Osteogenic Potential of Fast Set Bioceramic Cements: Molecular and In Vitro Study
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Osteogenic Differentiation
2.2. Biomaterial Preparation and Eluate Collection
2.3. Cell Attachment and Biocompatibility
- (a)
- 1 × 104 MG-63 cells cultured in 1 mL of NM (positive control);
- (b)
- 1 × 104 MG-63 cells cultured in 1 mL of EM, obtained, as previously described;
- (c)
- 1 × 104 MG-63 cells cultured in 1 mL of NM together with a pre-prepared bioceramic disc.
2.4. Osteogenic Differentiation
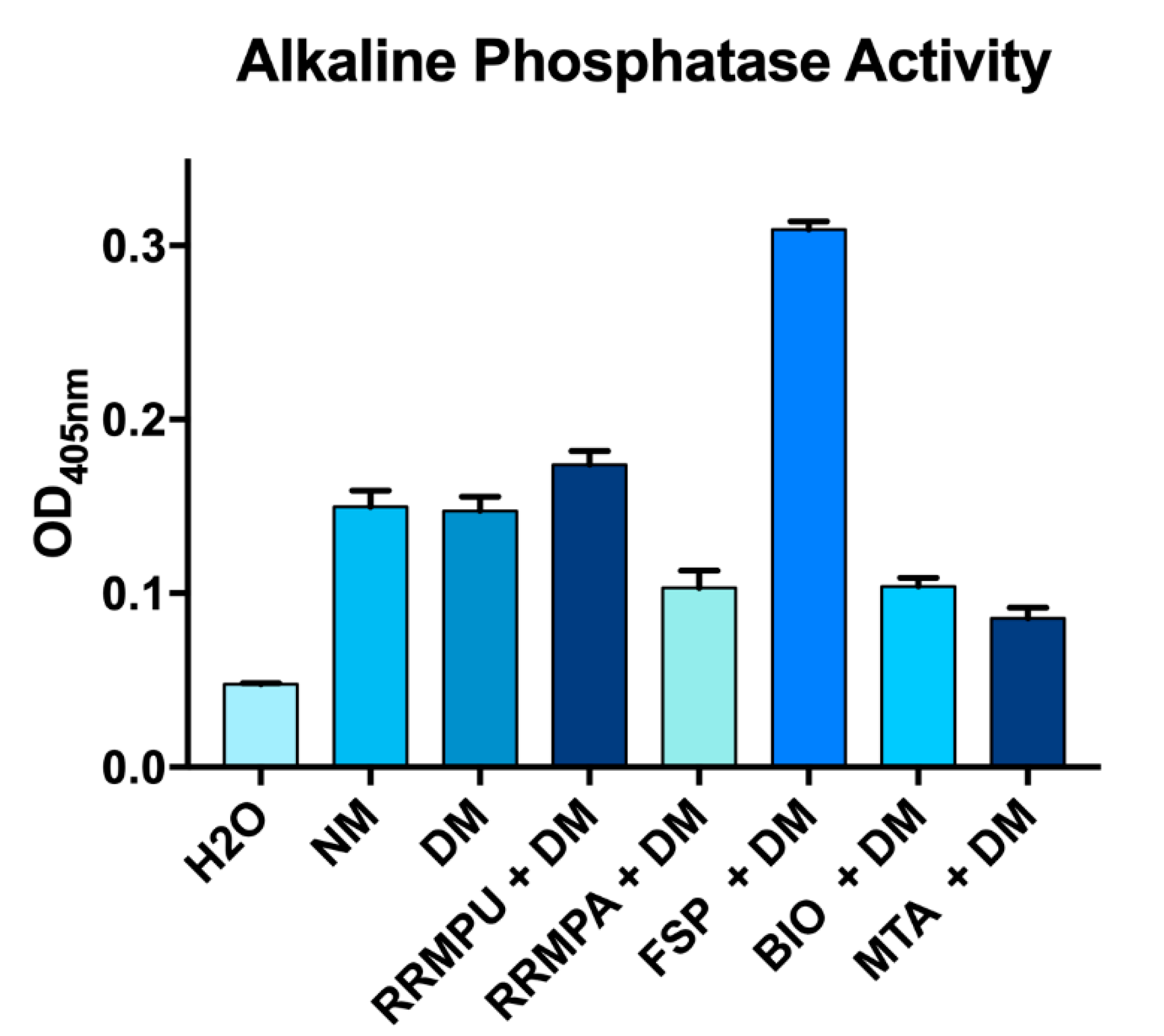
2.5. Alkaline Phosphatase Activity
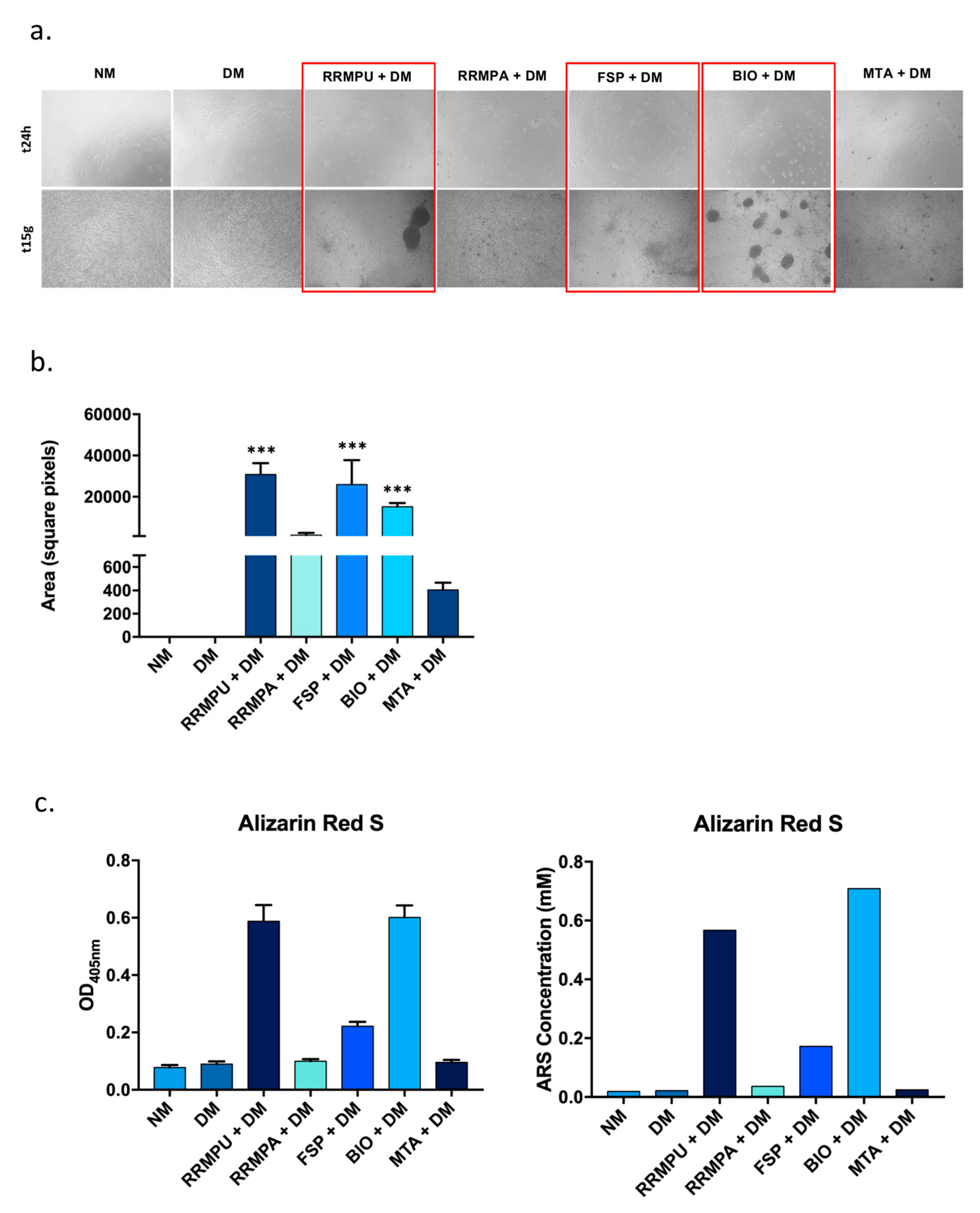
2.6. Mineralization and Alizarin Red S Staining
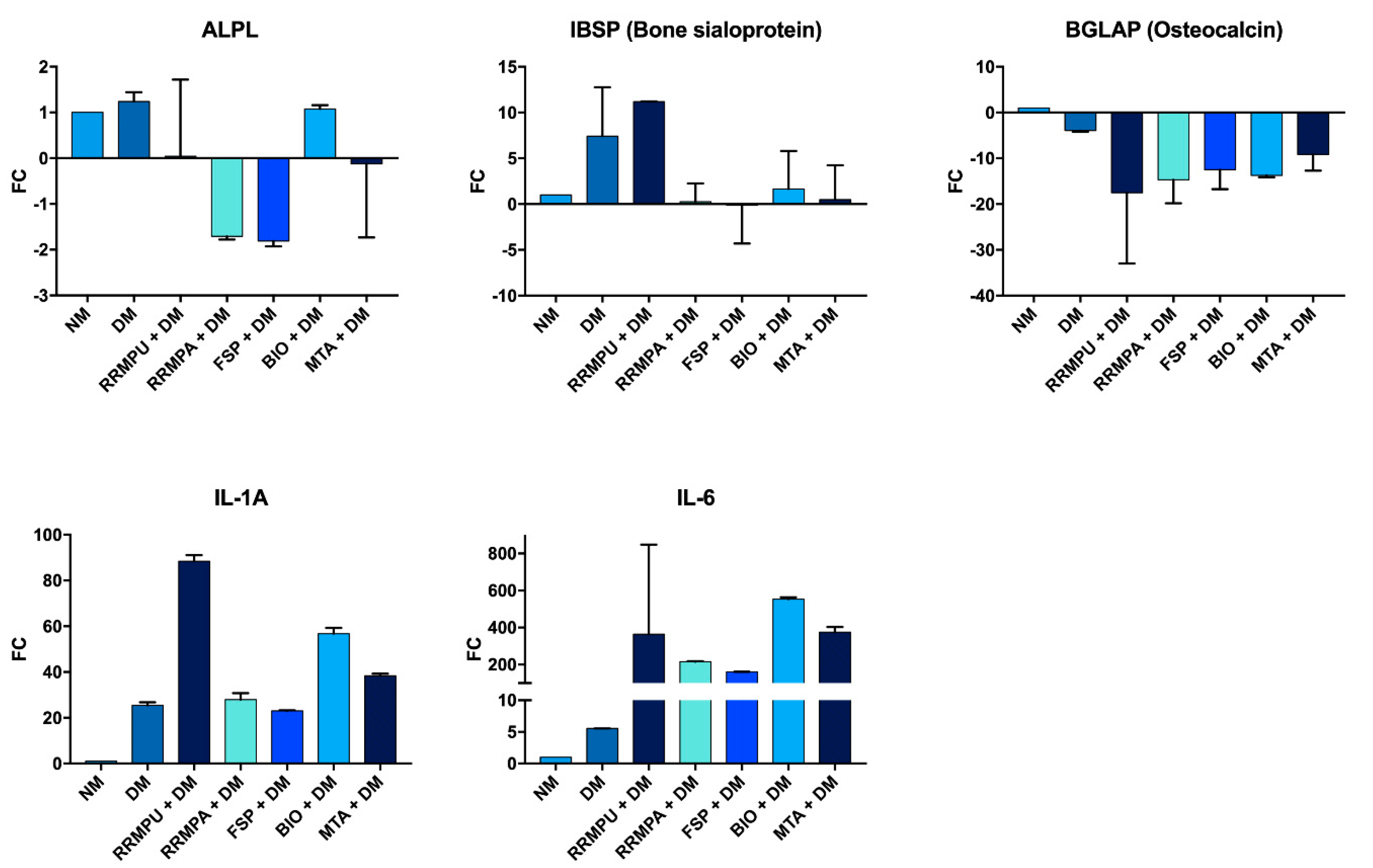
2.7. Quantitative Real-Time PCR (RT-qPCR)
2.8. Statistical Analysis
3. Results
3.1. Cellular Attachment and Biocompatibility
3.2. Osteogenic Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peters, L.B.; Wesselink, P.R.; Buijs, J.F.; Van Winkelhoff, A.J. Viable Bacteria in Root Dentinal Tubules of Teeth with Apical Periodontitis. J. Endod. 2001, 27, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ørstavik, D.; Pitt Ford, T.R. Essential Endodontology: Prevention and Treatment of Apical Periodontitis, 2nd ed.; Blackwell Munksgaard: Oxford, UK, 2008. [Google Scholar]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part I: Vital pulp therapy. Int. Endod. J. 2017, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2017, 51, 284–317. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Watson, T.; Ford, T.P. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J. Endod. 1993, 19, 591–595. [Google Scholar] [CrossRef]
- Yoshino, P.; Nishiyama, C.K.; Modena, K.C.D.S.; Santos, C.F.; Sipert, C.R. In vitro cytotoxicity of white MTA, MTA Fillapex® and portland cement on human periodontal ligament fibroblasts. Braz. Dent. J. 2013, 24, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef]
- Camilleri, J.; Sorrentino, F.; Damidot, D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent. Mater. 2013, 29, 580–593. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; Ginebra, M.-P.; Prati, C. Fluoride-containing nanoporous calcium-silicate MTA cements for endodontics and oral surgery: Early fluorapatite formation in a phosphate-containing solution. Int. Endod. J. 2011, 44, 938–949. [Google Scholar] [CrossRef]
- Trope, M.; Bunes, A.; Debelian, G. Root filling materials and techniques: Bioceramics a new hope? Endod. Top. 2015, 32, 86–96. [Google Scholar] [CrossRef]
- Haapasalo, M.; Parhar, M.; Huang, X.; Wei, X.; Lin, J.; Shen, Y. Clinical use of bioceramic materials. Endod. Top. 2015, 32, 97–117. [Google Scholar] [CrossRef]
- Wang, Z. Bioceramic materials in endodontics. Endod. Top. 2015, 32, 3–30. [Google Scholar] [CrossRef]
- Zhou, H.M.; Shen, Y.; Wang, Z.; Li, L.; Zheng, Y.; Häkkinen, L.; Haapasalo, M. In vitro cytotoxicity evaluation of a novel root repair material. J. Endod. 2013, 39, 478–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taha, N.A.; Safadi, R.A.; Alwedaie, M.S. Biocompatibility evaluation of Endosequence root repair paste in the connective tissue of rats. J. Endod. 2016, 42, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Willershausen, I.; Wolf, T.; Kasaj, A.; Weyer, V.; Willershausen, B.; Briseño-Marroquín, B. Influence of a bioceramic root end material and mineral trioxide aggregates on fibroblasts and osteoblasts. Arch. Oral Biol. 2013, 58, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Du, T.F.; Li, H.B.; Shen, Y.; Mobuchon, C.; Hieawy, A.; Wang, Z.J.; Yang, Y.; Ma, J.; Haapasalo, M. Physical properties and hydration behavior of a fast-setting bioceramic endodontic material. BMC Oral Health 2016, 16, 23. [Google Scholar] [CrossRef] [Green Version]
- Primus, C.M.; Tay, F.R.; Niu, L.-N. Bioactive tri/dicalcium silicate cements for treatment of pulpal and periapical tissues. Acta Biomater. 2019, 96, 35–54. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, Q.; Zhou, X.; Gao, Y.; Huang, D. A comparative study on root canal repair materials: A cytocompatibility assessment in L929 and MG63 cells. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.L.; Huang, T.H.; Ding, S.J.; Shie, M.Y.; Kao, C.T. Comparison of calcium and silicate cement and mineral trioxide aggregate biologic effects and bone markers expression in MG63 cells. J. Endod. 2009, 35, 682–685. [Google Scholar] [CrossRef]
- Pizzoferrato, A.; Ciapetti, G.; Stea, S.; Cenni, E.; Arciola, C.R.; Granchi, D. Cell culture methods for testing biocompatibility. Clin. Mater. 1994, 15, 173–190. [Google Scholar] [CrossRef]
- Zhu, Q.; Haglund, R.; Safavi, K.E.; Spangberg, L.S. Adhesion of human osteoblasts on root-end filling materials. J. Endod. 2000, 26, 404–406. [Google Scholar] [CrossRef]
- Bidar, M.; Afshari, J.T.; Shahrami, F. Evaluation of adhesion and morphology of human osteoblasts to white MTA and Portland cement. Iran. Endod. J. 2007, 2, 87–90. [Google Scholar] [PubMed]
- Gomes-Cornélio, A.L.; Rodrigues, E.M.; Salles, L.; Mestieri, L.B.; Faria, G.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Bioactivity of MTA Plus, Biodentine and an experimental calcium silicate-based cement on human osteoblast-like cells. Int. Endod. J. 2016, 50, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Margunato, S.; Taşlı, P.N.; Aydın, S.; Kazandag, M.K.; Şahin, F. In vitro evaluation of ProRoot MTA, Biodentine, and MM-MTA on human alveolar bone marrow stem cells in terms of biocompatibility and mineralization. J. Endod. 2015, 41, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.; Gunn, W.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Harrington, D.; D’Agostino, R.B.; Gatsonis, C.; Hogan, J.W.; Hunter, D.J.; Normand, S.L.T.; Drazen, J.M.; Hamel, M.B. New guidelines for statistical reporting in the journal. N. Engl. J. Med. 2019, 381, 285–286. [Google Scholar] [CrossRef]
- Cheng, L.; Ye, F.; Yang, R.; Lu, X.; Shi, Y.; Li, L.; Fan, H.; Bu, H. Osteoinduction of hydroxyapatite/beta-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomater. 2010, 6, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, Y.; Haapasalo, M. Dental materials with antibiofilm properties. Dent. Mater. 2014, 30, e1–e16. [Google Scholar] [CrossRef] [PubMed]
- Attik, G.N.; Villat, C.; Hallay, F.; Pradelle-Plasse, N.; Bonnet, H.; Moreau, K.; Colon, P.; Grosgogeat, B. In vitro biocompatibility of a dentine substitute cement on human MG63 osteoblasts cells: Biodentine™ versus MTA. Int. Endod. J. 2014, 47, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Ford, T.P.; Torabinejad, M.; McDonald, F. Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials 1999, 20, 167–173. [Google Scholar] [CrossRef]
- Chiang, T.Y.; Ding, S.J. Comparative physicochemical and biocompatible properties of radiopaque dicalcium silicate cement and mineral trioxide aggregate. J. Endod. 2010, 36, 1683–1687. [Google Scholar] [CrossRef]
- Clover, J.; Gowen, M. Are MG-63 and HOS TE85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone 1994, 15, 585–591. [Google Scholar] [CrossRef]
- Ding, S.J.; Kao, C.T.; Chen, C.L.; Shie, M.Y.; Huang, T.H. Evaluation of human osteosarcoma cell line genotoxicity effects of mineral trixoide aggregate and calcium silicate cements. J. Endod. 2010, 36, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Camps, J.; About, I. BiodentineTM induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Xie, Z.; Tang, S.; Ye, G.; Wang, P.; Li, J.; Liu, W.; Li, M.; Wang, S.; Wu, X.; Cen, S.; et al. Interleukin-6/interleukin-6 receptor complex promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 13. [Google Scholar] [CrossRef] [Green Version]

| Material | Composition | pH |
|---|---|---|
| Root Repair Material Putty (RRMPU, Brasseler) | Calcium silicates, zirconium oxide, tantalum oxide, calcium phosphate monobasic | >12 |
| Root Repair Material Paste (RRMPA, Brasseler) | Calcium silicates, zirconium oxide, tantalum oxide, calcium phosphate monobasic | >12 |
| Fast-set Putty (FSP, Brasseler) | Calcium silicates, zirconium oxide, tantalum oxide, calcium phosphate monobasic | >12 |
| Biodentine (BIO, Septodont, Saint-Maur-des-Fosses, France) | Powder: tricalcium silicate, dicalcium silicate, calcium carbonate, zirconium oxide, calcium oxide, iron oxide Liquid: calcium chloride, a hydrosoluble polymer, water | 9 |
| ProRoot® Mineral Trioxide Aggregate (MTA, Dentsply Maillefer, Ballaigues, Switzerland) | Powder: tricalcium silicate, dicalcium silicate, bismuth oxide, tricalcium aluminate, calcium sulfate dihydrate or gypsum Liquid: water | 9 |
| ARS | p-Value | ALP | p-Value | ||
|---|---|---|---|---|---|
| NM vs. DM | ns | 0.998 | NM vs. DM | ns | >0.99 |
| NM vs. RRMPU + DM | **** | <0.0001 | NM vs. RRMPU + DM | * | 0.02 |
| NM vs. RRMPA + DM | ns | 0.9549 | NM vs. RRMPA + DM | *** | <0.001 |
| NM vs. FSP + DM | *** | 0.0003 | NM vs. FSP + DM | *** | <0.001 |
| NM vs. BIO + DM | **** | <0.0001 | NM vs. BIO + DM | *** | <0.001 |
| NM vs. MTA + DM | ns | 0.9843 | NM vs. MTA + DM | *** | <0.001 |
| DM vs. RRMPU + DM | **** | <0.0001 | DM vs. RRMPU + DM | ** | 0.01 |
| DM vs. RRMPA + DM | ns | 0.9993 | DM vs. RRMPA + DM | *** | <0.001 |
| DM vs. FSP + DM | *** | 0.0008 | DM vs. FSP + DM | *** | <0.001 |
| DM vs. BIO + DM | **** | <0.0001 | DM vs. BIO + DM | *** | <0.001 |
| DM vs. MTA + DM | ns | >0.9999 | DM vs. MTA + DM | *** | <0.001 |
| RRMPU + DM vs. RRMPA + DM | **** | <0.0001 | RRMPU + DM vs. RRMPA + DM | *** | <0.001 |
| RRMPU + DM vs. FSP + DM | **** | <0.0001 | RRMPU + DM vs. FSP + DM | *** | <0.001 |
| RRMPU + DM vs. BIO + DM | ns | 0.9959 | RRMPU + DM vs. BIO + DM | *** | <0.001 |
| RRMPU + DM vs. MTA + DM | **** | <0.0001 | RRMPU + DM vs. MTA + DM | *** | <0.001 |
| RRMPA + DM vs. FSP + DM | ** | 0.0016 | RRMPA + DM vs. FSP + DM | *** | <0.001 |
| RRMPA + DM vs. BIO + DM | **** | <0.0001 | RRMPA + DM vs. BIO + DM | ns | >0.99 |
| RRMPA + DM vs. MTA + DM | ns | >0.9999 | RRMPA + DM vs. MTA + DM | ns | 0.13 |
| FSP + DM vs. BIO + DM | **** | <0.0001 | FSP + DM vs. BIO + DM | *** | <0.001 |
| FSP + DM vs. MTA + DM | ** | 0.0012 | FSP + DM vs. MTA + DM | *** | <0.001 |
| BIO + DM vs. MTA + DM | **** | <0.0001 | BIO + DM vs. MTA + DM | ns | 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasqualini, D.; Comba, A.; Annaratone, L.; Mola, V.; Alovisi, M.; Breschi, L.; Mazzoni, A.; Scotti, N.; Cassoni, P.; Berutti, E. Osteogenic Potential of Fast Set Bioceramic Cements: Molecular and In Vitro Study. Appl. Sci. 2020, 10, 6713. https://doi.org/10.3390/app10196713
Pasqualini D, Comba A, Annaratone L, Mola V, Alovisi M, Breschi L, Mazzoni A, Scotti N, Cassoni P, Berutti E. Osteogenic Potential of Fast Set Bioceramic Cements: Molecular and In Vitro Study. Applied Sciences. 2020; 10(19):6713. https://doi.org/10.3390/app10196713
Chicago/Turabian StylePasqualini, Damiano, Allegra Comba, Laura Annaratone, Virginia Mola, Mario Alovisi, Lorenzo Breschi, Annalisa Mazzoni, Nicola Scotti, Paola Cassoni, and Elio Berutti. 2020. "Osteogenic Potential of Fast Set Bioceramic Cements: Molecular and In Vitro Study" Applied Sciences 10, no. 19: 6713. https://doi.org/10.3390/app10196713





