Role of Surface-Treated Silica Nanoparticles on the Thermo-Mechanical Behavior of Poly(Lactide)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Processing
2.1.1. Nanofiller and Polymer Matrix
2.1.2. Preparation of the Samples
2.2. Characterization Techniques
3. Results and Discussion
3.1. Microstructural Analysis and Processability of the Materials
3.2. Thermal Properties
3.2.1. Thermogravimetric Analysis (TGA)
3.2.2. Calorimetric Characterization (DSC) and Kinetics Analysis of Crystallization
3.3. Dynamical Mechanical Analysis
3.3.1. Monofrequency DMTA Analysis and Vicat Grade
3.3.2. Multifrequency DMTA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Dedication
References
- Kulkarni, R.K.; Pani, K.C.; Neuman, C.; Leonard, F. Polylactic acid for surgical implants. Arch. Surg. 1966, 93, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Barrows, T.H. Degradable implant materials: A review of synthetic absorbable polymers and their application. Clin. Mater. 1986, 1, 233–257. [Google Scholar] [CrossRef]
- Chiellini, E.; Solaro, R. Recent Advances in Biodegradable Polymers and Plastics; Wiley-VCH Verlag: Weinheim, Germany, 2003. [Google Scholar]
- Tsuji, H. Polylactide. In Biopolymers Polyesters III Applications and Commercial Products; Doi, Y., Steinbuchel, A., Eds.; Wiley VCH Verlag: Weinheim, Germany, 2002; pp. 129–177. [Google Scholar]
- Auras, R.; Lim, L.T.; Selke, S.E.M.; Tsuji, H. Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Jiménez, A.; Peltzer, M.; Ruseckaite, R. Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications. Polymer Science, Series; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Fambri, L.; Migliaresi, C. Crystallization and thermal properties. In Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications; Auras, R., Lim, L.T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 113–124. [Google Scholar]
- Garlotta, D. A literature rewiew of poly(lactic acid). J. Polym. Environ. 2002, 9, 63–81. [Google Scholar] [CrossRef]
- Perego, G.; Cella, G.D.; Bastioli, C. Effect of molecular weight and crystallinity on poly(lactic acid) mechanical properties. J. Appl. Polym. Sci. 1996, 59, 37–43. [Google Scholar] [CrossRef]
- Auras, R.; Lim, L.T. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Oksman, K.; Skrifvars, M.; Selin, J.F. Natural fibres as reinforcement in polylactic acid (PLA) composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ogami, A.; Ueda, K. New Polylactide/Layered Silicate Nanocomposites. 3. High-Performance Biodegradable Materials. Chem. Mater. 2003, 15, 1456–1465. [Google Scholar] [CrossRef]
- Lewitus, D.; McCarthy, S.; Ophir, A.; Kenig, S. The effect of nanoclays on the properties of PLLA-modified polymers Part 1: Mechanical and thermal properties. J. Polym. Environ. 2006, 14, 171–177. [Google Scholar] [CrossRef]
- Singha, S.; Hedenqvist, M.S. A Review on Barrier Properties of Poly(Lactic Acid)/Clay Nanocomposites. Polymers 2020, 12, 1095. [Google Scholar] [CrossRef] [PubMed]
- Fambri, L.; Kesenci, K.; Migliaresi, C. Characterization of modulus and glass transition phenomena in poly(L-lactide)/hydroxyapatite composites. Polym. Compos. 2003, 24, 100–108. [Google Scholar] [CrossRef]
- Sun, J.; Shen, J.; Chen, S.; Cooper, M.A.; Fu, H.; Wu, D.; Yang, Z. Nanofiller Reinforced Biodegradable PLA/PHA Composites: Current Status and Future Trends. Polymers 2018, 10, 505. [Google Scholar] [CrossRef] [Green Version]
- Dorigato, A.; Pegoretti, A.; Kolarik, J. Nonlinear tensile creep of linear low density polyethylene/fumed silica nanocomposites: Time-strain superposition and creep prediction. Polym. Compos. 2010, 31, 1947–1955. [Google Scholar] [CrossRef]
- Dorigato, A.; D’Amato, M.; Pegoretti, A. Thermo-mechanical properties of high density polyethylene—Fumed silica nanocomposites: Effect of filler surface area and treatment. J. Polym. Res. 2012, 19, 9889. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A. Tensile creep behaviour of poly(methylpentene)-silica nanocomposites. Polym. Int. 2010, 59, 719–724. [Google Scholar] [CrossRef]
- Dorigato, A.; Govaert, L.E.; Pegoretti, A. Lifetime assessment of high-density polyethylene–silica nanocomposites. Nanomater. Nanotechnol. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dorigato, A.; Pegoretti, A. (Re)processing effects on polypropylene/silica nanocomposites. J. Appl. Polym. Sci. 2014, 131, 40242. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A.; Fambri, L.; Slouf, M.; Kolarik, J. Cycloolefin copolymer/fumed silica nanocomposites. J. Appl. Polym. Sci. 2011, 119, 3393–3402. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A.; Frache, A. Thermal stability of high density polyethylene—Fumed silica nanocomposites. J. Therm. Anal. Calorim. 2012, 109, 863–873. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A.; Penati, A. Linear low-density polyethylene/silica micro- and nanocomposites: Dynamic rheological measurements and modelling. Express Polym. Lett. 2010, 4, 115–129. [Google Scholar] [CrossRef]
- Dabrowska, I.; Fambri, L.; Pegoretti, A.; Slouf, M.; Vackova, T.; Kolarik, J. Spinning, drawing and physical properties of polypropylene nanocomposite fibers with fumed nanosilica. Express Polym. Lett. 2015, 9, 277–290. [Google Scholar] [CrossRef]
- Fambri, L.; Dabrowska, I.; Ceccato, R.; Pegoretti, A. Effects of Fumed Silica and Draw Ratio on Nanocomposite Polypropylene Fibers. Polymers 2017, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Cao, D.; Huang, Y.; Li, B.G. Poly(l-lactic acid)/SiO2 nanocomposites via in situ melt polycondensation of l-lactic acid in the presence of acidic silica sol: Preparation and characterization. Polymer 2008, 49, 742–748. [Google Scholar] [CrossRef]
- Cao, D.; Wu, L. Poly(L-lactic acid)/silicon dioxide nanocomposites prepared via in situ melt polycondensation of L-lactic acid in the presence of acidic silica sol: Isothermal crystallization and melting behaviors. J. Appl. Polym. Sci. 2009, 111, 1045–1050. [Google Scholar] [CrossRef]
- Nerantzaki, M.; Prokopiou, L.; Bikiaris, D.N.; Patsiaoura, D.; Chrissafis, K.; Klonos, P.; Kyritsis, A.; Pissis, P. In situ prepared poly(DL-lactic acid)/silica nanocomposites: Study of molecular composition, thermal stability, glass transition and molecular dynamics. Thermochim. Acta 2018, 669, 16–29. [Google Scholar] [CrossRef]
- Yan, S.F.; Yin, J.B.; Yang, Y.; Dai, Z.Z.; Ma, J.; Chen, X.S. Surface-grafted silica linked with L-lactic acid oligomer: A novel nanofiller to improve the performance of biodegradable poly(L-lactide). Polymer 2007, 46, 1688–1694. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, B.Y.; Liu, Q.S. Rheology and Crystallization of PLA Containing PLA-Grafted Nanosilica, Plast. Rubber Compos. 2014, 43, 309–314. [Google Scholar] [CrossRef]
- Sepulveda, J.; Villegas, C.; Torres, A.; Vargas, E.; Rodriguez, F.; Baltazar, S.; Prada, A.; Rojas, A.; Romero, J.; Faba, S.; et al. Effect of functionalized silica nanoparticles on the mass transfer process in active PLA nanocomposite films obtained by supercritical impregnation for sustainable food packaging. J. Supercrit. Fluids 2020, 161, 104844. [Google Scholar] [CrossRef]
- Zhu, A.; Diao, H.; Rong, Q.; Cai, A. Preparation and properties of polylactide–silica nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2866–2873. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Abbate, C.; Arena, M.; Rizzarelli, P. Preparation, characterization and biodegradation of biopolymer nanocomposites based on fumed silica. Eur. Polym. J. 2011, 47, 139–152. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Bian, J.; Han, L.; Dong, L.; Gao, G. Rheology and biodegradation of polylactide/silica nanocomposites. Polym. Compos. 2012, 33, 1719–1727. [Google Scholar] [CrossRef]
- Pilic, B.M.; Radusin, T.I.; Ristić, I.S.; Silvestre, C.; Lazić, V.L.; Baloš, S.S.; Duraccio, D. Hydrophobic silica nanoparticles as reinforcing filler for poly (lactic acid) polymer matrix. Hem. Ind. 2016, 70, 73–80. [Google Scholar] [CrossRef]
- Lai, J.C.H.; Rahman, M.R.; Hamdan, S. Comparative studies of thermo-mechanical and morphological properties of polylactic acid/fumed silica/clay (1.28E) and polylactic acid/fumed silica/clay (1.34TCN) nanocomposites. Polym. Bull. 2018, 75, 135–147. [Google Scholar] [CrossRef]
- Chen, B.-K.; Shih, C.-C.; Chen, A.F. Ductile PLA nanocomposites with improved thermal stability. Compos. Part A Appl. Sci. Manuf. 2012, 43, 2289–2295. [Google Scholar] [CrossRef]
- Wen, X.; Lin, Y.; Han, C.; Zhang, K.; Ran, X.; Li, Y.; Dong, L. Thermomechanical and Optical Properties of Biodegradable Poly(L-lactide)/Silica Nanocomposites by Melt Compounding. J. Appl. Polym. Sci. 2009, 114, 3379–3388. [Google Scholar] [CrossRef]
- Dorigato, A.; Sebastiani, M.; Pegoretti, A.; Fambri, L. Effect of silica nanoparticles on the mechanical performances of poly(lactic acid). J. Polym. Environ. 2012, 20, 713–725. [Google Scholar] [CrossRef]
- Georgiopoulos, P.; Kontou, E.; Niaounakis, M. Thermomechanical properties and rheological behavior of biodegradable composites. Polym. Compos. 2014, 35, 1140–1149. [Google Scholar] [CrossRef]
- Lv, H.; Song, S.; Sun, S.; Ren, L.; Zhang, H. Enhanced properties of poly(lactic acid) with silica nanoparticles. Polym. Adv. Technol. 2016, 27, 1156–1163. [Google Scholar] [CrossRef]
- Lertphirun, K.; Srikulkit, K. Properties of Poly(Lactic Acid) Filled with Hydrophobic Cellulose/SiO2 Composites. Int. J. Polym. Sci. 2019, 2019, 7835172. [Google Scholar] [CrossRef] [Green Version]
- Georgiopoulos, P.; Kontou, E.; Meristoudi, A.; Pispas, S.; Chatzinikolaidou, M. The effect of silica nanoparticles on the thermomechanical properties and degradation behavior of polylactic acid. J. Biomater. Appl. 2014, 29, 662–674. [Google Scholar] [CrossRef]
- Khankrua, R.; Pivsa-Art, S.; Hiroyuki, H.; Suttiruengwon, S. Thermal and Mechanical Properties of Biodegradable polyester/silica Nanocomposites. Energy Procedia 2013, 34, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Bikiaris, D.N. Nanocomposites of aliphatic polyesters: An overview of the effect of different nanofillers on enzymatic hydrolysis and biodegradation of polyesters. Polym. Degrad. Stab. 2013, 98, 1908–1928. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Achilias, D.S.; Nanakia, S.; Beslikas, T.; Bikiaris, D. PLA nanocomposites: Effect of filler type on non-isothermal crystallization. Thermochim. Acta 2010, 511, 129–139. [Google Scholar] [CrossRef]
- Myoung, S.H.; Im, S.S.; Kim, S.H. Non-isothermal crystallization behavior of PLA/acetylated cellulose nanocrystal/silica nanocomposites. Polym. Int. 2016, 65, 115–124. [Google Scholar] [CrossRef]
- Zhang, J.; Lou, J.; Ilias, S.; Krishnamachari, P.; Yan, J. Thermal properties of poly(lactic acid) fumed silica nanocomposites: Experiments and molecular dynamics simulations. Polymer 2008, 49, 2381–2386. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Avrami, M. Kynetics of Phase Change I, General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei J. Chem. Phys. 1940, 8, 212. [Google Scholar] [CrossRef]
- Jog, J.P. Crystallisation in polymer nanocomposites. Mater. Sci. Technol. 2006, 22, 797–806. [Google Scholar] [CrossRef]
- Riande, E.; Diaz-Calleja, R.; Prolongo, M.G.; Masegosa, R.M.; Salom, C. Polymer Viscoelasticity, Stress and Strain in Practice; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Wichmann, M.H.G.; Cascione, M.; Fiedler, B.; Quaresimin, M.; Schulte, K. Influence of surface treatment on mechancal behaviour of fumed silica/epoxy resin nano-composites. Compos. Interfaces 2006, 13, 699–715. [Google Scholar] [CrossRef]
- Fambri, L.; Bragagna, S.; Migliaresi, C. Biodegradable fibers of poly-L,DL-lactide 70/30 produced by melt spinning. Macromol. Symp. 2006, 234, 20–25. [Google Scholar] [CrossRef]
- Díaz-Díaz, A.M.; López-Beceiro, J.; Li, Y.; Cheng, Y.; Artiaga, R. Crystallization kinetics of a commercial poly(lactic acid) based on characteristic crystallization time and optimal crystallization temperature. J. Therm. Anal. Calorim. 2020, 1–8. [Google Scholar] [CrossRef]
- Iannace, S.; Nicolais, L. Isothermal Crystallization and Chain Mobility of Poly (L-lactide). J. Appl. Polym. Sci. 1997, 64, 911–919. [Google Scholar] [CrossRef]
- Di Lorenzo, M.R.; Androsch, R. Crystallization of poly(lactic acid). In Biodegradable Polyesters; Fakirov, S., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; p. 109. [Google Scholar] [CrossRef]
- Müller, A.J.; Ávila, M.; Saenz, G.; Salazar, J. Crystallization of PLA-based Materials. In Poly(Lactic Acid) Science and Technology: Processing, Properties, Additives and Applications; Polymer Science, Series; Jiménez, A., Peltzer, M., Ruseckaite, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2014; Chapter 3; pp. 66–98. [Google Scholar] [CrossRef]
- Hakim, R.H.; Cailloux, J.; Santana, O.O.; Bou, J.; Sanchez-Soto, M.; Odent, J.; Raquez, J.M.; Dubois, P.; Carrasco, F.; Maspoch, M.L. PLA/SiO2 composites: Influence of the filler modifications on the morphology, crystallization behavior, and mechanical properties. J. Appl. Polym. Sci. 2017, 134, 45367. [Google Scholar] [CrossRef]
- Sumita, M.; Tsukihi, H.; Miyasaka, K.; Ishikawa, K. Dynamic mechanical properties of polypropylene composites filled with ultrafine particles. J. Appl. Polym. Sci. 1984, 29, 1523–1530. [Google Scholar] [CrossRef]
- Fambri, L.; Dabrowska, I.; Ferrara, G.; Pegoretti, A. Morphology and viscoelastic properties of melt-spun HDPE/hydrotalcite nanocomposite fibers. Polym. Compos. 2016, 37, 288–298. [Google Scholar] [CrossRef]
- Crowley, K.J.; Zografi, G.D. The use of thermal methods for predicting glass-former fragility. Thermochim. Acta 2001, 380, 79–83. [Google Scholar] [CrossRef]


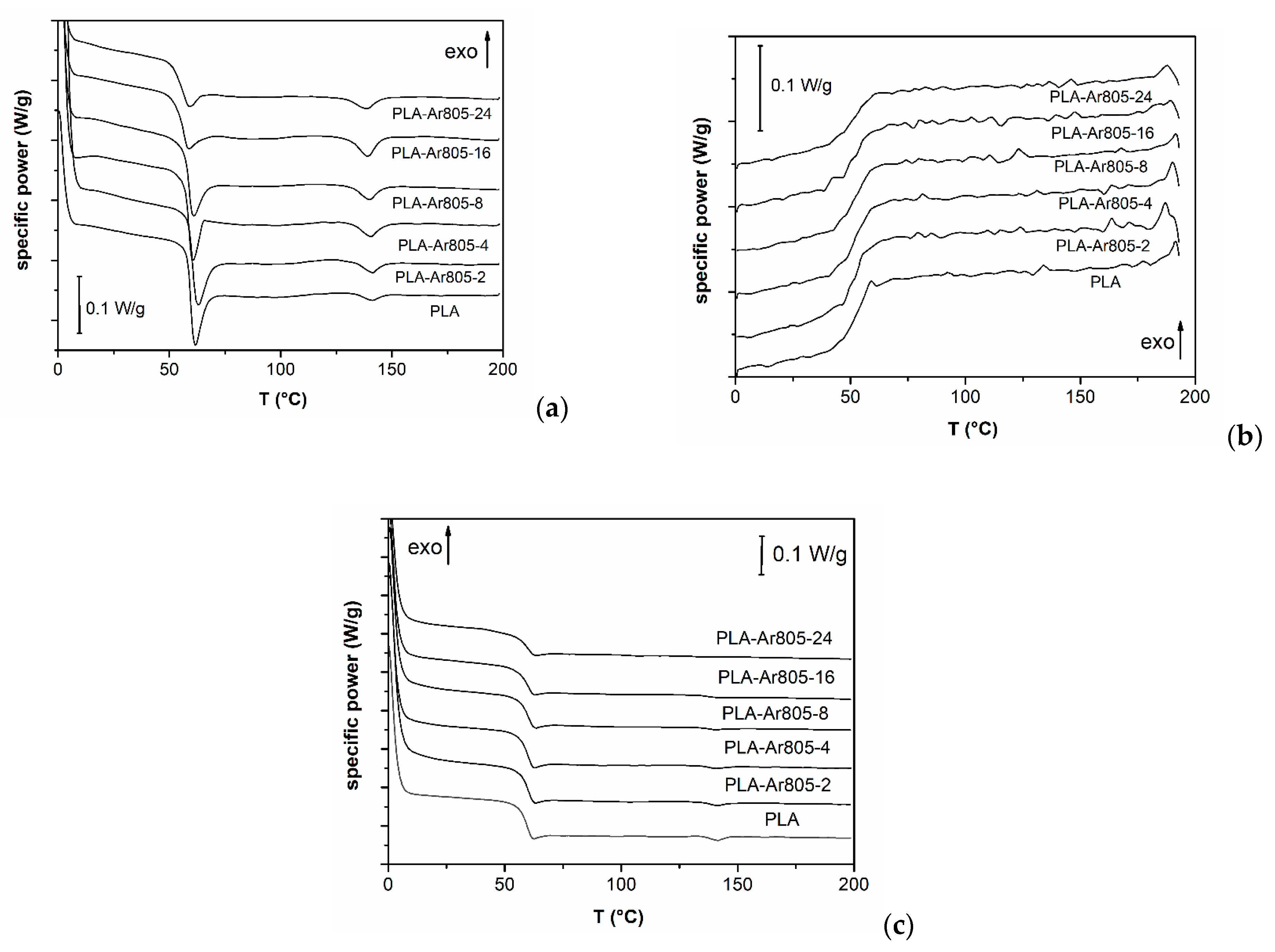

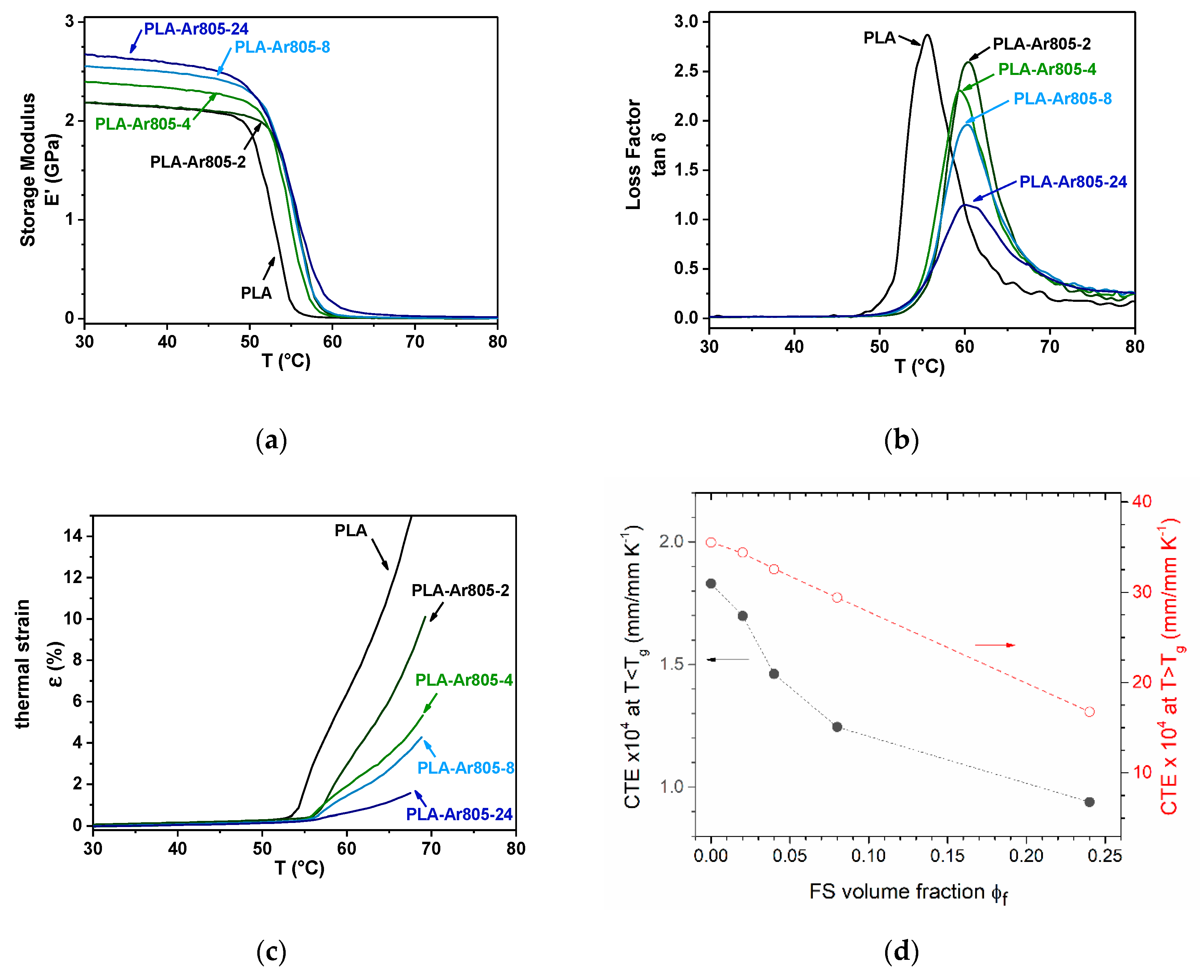
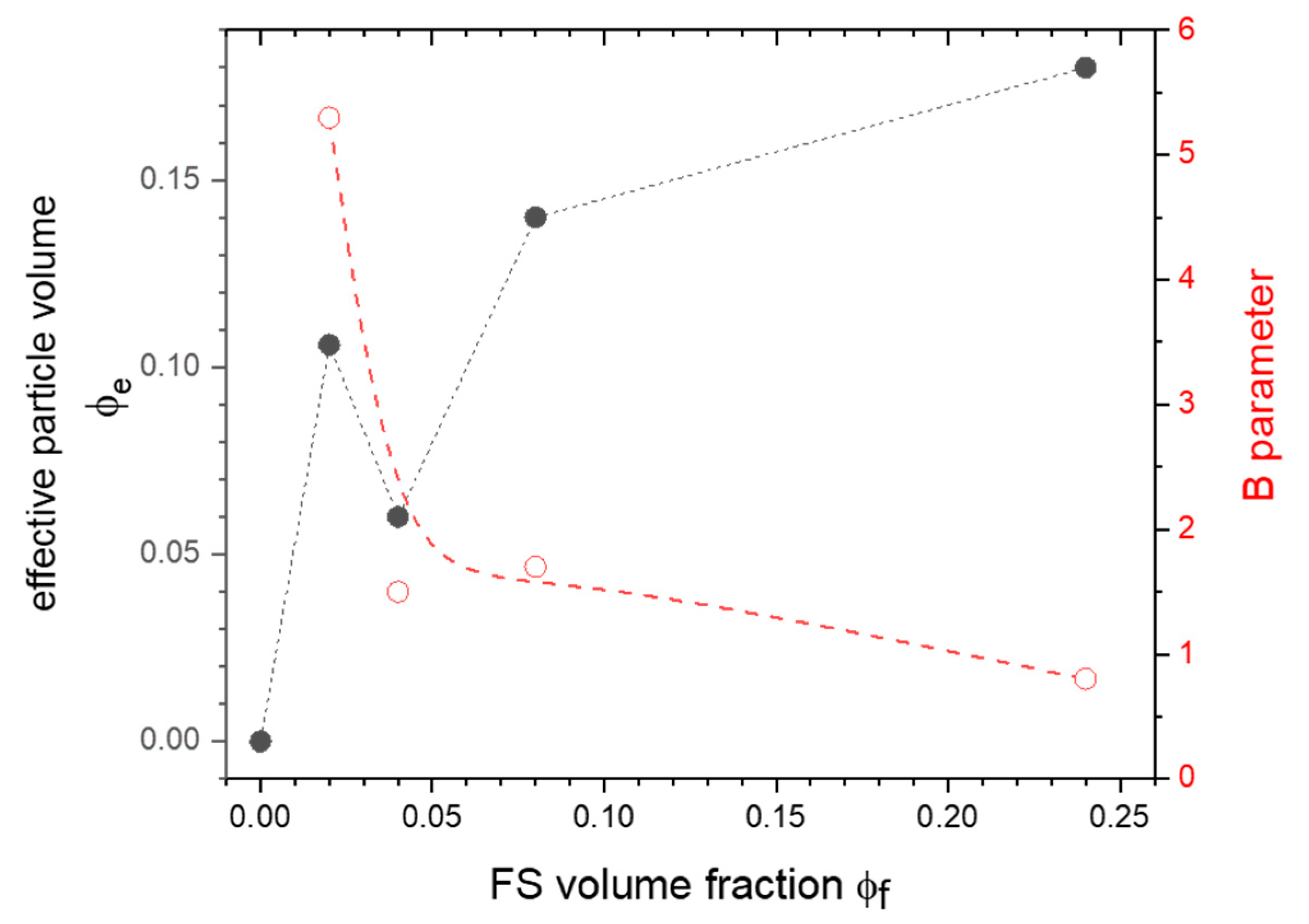

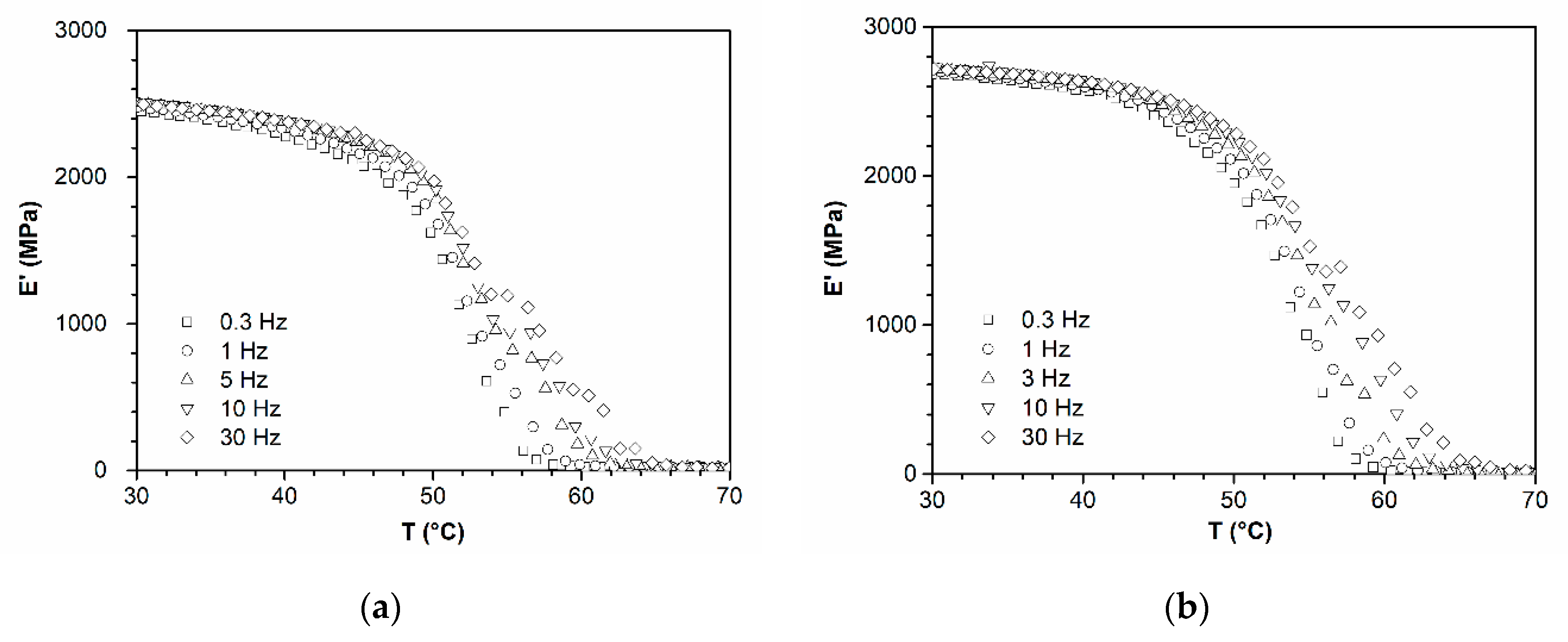

| Temperature and Kinetics | PLA | PLA-Ar805-2 | PLA-Ar805-4 | PLA-Ar805-8 | PLA-Ar805-24 |
|---|---|---|---|---|---|
| 170 °C | 5.7 ± 0.2 | 5.2 ± 0.5 | 3.7 ± 0.2 | 2.3 ± 0.2 | 0.8 ± 0.1 |
| 190 °C | 16.4 ± 1.3 | 14.1 ± 1.2 | 10.4 ± 0.3 | 6.6 ± 0.6 | 2.2 ± 0.5 |
| 210 °C | 38.7 ± 2.5 | 37.3 ± 1.5 | 25.4 ± 0.8 | 18.9 ± 1.0 | 5.2 ± 1.2 |
| Activation energy 1 (kJ/mol) | 85.3 ± 2.9 | 82.4 ± 4.6 | 84.8 ± 1.5 | 94.8 ± 1.4 | 81.9 ± 1.0 |
| Sample | T5% (°C) | Td (°C) | m700 (%) |
|---|---|---|---|
| PLA | 350.0 | 384.0 | 0.1 |
| PLA-Ar805-2 | 350.3 | 386.1 | 2.7 |
| PLA-Ar805-4 | 351.0 | 393.2 | 5.5 |
| PLA-Ar805-8 | 349.0 | 394.3 | 10.2 |
| PLA-Ar805-16 | 352.3 | 395.3 | 21.3 |
| PLA-Ar805-24 | 353.0 | 396.4 | 30.3 |
| Sample | Tg1 (°C) | Tm1 (°C) | Wc1 (%) | TgC (°C) | Tg2 (°C) |
|---|---|---|---|---|---|
| PLA | 59.6 | 141.2 | 0.6 | 57.0 | 59.4 |
| PLA-Ar805-1 | 59.7 | 140.8 | 1.2 | 53.7 | 60.0 |
| PLA-Ar805-2 | 60.4 | 141.2 | 1.0 | 53.8 | 59.9 |
| PLA-Ar805-4 | 58.8 | 140.5 | 1.2 | 51.5 | 59.7 |
| PLA-Ar805-8 | 59.9 | 140.8 | 1.5 | 51.0 | 60.8 |
| PLA-Ar805-16 | 58.9 | 139.9 | 1.2 | 54.4 | 60.0 |
| PLA-Ar805-24 | 56.6 | 139.1 | 1.5 | 50.1 | 59.7 |
| Sample | Storage Modulus E′ (MPa) at 25 °C/40 °C/60 °C | E″ Peak (°C) | Tanδ Peak Tg 1/Height/Width 2(°C/–/°C) | Thermal Strain at 65 °C (%) | VST (°C) |
|---|---|---|---|---|---|
| PLA | 2180/2100/11.1 | 55.6 | 56.6/2.99/6.2 | 11.2 | 62.6 + 0.2 |
| PLA-Ar805-2 | 2220/2120/41.3 | 56.8 | 60.6/2.61 /6.2 | 6.1 | 62.8 + 0.4 |
| PLA-Ar805-4 | 2410/2280/28.9 | 55.8 | 59.4/2.31/6.9 | 3.5 | 63.1 + 0.3 |
| PLA-Ar805-8 | 2580/2440/63.2 | 56.1 | 60.2/1.96/7.4 | 2.6 | 63.9 + 0.6 |
| PLA-Ar805-24 | 2710/2520/170 | 56.3 | 61.1/1.12/10.1 | 1.2 | 70.6 + 0.2 |
| Sample | ΔETg (J/mol K) | m 1 | D 1 |
|---|---|---|---|
| PLA | 623 ± 22 | 99.0 | 7.1 |
| PLA-Ar805-2 | 517 ± 12 | 81.0 | 9.1 |
| PLA-Ar805-4 | 547 ± 52 | 86.0 | 8.4 |
| PLA-Ar805-8 | 554 ± 7 | 86.8 | 8.3 |
| PLA-Ar805-24 | 576 ± 19 | 90.1 | 8.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fambri, L.; Dorigato, A.; Pegoretti, A. Role of Surface-Treated Silica Nanoparticles on the Thermo-Mechanical Behavior of Poly(Lactide). Appl. Sci. 2020, 10, 6731. https://doi.org/10.3390/app10196731
Fambri L, Dorigato A, Pegoretti A. Role of Surface-Treated Silica Nanoparticles on the Thermo-Mechanical Behavior of Poly(Lactide). Applied Sciences. 2020; 10(19):6731. https://doi.org/10.3390/app10196731
Chicago/Turabian StyleFambri, Luca, Andrea Dorigato, and Alessandro Pegoretti. 2020. "Role of Surface-Treated Silica Nanoparticles on the Thermo-Mechanical Behavior of Poly(Lactide)" Applied Sciences 10, no. 19: 6731. https://doi.org/10.3390/app10196731







