Experimental and Kinetic Investigation on NO Reduction by Rice Husk Char and Catalytically with CO
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
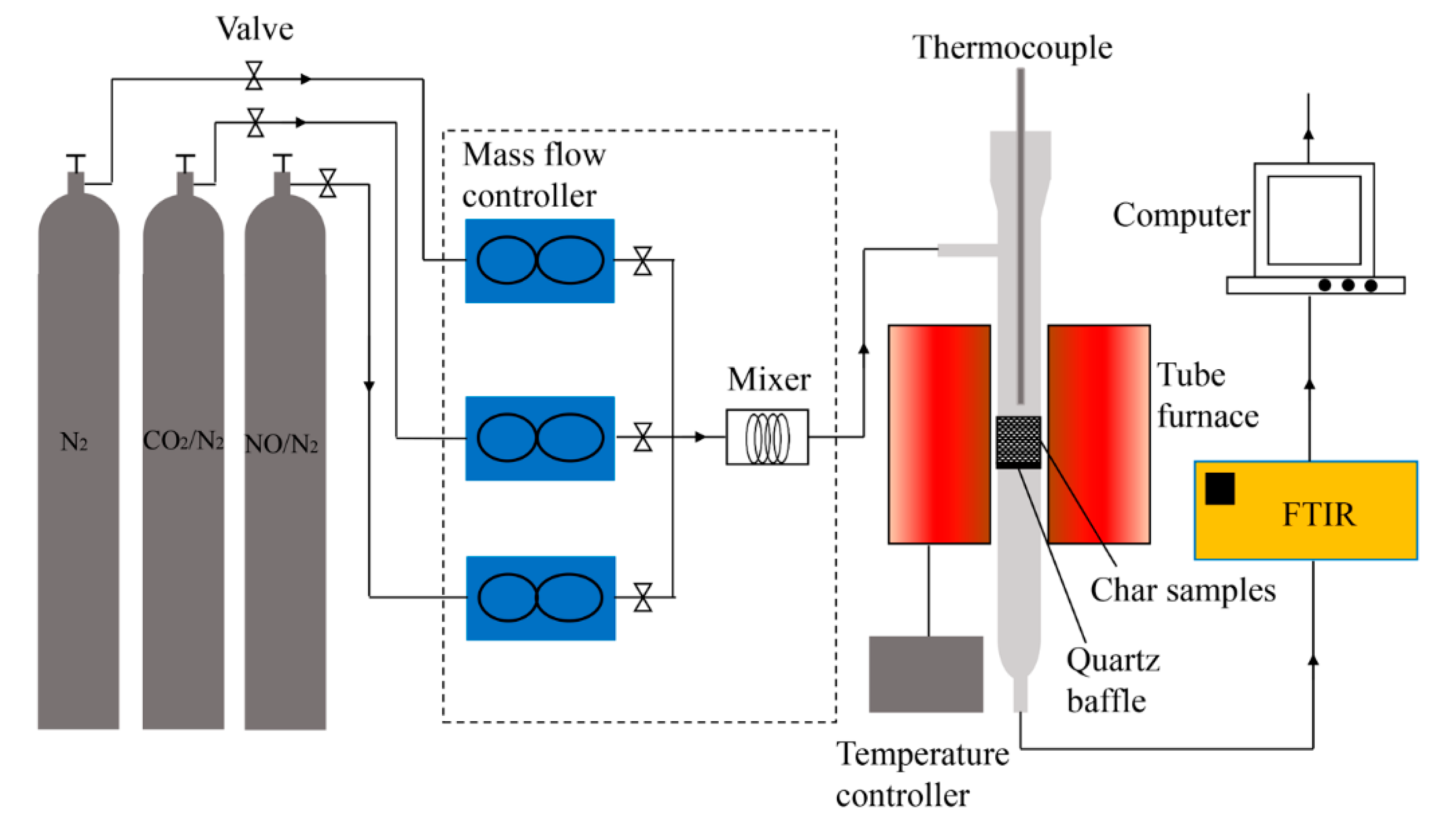
2.2. Experimental Set-Up and Procedure
3. Results
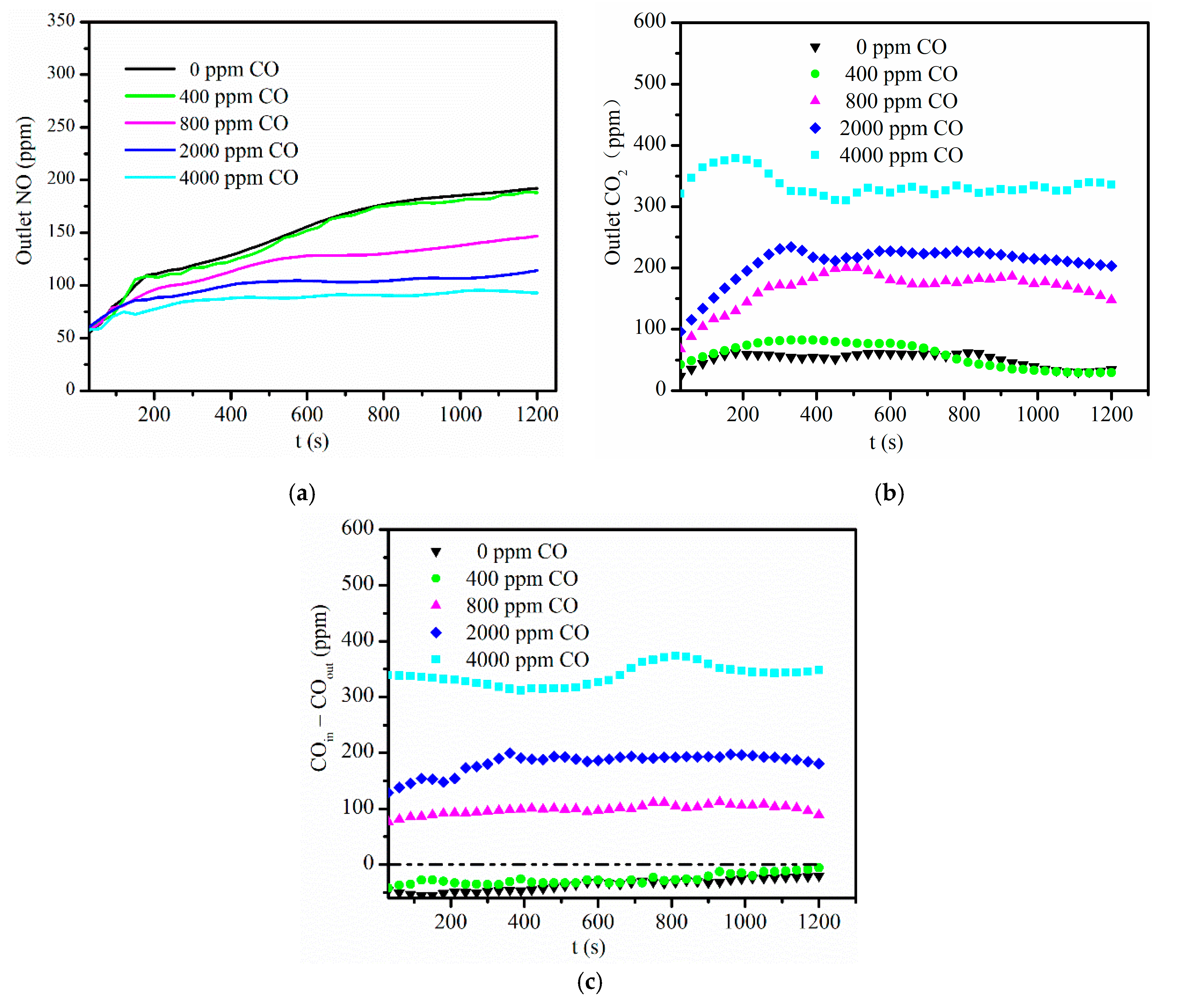
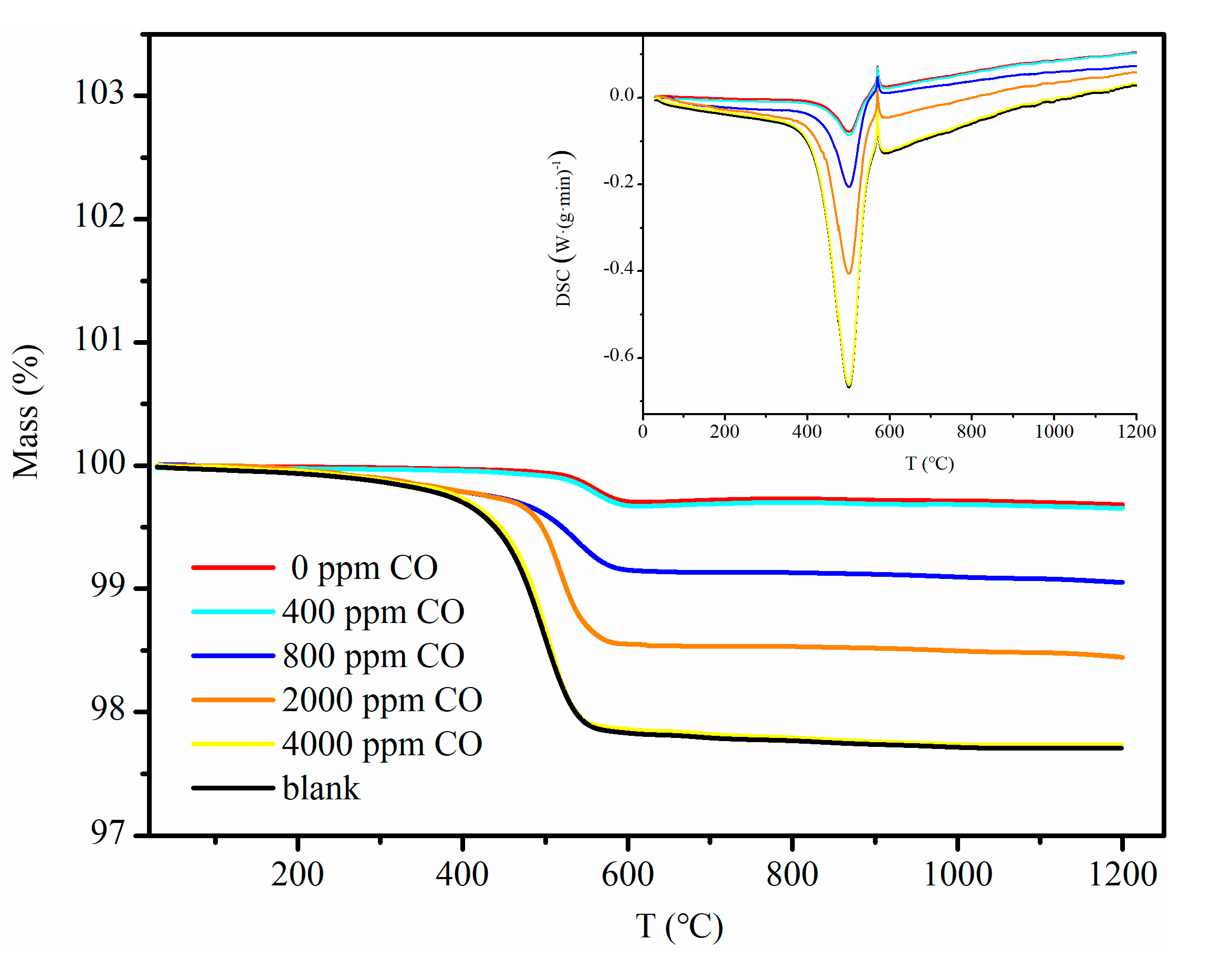
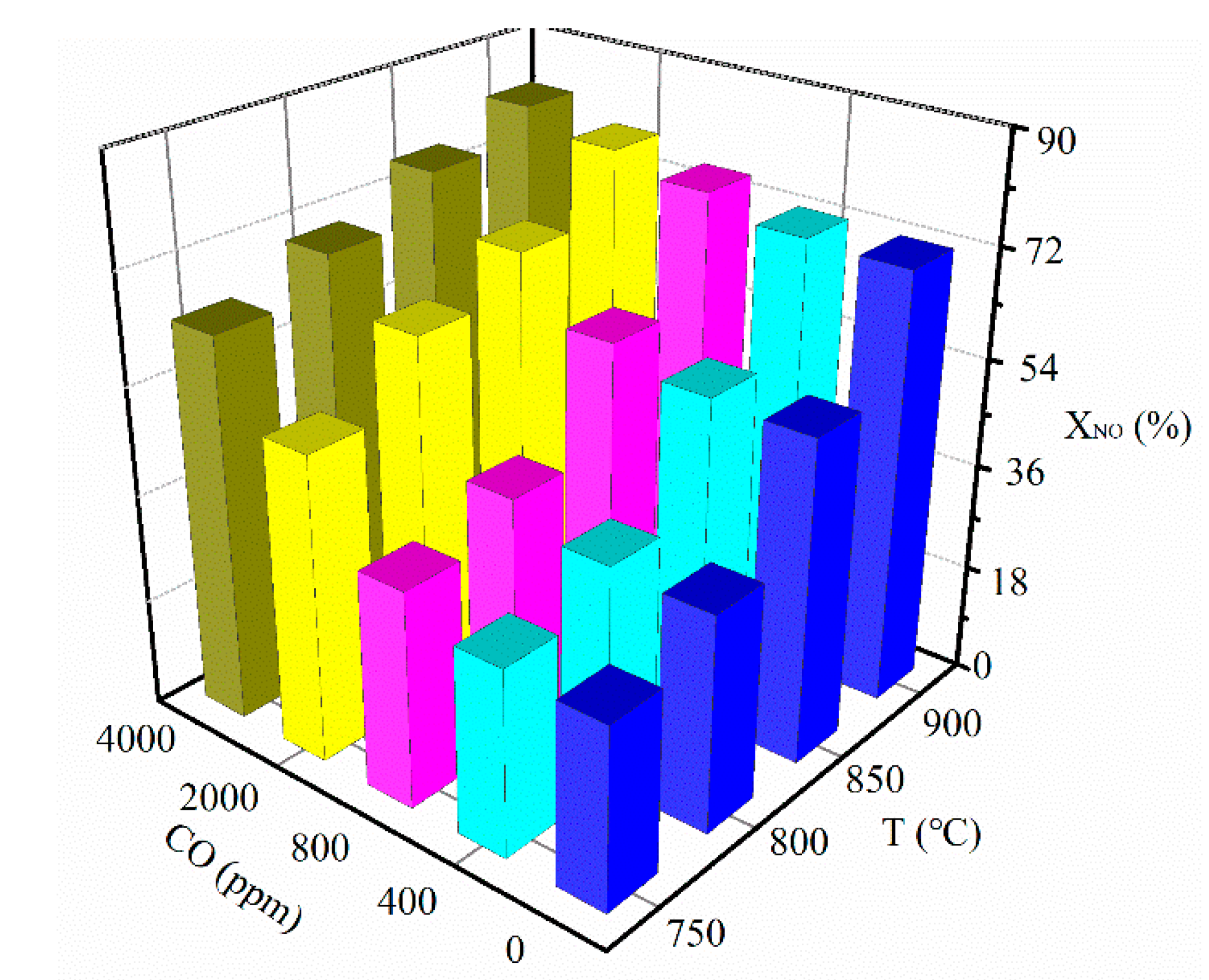
3.1. Effect of CO Concentration
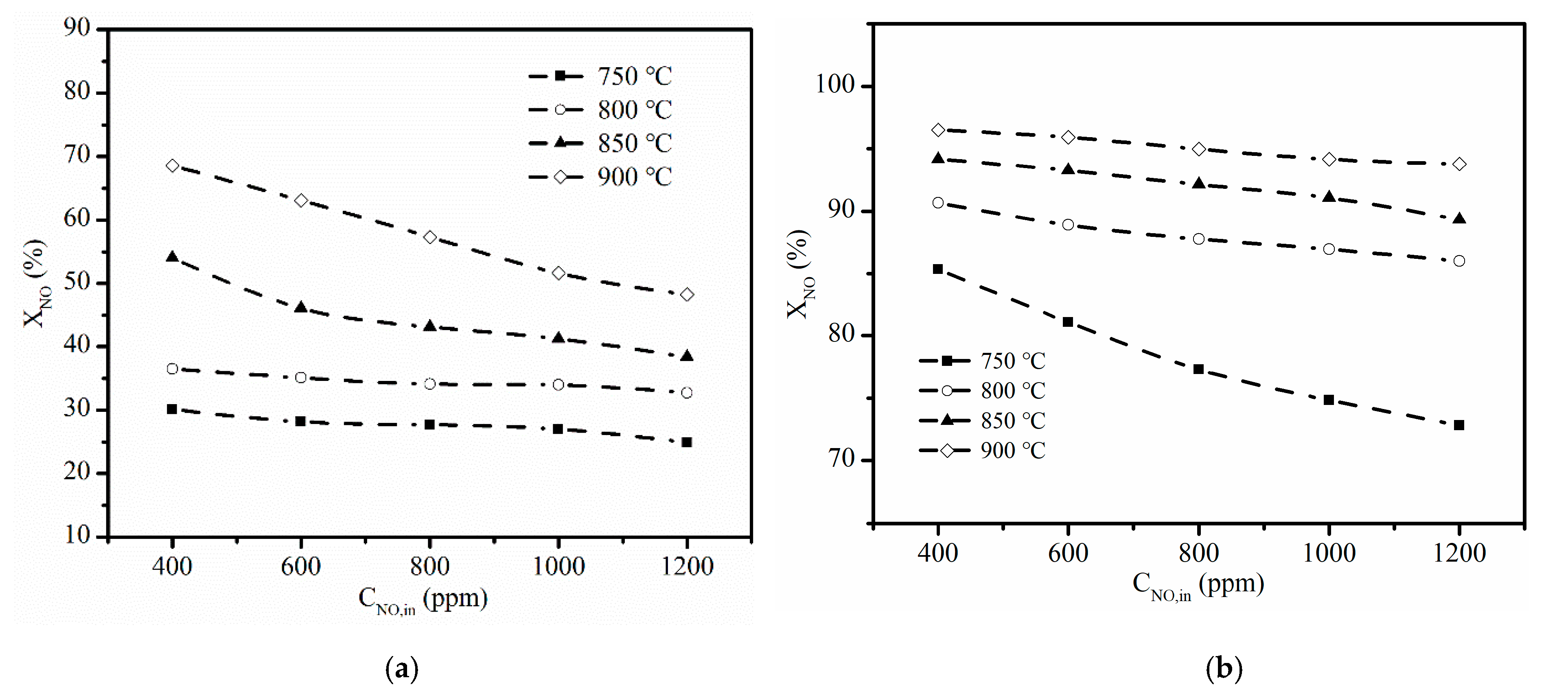
3.2. Effect of Reaction Temperature
3.3. Effect of Initial NO Concentration
3.4. Effect of Char Loading
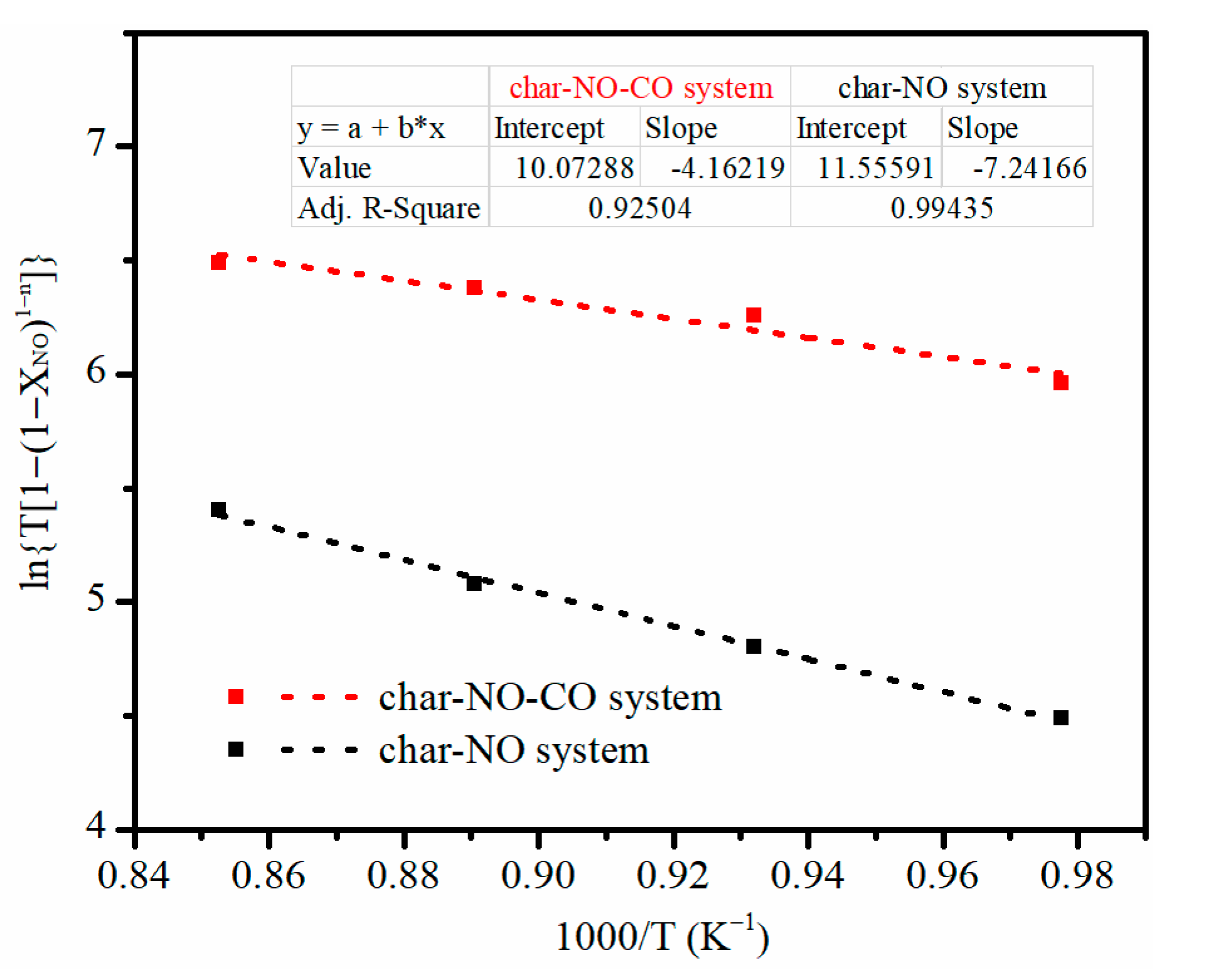
3.5. Determination of Reaction Order and Activation Energy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cui, S.; Hao, R.; Fu, D. Integrated method of non-thermal plasma combined with catalytical oxidation for simultaneous removal of SO2 and NO. Fuel 2019, 246, 365–374. [Google Scholar] [CrossRef]
- Gong, Z.; Shao, Y.; Pang, L.; Zhong, W.; Chen, C. Study on the emission characteristics of nitrogen oxides with coal combustion in pressurized fluidized bed. Chin. J. Chem. Eng. 2019, 27, 1177–1183. [Google Scholar] [CrossRef]
- Xu, M.; Li, S.; Wu, Y.; Jia, L. Reduction of recycled NO over char during oxy-fuel fluidized bed combustion: Effects of operating parameters. Appl. Energy 2017, 199, 310–322. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Hrynsphan, D.; Mei, Y.; Pan, H.; Wu, J.; Chen, J.-M.; Yao, J. FeII(EDTA)–NO Reduction by Mn Powder in Wet Flue Gas Denitrification Technology Coupled with Mn2+ Recycling: Performance, Kinetics, and Mechanism. Energy Fuels 2020, 34, 2590–2598. [Google Scholar] [CrossRef]
- Zheng, C.; Xiao, L.; Qu, R.; Liu, S.; Xin, Q.; Ji, P.; Song, H.; Wu, W.; Gao, X. Numerical simulation of selective catalytic reduction of NO and SO2 oxidation in monolith catalyst. Chem. Eng. J. 2019, 361, 874–884. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, Y.; Wang, Z.; Ma, C. Comparative study of coal based catalysts for NO adsorption and NO reduction by CO. Fuel 2018, 214, 230–241. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, X.; Wang, Z.; Ma, C.; Qin, Y. Investigation on Fe-Co binary metal oxides supported on activated semi-coke for NO reduction by CO. Appl. Catal. B Environ. 2017, 201, 636–651. [Google Scholar] [CrossRef]
- Shen, Q.; Liaw, S.B.; Costa, M.; Wu, H. Rapid Pyrolysis of Pulverized Biomass at a High Temperature: The Effect of Particle Size on Char Yield, Retentions of Alkali and Alkaline Earth Metallic Species, and Char Particle Shape. Energy Fuels 2020, 34, 7140–7148. [Google Scholar] [CrossRef]
- Wei, J.; Gong, Y.; Guo, Q.; Chen, X.; Ding, L.; Yu, G. A mechanism investigation of synergy behaviour variations during blended char co-gasification of biomass and different rank coals. Renew. Energy 2019, 131, 597–605. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, Y.; Wang, Z.; Huang, J.; Fang, Y. Interaction and its induced inhibiting or synergistic effects during co-gasification of coal char and biomass char. Bioresour. Technol. 2014, 173, 11–20. [Google Scholar] [CrossRef]
- Harding, N.; Adams, B.R. Biomass as a reburning fuel: A specialized cofiring application. Biomass Bioenergy 2000, 19, 429–445. [Google Scholar] [CrossRef]
- Wu, X.Y.; Song, Q.; Zhao, H.; Zhang, Z.H.; Yao, Q. Kinetic Modeling of Inherent Mineral Catalyzed NO Reduction by Biomass Char. Environ. Sci. Technol. 2014, 48, 4184–4190. [Google Scholar] [CrossRef] [PubMed]
- Aarna, I.; Suuberg, E.M. A review of the kinetics of the nitric oxide-carbon reaction. Fuel 1997, 76, 475–491. [Google Scholar] [CrossRef]
- Liu, J.; Tan, L.; Huang, L.; Wang, Q.; Liu, Y. Kinetic Monte Carlo Modeling for the NO–CO Reaction Mechanism on Rh(100) and Rh(111). Langmuir 2020, 36, 3127–3140. [Google Scholar] [CrossRef] [PubMed]
- Stanmore, B.; Tschamber, V.; Brilhac, J.-F. Oxidation of carbon by NOx, with particular reference to NO2 and N2O. Fuel 2008, 87, 131–146. [Google Scholar] [CrossRef]
- Fan, W.; Li, Y.; Xiao, M. Effect of Preoxidation O2 Concentration on the Reduction Reaction of NO by Char at High Temperature. Ind. Eng. Chem. Res. 2013, 52, 6101–6111. [Google Scholar] [CrossRef]
- Shu, Y.; Wang, H.; Zhu, J.; Tian, G.; Huang, J.; Zhang, F. An experimental study of heterogeneous NO reduction by biomass reburning. Fuel Process. Technol. 2015, 132, 111–117. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhang, W.; Zhao, J.; Wang, Z. Simultaneous SO2 and NO removal by pellets made of carbide slag and coal char in a bubbling fluidized-bed reactor. Process. Saf. Environ. Prot. 2020, 134, 83–94. [Google Scholar] [CrossRef]
- Aarna, I.; Suuberg, E.M. The Role of Carbon Monoxide in the NO−Carbon Reaction. Energy Fuels 1999, 13, 1145–1153. [Google Scholar] [CrossRef]
- Allen, D.; Hayhurst, A.N. The chemical reactions of nitric oxide with solid carbon and catalytically with gaseous carbon monoxide. Fuel 2015, 142, 260–267. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, X.; Wang, Z.; Ma, C.; Wang, M. Investigation on NO reduction and CO formation over coal char and mixed iron powder. Fuel 2019, 245, 52–64. [Google Scholar] [CrossRef]
- Wang, B.; Sheng, S.; Sun, L.S.; Hu, S.; Fei, H.; Lu, T.F.; Xiang, J. Investigation on Effect of CO on NO Heterogeneous Reduction with Char in O2/CO2 Atmosphere. J. Eng. Thermophys. 2012, 33, 336–338. [Google Scholar]
- López, D.; Calo, J. The NO−Carbon Reaction: The Influence of Potassium and CO on Reactivity and Populations of Oxygen Surface Complexes. Energy Fuels 2007, 21, 1872–1877. [Google Scholar] [CrossRef]
- Furusawa, T.; Tsunoda, M.; Tsujimura, M.; Adschiri, T. Nitric oxide reduction by char and carbon monoxide. Fuel 1985, 64, 1306–1309. [Google Scholar] [CrossRef]
- Wang, C.; Du, Y.; Che, D. Investigation on the NO Reduction with Coal Char and High Concentration CO during Oxy-fuel Combustion. Energy Fuels 2012, 26, 7367–7377. [Google Scholar] [CrossRef]
- Song, Y.H.; Beér, J.M.; Sarofim, A.F. Reduction of Nitric Oxide by Coal Char at Temperatures of 1250–1750 K. Combust. Sci. Technol. 1981, 25, 237–240. [Google Scholar] [CrossRef]
- Li, Y.; Radovic, L.; Lu, G.; Rudolph, V. A new kinetic model for the NO–carbon reaction. Chem. Eng. Sci. 1999, 54, 4125–4136. [Google Scholar] [CrossRef]
- Garijo, E.G.; Jensen, A.D.; Glarborg, P. Kinetic Study of NO Reduction over Biomass Char under Dynamic Conditions. Energy Fuels 2003, 17, 1429–1436. [Google Scholar] [CrossRef]
- Dong, L.; Gao, S.; Song, W.; Xu, G. Experimental study of NO reduction over biomass char. Fuel Process. Technol. 2007, 88, 707–715. [Google Scholar] [CrossRef]
- Dong, L.; Gao, S.; Xu, G. NO Reduction over Biomass Char in the Combustion Process. Energy Fuels 2010, 24, 446–450. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Geng, K.; Zhao, Y.; Wu, J.; Sun, R.; Sun, S.; Qiu, P. Investigation of Heterogeneous NO Reduction by Biomass Char and Coal Char Blends in a Microfluidized Bed Reaction Analyzer. Energy Fuels 2020, 34, 6317–6325. [Google Scholar] [CrossRef]
- Schönenbeck, C.; Gadiou, R.; Schwartz, D. A kinetic study of the high temperature NO–char reaction. Fuel 2004, 83, 443–450. [Google Scholar] [CrossRef]
- Wang, X.; Si, J.; Tan, H.; Zhao, Q.; Xu, T. Kinetics investigation on the reduction of NO using straw char based on physicochemical characterization. Bioresour. Technol. 2011, 102, 7401–7406. [Google Scholar] [CrossRef] [PubMed]
- Zevenhoven, R.; Hupa, M. The reactivity of chars from coal, peat and wood towards NO, with and without CO. Fuel 1998, 77, 1169–1176. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Wu, J.; Liu, Y. Novel carbon-based sorbents for elemental mercury removal from gas streams: A review. Chem. Eng. J. 2020, 391, 123514. [Google Scholar] [CrossRef]
- Fan, X.-D.; Wu, Y.-J.; Tu, R.; Sun, Y.; Jiang, E.-C.; Xu, X.-W. Hydrodeoxygenation of guaiacol via rice husk char supported Ni based catalysts: The influence of char supports. Renew. Energy 2020, 157, 1035–1045. [Google Scholar] [CrossRef]
- Sørensen, C.O.; Johnsson, J.E.; Jensen, A.D. Reduction of NO over Wheat Straw Char. Energy Fuels 2001, 15, 1359–1368. [Google Scholar] [CrossRef]
- Chan, L.; Sarofim, A.; Beér, J. Kinetics of the NO carbon reaction at fluidized bed combustor conditions. Combust. Flame 1983, 52, 37–45. [Google Scholar] [CrossRef]
- Chambrion, P.; Orikasa, H.; Suzuki, T.; Kyotani, T.; Tomita, A. A study of the C–NO reaction by using isotopically labelled C and NO. Fuel 1997, 76, 493–498. [Google Scholar] [CrossRef]
- Guerrero, M.; Millera, A.; Alzueta, M.; Bilbao, R. Experimental and Kinetic Study at High Temperatures of the NO Reduction over Eucalyptus Char Produced at Different Heating Rates. Energy Fuels 2011, 25, 1024–1033. [Google Scholar] [CrossRef]
- Kapteijn, F.; Mierop, A.J.C.; Abbel, G.; Moulijn, J.A. Reduction of NOxover alkali metal–carbon systems. J. Chem. Soc. Chem. Commun. 1984, 16, 1085–1086. [Google Scholar] [CrossRef]
- Wu, S.L.; Iisa, K. Kinetics of NO Reduction by Black Liquor Char. Energy Fuels 1998, 12, 457–463. [Google Scholar] [CrossRef]
- Aarna, I.; Suuberg, E.M. A study of the reaction order of the NO-carbon gasification reaction. Symp. Combust. 1998, 27, 3061–3068. [Google Scholar] [CrossRef]
- Suzuki, T.; Kyotani, T.; Tomita, A. Study on the Carbon-Nitric Oxide Reaction in the Presence of Oxygen. Ind. Eng. Chem. Res. 1994, 33, 2840–2845. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Gathitu, B.B. Design of mixed fuel for heterogeneous reburning. Fuel 2006, 85, 1781–1793. [Google Scholar] [CrossRef]
- Jensen, L.S.; Jannerup, H.E.; Glarborg, P.; Jensen, A.D.; Dam-Johansen, K. Experimental investigation of no from pulverized char combustion. Proc. Combust. Inst. 2000, 28, 2271–2278. [Google Scholar] [CrossRef]
- He, J.; Song, W.; Gao, S.; Dong, L.; Barz, M.; Li, J.; Lin, W. Experimental study of the reduction mechanisms of NO emission in decoupling combustion of coal. Fuel Process. Technol. 2006, 87, 803–810. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, Y.; Tan, H.; Liu, Y.; Niu, Y.; Xu, T. Influences of CO and O2 on the Reduction of N2O by Biomass Char. Energy Fuels 2012, 26, 3125–3131. [Google Scholar] [CrossRef]
- Garijo, E.; Jensen, A.D.; Glarborg, P. Reactivity of coal char in reducing NO. Combust. Flame 2004, 136, 249–253. [Google Scholar] [CrossRef]


| Proximate Analysis (ad, %) | Ultimate Analysis (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fixed Carbon | Ash | Volatile Matter | Moisture | C | H | N | O | S |
| 48.60 | 42.79 | 4.22 | 4.39 | 48.85 | 0.56 | 0.44 | 2.94 | 0.03 |
| Surface Areas (m2·g−1) | Pore Volume (cm3·g−1) | Pore Size (nm) |
|---|---|---|
| 135.83 | 0.031 | 6.382 |
| CO (ppm) | Blank | 0 | 400 | 800 | 2000 | 4000 |
| Mass loss (%) | 2.29 | 0.32 | 0.35 | 0.95 | 1.55 | 2.27 |
| Temp (°C) | CNO,in − CNO,out (ppm) | CCO,in − CCO,out (ppm) | CCO2,out (ppm) |
|---|---|---|---|
| 750 | 260 | 239 | 246 |
| 800 | 283 | 247 | 254 |
| 850 | 298 | 310 | 315 |
| 900 | 313 | 329 | 335 |
| Char–NO | Char–NO–CO | |
|---|---|---|
| n | 0.71 | 0.82 |
| Eα (kJ·mol−1) | 60.21 | 34.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Wang, Y.; Cui, S.; Jin, X. Experimental and Kinetic Investigation on NO Reduction by Rice Husk Char and Catalytically with CO. Appl. Sci. 2020, 10, 6715. https://doi.org/10.3390/app10196715
Li N, Wang Y, Cui S, Jin X. Experimental and Kinetic Investigation on NO Reduction by Rice Husk Char and Catalytically with CO. Applied Sciences. 2020; 10(19):6715. https://doi.org/10.3390/app10196715
Chicago/Turabian StyleLi, Nan, Yali Wang, Suping Cui, and Xiaodong Jin. 2020. "Experimental and Kinetic Investigation on NO Reduction by Rice Husk Char and Catalytically with CO" Applied Sciences 10, no. 19: 6715. https://doi.org/10.3390/app10196715
APA StyleLi, N., Wang, Y., Cui, S., & Jin, X. (2020). Experimental and Kinetic Investigation on NO Reduction by Rice Husk Char and Catalytically with CO. Applied Sciences, 10(19), 6715. https://doi.org/10.3390/app10196715





