure 11.
Effect of composition on the (a) compressive strength and (b) Young’s modulus of TGPFs at a constant curing temperature.
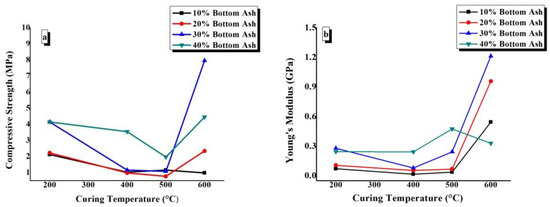
Figure 12.
Effect of curing temperature on the (a) compressive strength and (b) Young’s modulus of TGPFs of the same composition.
It can be seen from Figure 11 that there is a general trend of increasing compressive strength and Young’s modulus with increasing BA or decreasing liquid/solid ratio at all CTs, with an exception of a decrease in compressive strength and Young’s modulus values at 600 °C as we increase BA content from 30% to 40%, which is due to irregular foaming of 40% BA composition cured at 600 °C; this result is in accordance with the bulk density values, as bulk density also decreases when BA content is increased from 30% to 40% at 600 °C CT, as mentioned earlier in 4.4. Figure 11 shows that the maximum compressive strength and Young’s modulus values are presented by the TGPFs cured at 600 °C. The maximum Young’s modulus values at the curing temperature of 600 °C suggests the catastrophic failure of TGPFs cured at 600 °C, and this effect was observed during the mechanical testing. The curves at 400 °C and 500 °C CTs shows the minimum compressive strength and Young’s modulus. This behaviour is in accordance with the bulk density, WLI, and FTIR analysis results, which show the minimum degree of geopolymerization for TGPFs cured at 400 °C and 500 °C, as shown earlier in Figure 5b.
Figure 12a shows that there is a general trend of decrease in compressive strength as CT increases from 200 °C to 500 °C, and then there is a sharp increase in the compressive strength as we move from 500 °C to 600 °C. This behaviour is due to the viscous phase transition while moving from 500 °C to 600 °C, as mentioned earlier. Figure 11b shows that the Young’s modulus values decrease from 200 °C to 400 °C and then increase again up to 600 °C CT for all the compositions except for 40%, in which the Young’s modulus decreases as the CT is increased from 500 °C to 600 °C. It is clear from Figure 11(a and b) that the minimum compressive strength and Young’s modulus values are obtained by 10% and 20% BA compositions, and this effect is in accordance with the bulk density, FTIR, and WLI values, which show the minimum degree of geopolymerization for the10% and 20% compositions, as shown earlier in Figure 6. The maximum compressive strength and Young’s modulus values are 7.96MPa and 1.21GPa, which are obtained by BA30-600.
5. Conclusions
Aerated geopolymer foams were obtained by thermal process. Sodium silicate was used as an alkaline activator for geopolymerization, and temperature activated the foaming. Rapid heating at temperature above 120 °Ctransformed the viscous slurry into a lightweight foam due to the endothermic process associated with the emission of water vapour. Vapour was trapped into the sticky viscous slurry, resulting in small bubbles and closed pore foam. The foam became rigid as the hydrated silicate condensed. Subsequently, it acquired greater mechanical strength with the development of geopolymerization reactions.
A systematic investigation on the correlation of processing to properties was carried out. The bottom ash (BA)fraction was observed to influence compressive strength, bulk density, and the degree of geopolymerization, which increase with BA content, while WAI, WLI, and foamability decrease. A sample from batch BA10-500 showed the maximum foamability (171%) and high WLI (74%). The curing temperature was observed to affect compressive strength, bulk density, degree of geopolymerization, and foamability. From 200 to 500 °C, compressive strength, bulk density, and foaming were observed to decrease, while WAI, WLI, and foamability were observed to increase. At temperatures above 500 °C, compressive strength, bulk density, and degree of geopolymerization were observed to increase and WAI, WLI, and foamability were observed to decrease. Above 500 °C, the system crossed its glass transition temperature, therefore due to viscous flow, densification and strengthening occurred. The maximum compressive strength of8MPa was shown by BA30-600, with 39% foamability and 6% WLI.
As evidenced from the results of this study, a wide range of properties can be obtained depending on the different processes and compositions used, thus allowing for a wide range of potential applications.
Author Contributions
Conceptualization, A.L., E.U.H. and S.K.P.; methodology, A.L., E.U.H. and M.S.A.; software, M.S.A.; validation, E.U.H. and M.S.A.; formal analysis, M.S.A. and K.R.; investigation, M.S.A. and K.R.; data curation, M.S.A. writing—original draft preparation, E.U.H. and M.S.A.; writing—review and editing, A.L and S.K.P.; visualization, E.U.H and K.R.; supervision, E.U.H and K.R.; project administration, E.U.H and K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kalpana, M.; Mohith, S. Study on autoclaved aerated concrete: Review. Mater. Today Proc. 2020, 22, 894–896. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, J.; Lu, X.; Liu, M.; Lin, Y.; Gong, W.; Ning, G. Preparation of sintered foam materials by alkali-activated coal fly ash. J. Hazard. Mater. 2010, 174, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Hardjito, D.; Fung, S. FLY ASH-BASED GEOPOLYMER MORTAR INCORPORATING BOTTOM ASH. Mod. Appl. Sci. 2009, 4, 44. [Google Scholar] [CrossRef]
- Revathi, V.; Thaarrini, J.; Rao, M.V. A Prospective Study on Alkali Activated Bottom Ash-GGBS Blend in Paver Blocks. Int. J. Civ. Environ. Eng. 2014, 8, 291–298. [Google Scholar]
- Cox, M.; Nugteren, H.; Janssen-Jurkovičová, M. Combustion Residues: Current, Novel and Renewable Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-09443-3. [Google Scholar]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Chen, B.; Wang, K.; Chen, X.; Lu, A. Study of Foam Glass with High Content of Fly Ash Using Calcium Carbonate as Foaming Agent. Mater. Lett. 2012, 79, 263–265. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Tulyaganov, D.U.; Ferreira, J.M.F. Preparation and characterization of foams from sheet glass and fly ash using carbonates as foaming agents. Ceram. Int. 2009, 35, 229–235. [Google Scholar] [CrossRef]
- Chen, X.; Lu, A.; Qu, G. Preparation and characterization of foam ceramics from red mud and fly ash using sodium silicate as foaming agent. Ceram. Int. 2013, 39, 1923–1929. [Google Scholar] [CrossRef]
- Ul Haq, E.; Kunjalukkal Padmanabhan, S.; Licciulli, A. Microwave synthesis of thermal insulating foams from coal derived bottom ash. Fuel Process. Technol. 2015, 130, 263–267. [Google Scholar] [CrossRef]
- Lopes, A.V.; Lopes, S.M.R.; Pinto, I. Influence of the Composition of the Activator on Mechanical Characteristics of a Geopolymer. Appl. Sci. 2020, 10, 3349. [Google Scholar] [CrossRef]
- Xie, T.; Ozbakkaloglu, T. Behavior of low-calcium fly and bottom ash-based geopolymer concrete cured at ambient temperature. Ceram. Int. 2015, 41, 5945–5958. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Jamaludin, L.; Hussin, K.; Bnhussain, M.; Ghazali, C.M.R.; Ahmad, M.I. Fly Ash Porous Material using Geopolymerization Process for High Temperature Exposure. Int. J. Mol. Sci. 2012, 13, 4388–4395. [Google Scholar] [CrossRef] [PubMed]
- Emdadi, Z.; Asim, N.; Amin, M.H.; Ambar Yarmo, M.; Maleki, A.; Azizi, M.; Sopian, K. Development of Green Geopolymer Using Agricultural and Industrial Waste Materials with High Water Absorbency. Appl. Sci. 2017, 7, 514. [Google Scholar] [CrossRef]
- Lancellotti, I.; Ponzoni, C.; Barbieri, L.; Leonelli, C. Alkali activation processes for incinerator residues management. Waste Manag. 2013, 33, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Belova, E.V.; Kolyagin, Y.A.; Uspenskaya, I.A. Structure and glass transition temperature of sodium-silicate glasses doped with iron. J. Non-Cryst. Solids 2015, 423–424, 50–57. [Google Scholar] [CrossRef]
- ul Haq, E.; Kunjalukkal Padmanabhan, S.; Licciulli, A. Synthesis and characteristics of fly ash and bottom ash based geopolymers–A comparative study. Ceram. Int. 2014, 40, 2965–2971. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. Use of Infrared Spectroscopy to Study Geopolymerization of Heterogeneous Amorphous Aluminosilicates. Langmuir 2003, 19, 8726–8734. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).