Evaluation and Origin Discrimination of Two Monocultivar Extra Virgin Olive Oils, Cultivated in the Coastline Part of North-Western Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Oil Sampling
2.2. Analysis of the Quality Parameters
2.3. Analysis of the Sterolic Profile
2.4. Analysis of the Fatty Acid Profile
2.5. Statistical Analysis
3. Results and Discussion
3.1. Quality Parameters of the Examined Olive Oils
3.2. Fatty Acid Profile of the Two Monocultivar Olive Oils
3.3. Sterolic Profile of the Two Monocultivar Olive Oils
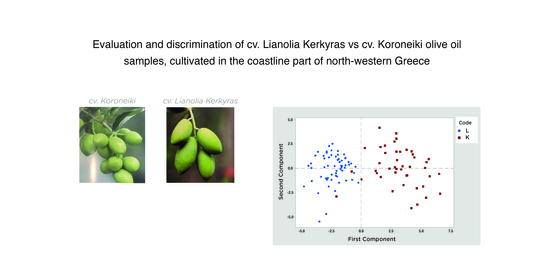
3.4. Chemometric Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boskou, D. Olive Oil: Chemistry and Technology, 2nd ed.; AOCS Press: Champaign, IL, USA, 2006; pp. 45–93. [Google Scholar]
- Preddy, V.; Watson, R. Olives and Olive Oil in Health and Disease Prevention, 1st ed.; Academic Press: Oxford, UK, 2010; pp. 1100–1220. [Google Scholar]
- Roche, H.M.; Gibney, M.J.; Kafatos, A.; Zampelas, A.; Williams, C.M. Beneficial properties of olive oil. Food Res. Int. 2000, 33, 227–231. [Google Scholar] [CrossRef]
- Official Website of the European Union. An Overview of the Production and Marketing of Olive Oil in the EU. Available online: https://ec.europa.eu/info/food-farming-fisheries/plants-and-plant-products/plant-products/olive-oil2020 (accessed on 20 July 2020).
- International Olive Council Database. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotionunit/#exports (accessed on 10 July 2020).
- Economic Affairs & Promotion Unit, International Olive Council. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/ (accessed on 12 July 2020).
- Council Regulation (EC). No. 2081/92 of 14 July 1992 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs. Off. J. Eur. Union 1992, L208, 1–8. [Google Scholar]
- Council Regulation (EC). No. 2082/92 of 14 July 1992 on certificates of specific character for agricultural products and foodstuffs. Off. J. Eur. Union 1992, L208, 9–14. [Google Scholar]
- Council Regulation (EC). No. 510/2006 of 20 March 2006 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs. Off. J. Eur. Union 2006, L93, 12–25. [Google Scholar]
- Council Regulation (EC). No. 1898/2006 of 14 December 2006 laying down detailed rules of implementation of Council Regulation (EC) no. 510/2006 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs. Off. J. Eur. Union 2006, L369, 1–23. [Google Scholar]
- Council Regulation (EC). No. 182/2009 of 6 March 2009 amending Regulation (EC) no. 1019/2002 on marketing standards for olive oil. Off. J. Eur. Union 2009, L63, 6–8. [Google Scholar]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; García-González, D.L.; Moreda, W.; et al. Olive oil quality and authenticity: A review of current EU legislation, standards, relevant methods of analyses, their drawbacks and recommendations for the future. Trends Food Sci. Technol. 2019, (in press). [Google Scholar] [CrossRef]
- Aparicio, R.; Luna, G. Characterization of monovarietal virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Montet, D.; Ray, R. Food Traceability and Authenticity, 1st ed; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Montealegre, C.; Alegre, M.; García-Ruiz, C. Traceability markers to the botanical origin in olive oils. J. Agric. Food Chem. 2009, 58, 28–38. [Google Scholar] [CrossRef]
- Bajoub, A.; Medina-Rodríguez, S.; Gómez-Romero, M.; Ajal, E.A.; Bagur-González, M.G.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Assessing the varietal origin of extra-virgin olive oil using liquid chromatography fingerprints of phenolic compound, data fusion and chemometrics. Food Chem. 2017, 215, 245–255. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Moreno-Rojas, J.M.; Holland, M.V.; Reniero, F.; Guillou, C.; Héberger, K. Virgin olive oil authentication by multivariate analyses of 1H NMR fingerprints and δ13C and δ2H data. J. Agric. Food Chem. 2010, 58, 5586–5596. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, F.; Ventrella, A.; Napoli, C.; Humpfer, E.; Schütz, B.; Schäfer, H.; Kontominas, M.G.; Sacco, D. Classification of olive oils according to geographical origin by using 1H NMR fingerprinting combined with multivariate analysis. Food Chem. 2012, 130, 177–183. [Google Scholar] [CrossRef]
- Becerra-Herrera, M.; Vélez-Martín, A.; Ramos-Merchante, A.; Richter, P.; Beltrán, R.; Sayago, A. Characterization and evaluation of phenolic profiles and color as potential discriminating features among Spanish extra virgin olive oils with protected designation of origin. Food Chem. 2018, 241, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Kostelenos, G. Olive Fruit Data, History, Description and Geographical Distribution of Olive Varieties in Greece, 1st ed.; Kostelenos Georgios: Poros island, Greece, 2011; pp. 1–436. (In Greek) [Google Scholar]
- Kostelenos, G. Greek olive varieties. In Elaiokomia Egkiklopedia-The Olive Oil, 1st ed.; Zambounis, V., Ed.; Axion Ekdotiki: Athens, Greece, 2017; Volume 2, pp. 39–53. (In Greek) [Google Scholar]
- Pouliarekou, E.; Badeka, A.; Tasioula-Margaria, M.; Kontakos, S.; Longobardi, F.; Kontominas, M.G. Characterization and classification of Western Greek olive oils according to cultivar and geographical origin based on volatile compounds. J. Chromatogr. A 2011, 1218, 7534–7542. [Google Scholar] [CrossRef]
- Longobardi, F.; Ventrella, A.; Casielloa, G.; Sacco, D.; Tasioula-Margari, A.K.; Kiritsakis, A.K.; Kontominas, M.G. Characterisation of the geographical origin of Western Greek virgin olive oils based on instrumental and multivariate statistical analysis. Food Chem. 2012, 133, 1169–1175. [Google Scholar] [CrossRef]
- Karabagias, I.; Michos, C.; Badeka, A.; Kontakos, S.; Stratis, I.; Kontominas, M.G. Classification of Western Greek virgin olive oils according to geographical origin based on chromatographic, spectroscopic, conventional and chemometric analyses. Food Res. Int. 2013, 54, 1950–1958. [Google Scholar] [CrossRef]
- Commission Regulation (EEC). No. 2568/91 of 14 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union 1991, L208, 1–8. [Google Scholar]
- Kiritsakis, A.; Christie, W. Analysis of edible oil. In Handbook of Olive Oil; Harwood, J., Aparicio, R., Eds.; Springer: Gaithersburg, MD, USA, 2000; pp. 129–158. [Google Scholar]
- Boskou, D. Olive oil. In More on Mediterranean Diets; Simopoulos, A.P., Visioli, F., Eds.; Karger: Basel, Switzerland, 2007; Volume 97, pp. 180–210. [Google Scholar]
- Di Bella, G.; Maisano, R.; La Pera, L.; Lo Turco, V.; Salvo, F.; Dugo, G. Statistical characterization of Sicilian olive oils from the Peloritana and Maghrebian zones according to the fatty acid profile. J. Agric. Food Chem. 2007, 55, 6568–6574. [Google Scholar] [CrossRef]
- Ollivier, D.; Artaud, J.; Pinatel, C.; Durbec, J.P.; Guerere, M. Triacylglycerol and fatty acid compositions of French virgin olive oils. Characterization by chemometrics. J. Agric. Food Chem. 2003, 51, 5723–5731. [Google Scholar] [CrossRef]
- D’Imperio, M.; Dugo, G.; Alfa, M.; Mannina, L.; Segre, A.L. Statistical analysis on Sicilian olive oils. Food Chem. 2007, 102, 956–965. [Google Scholar] [CrossRef]
- Skiada, V.; Tsarouhas, P.; Varzakas, T. Comparison and discrimination of two major monocultivar extra virgin olive oils in the southern region of Peloponnese, according to specific compositional/traceability markers. Foods 2020, 9, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanoudaki, E.; Kotsifaki, F.; Koutsaftakis, A. Classification of virgin olive oils of the two major Cretan cultivars based on their fatty acid composition. J. Am. Oil Chem. Soc. 1999, 76, 623–626. [Google Scholar] [CrossRef]
- Krichene, D.; Taamalli, W.; Daoud, D.; Salvador, M.; Fregapane, G.; Zarrouk, M. Phenolic compounds, tocopherols and other minor components in virgin olive oils of some Tunisian varieties. J. Food Biochem. 2007, 31, 179–194. [Google Scholar] [CrossRef]
- Alves, E.; Simoes, A.; Rosário Domingues, M. Fruit seeds and their oils as promising sources of value-added lipids from agro-industrial byproducts: Oil content, lipid composition, lipid analysis, biological activity and potential biotechnological applications. Crit. Rev. Food Sci. Nutr. 2020. [CrossRef]
- Moreau, R.A.; Norton, R.A.; Hicks, K.B. Phytosterols and phytostanols lower cholesterol. Inform (Champaign) 1999, 10, 572–577. [Google Scholar]
- Boskou, D.; Tsimidou, M.; Blekas, D. Olive Oil Chemistry and Technology; AOCS Press: Champaign, IL, USA, 2006; pp. 41–72. [Google Scholar]
- López-Miranda, J.; Pérez-Jiménez, F.; Ros, E.; De Caterina, R.; Badimón, L.; Covas, M.I.; Escrich, E.; Ordovás, J.M.; Soriguer, F.; Abiá, R.; et al. Olive oil and health: In Proceedings of the II international conference on olive oil and health consensus report, Jaén and Córdoba, Spain. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [CrossRef]
- Lukic, M.; Lukic, I.; Krapac, M.; Sladonja, B.; Pilizota, V. Sterols and triterpene diols in olive oil as indicators of variety and degree of ripening. Food Chem. 2013, 136, 251–258. [Google Scholar] [CrossRef]
- Bucci, R.; Magrı’, A.D.; Magrı, A.L.; Marini, D.; Marini, F. Chemical authentication of extra virgin olive oil varieties by supervised chemometric procedures. J. Agric. Food Chem. 2002, 50, 413–418. [Google Scholar] [CrossRef]
- Matos, L.C.; Cunha, S.C.; Amaral, J.S.; Pereira, J.A.; Andrade, P.B.; Seabra, R.M.; Oliveira, B. Chemometric characterization of three varietal olive oils (cvs. Cobrancosa, Madural and Verdeal Transmontana) extracted from olives with different maturation indices. Food Chem. 2007, 102, 406–414. [Google Scholar] [CrossRef]
- Brescia, M.A.; Alviti, G.; Liuzzi, V.; Sacco, A. Chemometrics classification of olive cultivars based on compositional data of oils. J. Am. Oil. Chem. Soc. 2003, 80, 945–950. [Google Scholar] [CrossRef]
- Lorenzo, I.M.; Perez Pavon, J.L.; Fernandez Laespada, M.E.; Garcıa Pinto, C.; Moreno Cordero, B.; Henriques, L.R.; Peres, M.F.; Simoes, M.P.; Lopes, P.S. Application of headspace-mass spectrometry for differentiating sources of olive oils. Anal. Bioanal. Chem. 2002, 374, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.B.; Rocchetti, G.; Montesano, D.; Ali, S.B.; Guasmi, F.; Grati Kamoun, N.; Lucini, L. Discrimination of Tunisian and Italian extra-virgin olive oils according to their phenolic and sterolic fingerprints. Food Res. Int. 2018, 106, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Skiada, V.; Tsarouhas, P.; Varzakas, T. Preliminary study and observation of “Kalamata PDO” extra virgin olive oil, in the Messinia region, southwest of Peloponnese, Greece. Foods 2019, 8, 610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, J.S.; Bueno, E.O.; Garcia, A.M.M.; Cano, M.M. Sterol and erythrodiol uvaol content of virgin olive oils from cultivars of Extramadura (Spain). Food Chem. 2004, 87, 225–230. [Google Scholar]
- Rivera del Alamo, R.M.; Fregapane, G.; Aranda, F.; Gomez-Alonso, S.; Salvador, M.D. Sterol and alcohol composition of Cornicabra virgin olive oil: The campesterol content exceeds the upper limit of 4% established by EU regulations. Food Chem. 2004, 84, 533–537. [Google Scholar] [CrossRef]
- Mailer, R.J.; Ayton, J.; Graham, K. The Influence of growing region, cultivar and harvest timing on the diversity of Australian olive oil. J. Am. Oil. Chem. Soc. 2010, 87, 877–884. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Chartzoulakis, K.; Koutsaftakis, A.; Kotsifaki, F. Effect of drought stress on qualitative characteristics of olive oil of cv Koroneiki. Grasas Aceites 2001, 52, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Awad, A.B.; Chan, K.C.; Downie, A.C.; Fink, C.S. Peanuts as a source of β-sitosterol, a sterol with anticancer properties. Nutr. Cancer 2000, 36, 238–241. [Google Scholar] [CrossRef]
- Awad, A.B.; Chen, Y.C.; Fink, C.S.; Hennessey, T. Beta-Sitosterol inhibits HT-29 human colon cancer cell growth and alters membrane lipids. Anticancer Res. 1996, 16, 2797–2804. [Google Scholar]



| Parameter | cv. Koroneiki (N = 44) | cv. Lianolia Kerkyras (N = 60) | EEC Limit for Extra Virgin Olive Oil (EVOO) Category | ||
|---|---|---|---|---|---|
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | ||
| Free acidity (%) | 0.24 ± 0.10 | 0.13–0.55 | 0.27 ± 0.12 | 0.12–0.75 | ≤0.80 |
| Peroxide value (meqO2/kg) | 6.64 ± 1.26 | 3.81–9.66 | 5.21 ± 1.12 | 3.41–8.64 | ≤20 |
| K232 | 1.56 ± 0.14 | 1.39–2.04 | 1.61 ± 0.15 | 1.25–1.95 | ≤2.50 |
| K268 | 0.14 ± 0.01 | 0.11–0.19 | 0.14 ± 0.02 | 0.11–0.21 | ≤0.22 |
| Parameter | cv. Koroneiki (N = 44) | cv. Lianolia Kerkyras (N = 60) | Calculated p-Value | EEC Limit for EVOO Category | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | |||
| Myristic C14:0 (%) | 0.009 ± 0.002 | 0.006–0.018 | 0.008 ± 0.004 | 0.003–0.04 | n.s | ≤0.03 |
| Palmitic C16:0 (%) | 13.17 ± 1.01 | 11.16–17.59 | 14.76 ± 0.91 | 12.97–16.71 | 0.000 | 7.50–20.00 |
| Palmitoleic C16:1 (%) | 1.07 ± 0.17 | 0.83–1.69 | 1.47 ± 0.19 | 0.97–1.91 | 0.000 | 0.30–3.50 |
| Heptadecanoic C17:0 (%) | 0.04 ± 0.01 | 0.02–0.06 | 0.04 ± 0.01 | 0.02–0.07 | n.s | ≤0.40 |
| Heptadecenoic C17:1 (%) | 0.07 ± 0.01 | 0.05–0.12 | 0.08 ± 0.01 | 0.05–0.13 | 0.003 | ≤0.60 |
| Stearic C18:0 (%) | 2.51 ± 0.24 | 2.03–2.98 | 2.04 ± 0.15 | 1.78–2.64 | 0.000 | 0.50–5.00 |
| Oleic C18:1 (%) | 75.07 ± 1.71 | 69.76–77.96 | 69.55 ± 1.71 | 65.39–73.00 | 0.000 | 55.00–83.00 |
| Linoleic C18:2 (%) | 6.43 ± 1.27 | 4.21–9.55 | 10.40 ± 0.91 | 8.30–12.80 | 0.000 | 2.50–21.00 |
| Linolenic C18:3 (%) | 0.72 ± 0.07 | 0.63–0.88 | 0.79 ± 0.08 | 0.60–0.99 | 0.000 | ≤1.00 |
| Arachidic C20:0 (%) | 0.45 ± 0.03 | 0.34–0.53 | 0.40 ± 0.02 | 0.30–0.49 | 0.000 | ≤0.60 |
| Eicosenoic C20:1 (%) | 0.29 ± 0.04 | 0.23–0.37 | 0.28 ± 0.03 | 0.20–0.33 | n.s | ≤0.50 |
| Behenic C22:0 (%) | 0.13 ± 0.02 | 0.09–0.18 | 0.13 ± 0.02 | 0.09–0.18 | n.s | ≤0.20 |
| Lignoceric C24:0 (%) | 0.05 ± 0.02 | 0.01–0.10 | 0.05 ± 0.01 | 0.03–0.09 | 0.009 | ≤0.20 |
| cv. Koroneiki (N = 44) | cv. Lianolia Kerkyras (N = 60) | Calculating p-Value | EEC Limit for EVOO Category | |
|---|---|---|---|---|
| Sterols and Triterpene Diols | Mean ± SD | Mean ± SD | ||
| Cholesterol (%) | 0.10 ± 0.08 | 0.12 ± 0.06 | n.s | ≤0.5 |
| 24-methylene-cholesterol % | 0.23 ± 0.09 | 0.08 ± 0.04 | 0.000 | |
| Campesterol % | 3.82 ± 0.35 | 3.42 ± 0.17 | 0.000 | ≤4.0 |
| Campestanol % | 0.07 ± 0.03 | 0.04 ± 0.02 | 0.000 | <campesterol |
| Stigmasterol % | 0.63 ± 0.18 | 0.49 ± 0.15 | 0.000 | |
| Chlerosterol % | 0.81 ± 0.20 | 0.81 ± 0.16 | n.s | |
| β-Sitosterol % | 85.95 ± 2.68 | 89.21 ± 1.27 | 0.000 | |
| Sitostanol % | 0.48 ± 0.24 | 0.69 ± 0.17 | 0.000 | |
| Δ-5-avenasterol % | 6.93 ± 2.38 | 4.31 ± 1.27 | 0.001 | |
| Δ-5, 24-stigm/dienol % | 0.29 ± 0.14 | 0.22 ± 0.11 | 0.002 | |
| Δ-7-stigmastenol % | 0.32 ± 0.15 | 0.29 ± 0.11 | n.s | ≤0.5 |
| Δ-7-avenasterol % | 0.25 ± 0.16 | 0.26 ± 0.11 | n.s | |
| Apparent b-Sitosterol % | 94.63 ± 0.70 | 95.28 ± 0.35 | 0.000 | ≥93.0 |
| Total Erythrodiol % | 2.76 ± 1.07 | 1.43 ± 0.45 | 0.000 | ≤4.5 |
| Total sterols (mg/kg) | 1020.8 ± 120.7 | 1343.7 ± 115.1 | 0.000 | ≥1000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skiada, V.; Agriopoulou, S.; Tsarouhas, P.; Katsaris, P.; Stamatelopoulou, E.; Varzakas, T. Evaluation and Origin Discrimination of Two Monocultivar Extra Virgin Olive Oils, Cultivated in the Coastline Part of North-Western Greece. Appl. Sci. 2020, 10, 6733. https://doi.org/10.3390/app10196733
Skiada V, Agriopoulou S, Tsarouhas P, Katsaris P, Stamatelopoulou E, Varzakas T. Evaluation and Origin Discrimination of Two Monocultivar Extra Virgin Olive Oils, Cultivated in the Coastline Part of North-Western Greece. Applied Sciences. 2020; 10(19):6733. https://doi.org/10.3390/app10196733
Chicago/Turabian StyleSkiada, Vasiliki, Sofia Agriopoulou, Panagiotis Tsarouhas, Panagiotis Katsaris, Eygenia Stamatelopoulou, and Theodoros Varzakas. 2020. "Evaluation and Origin Discrimination of Two Monocultivar Extra Virgin Olive Oils, Cultivated in the Coastline Part of North-Western Greece" Applied Sciences 10, no. 19: 6733. https://doi.org/10.3390/app10196733