Analysis of Photosynthetic Systems and Their Applications with Mathematical and Computational Models
Abstract
:1. Introduction
2. Methodology
2.1. Calculation of Nuclear Magnetic Resonance (NMR) and Nuclear Quadrupole Resonance (NQR) Properties
2.2. Quantum Entanglement
2.3. Hierarchical Equations of Motion (HEOM)
2.4. Calculation of Energies and Spectral Density of FMO Complex
2.5. Normal Mode Analysis
2.6. Time-Dependent Density Functional Theory
3. Energetic and Spectroscopic Properties Using DFT
4. Applications of Photosynthetic Systems
4.1. Environmental Science Applications
4.2. Biomimetic Applications
4.3. Health and Applications in Medicine
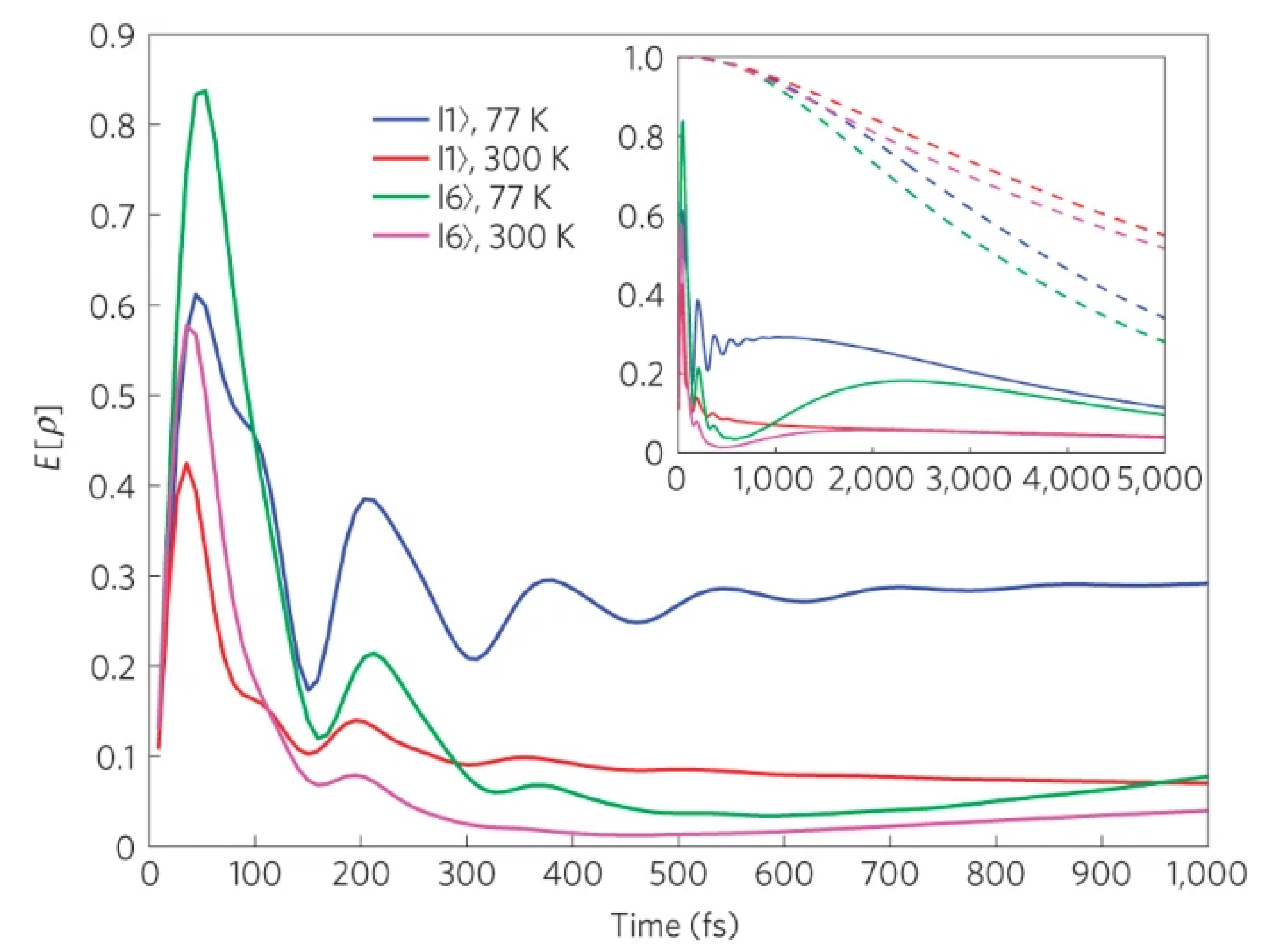
4.4. Enhancing the Quantum Efficiency of Excitonic Energy Transfer and Ultrafast Processes in Light Harvesting Complexes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wen, J.; Tsukatani, Y.; Cui, W.; Zhang, H.; Gross, M.L.; Bryant, D.A.; Blankenship, R.E. Structural model and spectroscopic characteristics of the FMO antenna protein from the aerobic chlorophototroph, Candidatus Chloracidobacterium thermophilum. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, L.A.; Habershon, S. Photosynthetic pigment-protein complexes as highly connected networks: Implications for robust energy transport. Proc. R. Soc. A Math. Phys. Eng. Sci. 2017, 473, 20170112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.; Zhang, H.; Gross, M.L.; Blankenship, R.E. Membrane orientation of the FMO antenna protein from Chlorobaculum tepidum as determined by mass spectrometry-based footprinting. Proc. Natl. Acad. Sci. USA 2009, 106, 6134–6139. [Google Scholar] [CrossRef] [Green Version]
- Leng, X.; Yan, Y.M.; Zhu, R.D.; Song, K.; Weng, Y.X.; Shi, Q. Simulation of the two-dimensional electronic spectroscopy and energy transfer dynamics of light-harvesting complex ii at ambient temperature. J. Phys. Chem. B 2018, 122, 4642–4652. [Google Scholar] [CrossRef]
- Nielsen, A.Z.; Ziersen, B.; Jensen, K.; Lassen, L.M.; Olsen, C.E.; Møller, B.L.; Jensen, P.E. Redirecting Photosynthetic Reducing Power toward Bioactive Natural Product Synthesis. ACS Synth. Biol. 2013, 2, 308–315. [Google Scholar] [CrossRef]
- Sasaki, K.; Watanabe, M.; Suda, Y.; Ishizuka, A.; Noparatnaraporn, N. Applications of photosynthetic bacteria for medical fields. J. Biosci. Bioeng. 2005, 100, 481–488. [Google Scholar] [CrossRef]
- Suchkov, S.; Herrera, A.S. The role of human photosynthesis in predictive, preventive and personalized medicine. EPMA J 2014, 5, A146. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, G.; Peng, M.; Zhou, Q.; Li, J.; Xu, H.; Meng, F. Synthetic white spirit wastewater treatment and biomass recovery by photosynthetic bacteria: Feasibility and process influence factors. Int. Biodeterior. Biodegrad. 2016, 113, 134–138. [Google Scholar] [CrossRef]
- Hohmann-Marriott, M.F.; Blankenship, R.E. Variable fluorescence in green sulfur bacteria. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Bína, D.; Gardian, Z.; Vácha, F.; Litvín, R. Native FMO-reaction center supercomplex in green sulfur bacteria: An electron microscopy study. Photosynth. Res. 2016, 128, 93–102. [Google Scholar] [CrossRef]
- Kramer, T.; Rodriguez, M. Two-dimensional electronic spectra of the photosynthetic apparatus of green sulfur bacteria. Sci. Rep. 2017, 7, 45245. [Google Scholar] [CrossRef] [Green Version]
- Karafyllidis, I.G. Quantum transport in the FMO photosynthetic light-harvesting complex. J. Biol. Phys. 2017, 43, 239–245. [Google Scholar] [CrossRef]
- Kramer, T.; Noack, M.; Reinefeld, A.; Rodríguez, M.; Zelinskyy, Y. Efficient calculation of open quantum system dynamics and time-resolved spectroscopy with distributed memory HEOM (DM-HEOM). J. Comput. Chem. 2018, 39, 1779–1794. [Google Scholar] [CrossRef] [Green Version]
- Rathbone, H.W.; Davis, J.A.; Michie, K.A.; Goodchild, S.C.; Robertson, N.O.; Curmi, P.M. Coherent phenomena in photosynthetic light harvesting: Part one—Theory and spectroscopy. Biophys. Rev. 2018, 10, 1427–1441. [Google Scholar] [CrossRef]
- Rathbone, H.W.; Davis, J.A.; Michie, K.A.; Goodchild, S.C.; Robertson, N.O.; Curmi, P.M. Coherent phenomena in photosynthetic light harvesting: Part two—Observations in biological systems. Biophys. Rev. 2018, 10, 1443–1463. [Google Scholar] [CrossRef]
- Saer, R.G.; Schultz, R.L.; Blankenship, R.E. The influence of quaternary structure on the stability of Fenna–Matthews–Olson (FMO) antenna complexes. Photosynth. Res. 2019, 140, 39–49. [Google Scholar] [CrossRef]
- Tronrud, D.E.; Wen, J.; Gay, L.; Blankenship, R.E. The structural basis for the difference in absorbance spectra for the FMO antenna protein from various green sulfur bacteria. Photosynth. Res. 2009, 100, 79–87. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, H.; Gross, M.L.; Blankenship, R.E. Native Electrospray Mass Spectrometry Reveals the Nature and Stoichiometry of Pigments in the FMO Photosynthetic Antenna Protein. Biochemistry 2011, 50, 3502–3511. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Yan, Y.; Wei, J. Theoretical Study on the Effect of Environment on Excitation Energy Transfer in Photosynthetic Light-Harvesting Systems. J. Phys. Chem. B 2020, 124, 2354–2362. [Google Scholar] [CrossRef]
- Kim, Y.; Morozov, D.; Stadnytskyi, V.; Savikhin, S.; Slipchenko, L.V. Predictive First-Principles Modeling of a Photosynthetic Antenna Protein: The Fenna–Matthews–Olson Complex. J. Phys. Chem. Lett. 2020, 11, 1636–1643. [Google Scholar] [CrossRef]
- Friemann, R.; Lee, K.; Brown, E.N.; Gibson, D.T.; Eklund, H.; Ramaswamy, S. Structures of the multicomponent Rieske non-heme iron toluene 2,3-dioxygenase enzyme system. Acta Crystallogr. Sect. D 2009, 65, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Camara-Artigas, A.; Blankenship, R.E.; Allen, J.P. The structure of the FMO protein from Chlorobium tepidum at 2.2 Ã resolution. Photosynth. Res. 2003, 75, 49–55. [Google Scholar] [CrossRef]
- Jia, X.; Mei, Y.; Zhang, J.Z.H.; Mo, Y. Hybrid QM/MM study of FMO complex with polarized protein-specific charge. Sci. Rep. 2015, 5, 17096. [Google Scholar] [CrossRef] [Green Version]
- Gammeren, A.J.V.; Hulsbergen, F.B.; Erkelens, C.; Groot, H.J.M.D. Synthetic analogues of the histidine–chlorophyll complex: A NMR study to mimic structural features of the photosynthetic reaction center and the light-harvesting complex. J. Biol. Inorg. Chem. 2004, 9, 109–117. [Google Scholar] [CrossRef]
- Makarska-Bialokoz, M.; Kaczor, A.A. Computational Analysis of Chlorophyll Structure and UV-Vis Spectra: A Student Research Project on the Spectroscopy of Natural Complexes. Spectrosc. Lett. 2014, 47, 147–152. [Google Scholar] [CrossRef]
- Sinnecker, S.; Koch, W.; Lubitz, W. Chlorophyll a Radical Ions: A Density Functional Study. J. Phys. Chem. B 2002, 106, 5281–5288. [Google Scholar] [CrossRef]
- Sundholm, D. Density functional theory calculations of the visible spectrum of chlorophyll a. Chem. Phys. Lett. 1999, 302, 480–484. [Google Scholar] [CrossRef]
- Taguchi, A.T.; O’Malley, P.J.; Wraight, C.A.; Dikanov, S.A. Nuclear Hyperfine and Quadrupole Tensor Characterization of the Nitrogen Hydrogen Bond Donors to the Semiquinone of the QB Site in Bacterial Reaction Centers: A Combined X- and S-Band 14,15N ESEEM and DFT Study. J. Phys. Chem. B 2014, 118, 1501–1509. [Google Scholar] [CrossRef]
- Xu, J.; Terskikh, V.V.; Huang, Y. 25Mg Solid-State NMR: A Sensitive Probe of Adsorbing Guest Molecules on a Metal Center in Metal-Organic Framework CPO-27-Mg. J. Phys. Chem. Lett. 2013, 4, 7–11. [Google Scholar] [CrossRef]
- Kacprzak, S.; Kaupp, M. Electronic g-Tensors of Semiquinones in Photosynthetic Reaction Centers. A Density Functional Study. J. Phys. Chem. B 2004, 108, 2464–2469. [Google Scholar] [CrossRef]
- Lumpkin, O. 25Mg and 14N nuclear quadrupole resonances in chlorophyll-a and magnesium phthalocyanine. J. Chem. Phys. 1975, 62, 3281–3283. [Google Scholar] [CrossRef]
- Hoyer, S.; Sarovar, M.; Whaley, K.B. Limits of quantum speedup in photosynthetic light harvesting. New J. Phys. 2010, 12, 065041. [Google Scholar] [CrossRef]
- Ishizaki, A.; Fleming, G.R. On the Interpretation of Quantum Coherent Beats Observed in Two-Dimensional Electronic Spectra of Photosynthetic Light Harvesting Complexes. J. Phys. Chem. B 2011, 115, 6227–6233. [Google Scholar] [CrossRef]
- Scholes, G.D. Quantum-Coherent Electronic Energy Transfer: Did Nature Think of It First? J. Phys. Chem. Lett. 2010, 1, 2–8. [Google Scholar] [CrossRef]
- Shim, S.; Rebentrost, P.; Valleau, S.; Aspuru-Guzik, A. Atomistic Study of the Long-Lived Quantum Coherences in the Fenna–Matthews–Olson Complex. Biophys. J. 2012, 102, 649–660. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Bishop, M.M.; Roscioli, J.D.; LaFountain, A.M.; Frank, H.A.; Beck, W.F. Excitation Energy Transfer by Coherent and Incoherent Mechanisms in the Peridinin-Chlorophyll a Protein. J. Phys. Chem. Lett. 2017, 8, 463–469. [Google Scholar] [CrossRef]
- Rafiq, S.; Scholes, G.D. From Fundamental Theories to Quantum Coherences in Electron Transfer. J. Am. Chem. Soc. 2018, 141, 708–722. [Google Scholar] [CrossRef]
- Thyrhaug, E.; Tempelaar, R.; Alcocer, M.J.; Žídek, K.; Bína, D.; Knoester, J.; Jansen, T.L.; Zigmantas, D. Identification and characterization of diverse coherences in the Fenna–Matthews–Olson complex. Nat. Chem. 2018, 10, 780. [Google Scholar] [CrossRef]
- Irgen-Gioro, S.; Spencer, A.P.; Hutson, W.O.; Harel, E. Coherences of bacteriochlorophyll a uncovered using 3D-electronic spectroscopy. J. Phys. Chem. Lett. 2018, 9, 6077–6081. [Google Scholar] [CrossRef]
- Maiuri, M.; Ostroumov, E.E.; Saer, R.G.; Blankenship, R.E.; Scholes, G.D. Coherent wavepackets in the Fenna–Matthews–Olson complex are robust to excitonic-structure perturbations caused by mutagenesis. Nat. Chem. 2018, 10, 177. [Google Scholar] [CrossRef]
- Jumper, C.C.; Rafiq, S.; Wang, S.; Scholes, G.D. From coherent to vibronic light harvesting in photosynthesis. Curr. Opin. Chem. Biol. 2018, 47, 39–46. [Google Scholar] [CrossRef]
- Meneghin, E.; Pedron, D.; Collini, E. Characterization of the coherent dynamics of bacteriochlorophyll a in solution. Chem. Phys. 2019, 519, 85–91. [Google Scholar] [CrossRef]
- Vassiliev, I.R.; Kjær, B.; Schorner, G.L.; Scheller, H.V.; Golbeck, J.H. Photoinduced Transient Absorbance Spectra of P840/P840+ and the FMO Protein in Reaction Centers of Chlorobium vibrioforme. Biophys. J. 2001, 81, 382–393. [Google Scholar] [CrossRef] [Green Version]
- Saer, R.G.; Stadnytskyi, V.; Magdaong, N.C.; Goodson, C.; Savikhin, S.; Blankenship, R.E. Probing the excitonic landscape of the Chlorobaculum tepidum Fenna-Matthews-Olson (FMO) complex: A mutagenesis approach. Biochim. Biophys. Acta (BBA)-Bioenerg. 2017, 1858, 288–296. [Google Scholar] [CrossRef]
- Saer, R.; Orf, G.S.; Lu, X.; Zhang, H.; Cuneo, M.J.; Myles, D.A.A.; Blankenship, R.E. Perturbation of bacteriochlorophyll molecules in Fenna–Matthews–Olson protein complexes through mutagenesis of cysteine residues. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016, 1857, 1455–1463. [Google Scholar] [CrossRef]
- Rolczynski, B.S.; Navotnaya, P.; Sussman, H.R.; Engel, G.S. Cysteine-mediated mechanism disrupts energy transfer to prevent photooxidation. Proc. Natl. Acad. Sci. USA 2016, 113, 8562–8564. [Google Scholar] [CrossRef] [Green Version]
- Orf, G.S.; Saer, R.G.; Niedzwiedzki, D.M.; Zhang, H.; McIntosh, C.L.; Schultz, J.W.; Mirica, L.M.; Blankenship, R.E. Evidence for a cysteine-mediated mechanism of excitation energy regulation in a photosynthetic antenna complex. Proc. Natl. Acad. Sci. USA 2016, 113, E4486–E4493. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cai, Z.L.; Chen, M. Spectroscopic Properties of Chlorophyll f. J. Phys. Chem. B 2013, 117, 11309–11317. [Google Scholar] [CrossRef]
- O’Malley, P.J.; Collins, S.J. The Effect of Axial Mg Ligation on the Geometry and Spin Density Distribution of Chlorophyll and Bacteriochlorophyll Cation Free Radical Models: A Density Functional Study. J. Am. Chem. Soc. 2001, 123, 11042–11046. [Google Scholar] [CrossRef]
- Adolphs, J.; Renger, T. How Proteins Trigger Excitation Energy Transfer in the FMO Complex of Green Sulfur Bacteria. Biophys. J. 2006, 91, 2778–2797. [Google Scholar] [CrossRef] [Green Version]
- Kramer, T.; Kreisbeck, C. Modelling excitonic-energy transfer in light-harvesting complexes. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2014; Volume 1575, pp. 111–135. [Google Scholar]
- Kreisbeck, C.; Kramer, T. Long-Lived Electronic Coherence in Dissipative Exciton Dynamics of Light-Harvesting Complexes. J. Phys. Chem. Lett. 2012, 3, 2828–2833. [Google Scholar] [CrossRef] [Green Version]
- Kreisbeck, C.; Kramer, T.; Rodríguez, M.; Hein, B. High-performance solution of hierarchical equations of motion for studying energy transfer in light-harvesting complexes. J. Chem. Theory Comput. 2011, 7, 2166–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Songi, L.; Shia, Q. Calculation of correlated initial state in the hierarchical equations of motion method using an imaginary time path integral approach. J. Chem. Phys. 2015, 143, 194106. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Rhee, Y.M. Constructing an Interpolated Potential Energy Surface of a Large Molecule: A Case Study with Bacteriochlorophyll a Model in the Fenna–Matthews–Olson Complex. J. Chem. Theory Comput. 2016, 12, 5235–5246. [Google Scholar] [CrossRef]
- Albaugh, A.; Boateng, H.A.; Bradshaw, R.T.; Demerdash, O.N.; Dziedzic, J.; Mao, Y.; Margul, D.T.; Swails, J.; Zeng, Q.; Case, D.A.; et al. Advanced Potential Energy Surfaces for Molecular Simulation. J. Phys. Chem. B 2016, 120, 9811–9832. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.K.; Huo, P.; Coker, D.F. Semiclassical Path Integral Dynamics: Photosynthetic Energy Transfer with Realistic Environment Interactions. Annu. Rev. Phys. Chem. 2016, 67, 639–668. [Google Scholar] [CrossRef]
- Lee, M.H.; Troisi, A. Vibronic enhancement of excitation energy transport: Interplay between local and non-local exciton-phonon interactions. J. Chem. Phys. 2017, 146, 075101. [Google Scholar] [CrossRef]
- Varsano, D.; Caprasecca, S.; Coccia, E. Theoretical description of protein field effects on electronic excitations of biological chromophores. J. Phys. Condens. Matter 2017, 29, 013002. [Google Scholar] [CrossRef] [Green Version]
- Renger, T.; Klinger, A.; Steinecker, F.; Schmidt Busch, M.; Numata, J.; Müh, F. Normal mode analysis of the spectral density of the Fenna–Matthews–Olson light-harvesting protein: How the protein dissipates the excess energy of excitons. J. Phys. Chem. B 2012, 116, 14565–14580. [Google Scholar] [CrossRef]
- Liguori, N.; Croce, R.; Marrink, S.J.; Thallmair, S. Molecular dynamics simulations in photosynthesis. Photosynth. Res. 2020, 144, 273–295. [Google Scholar] [CrossRef] [Green Version]
- Higashi, M.; Saito, S. Quantitative Evaluation of Site Energies and Their Fluctuations of Pigments in the Fenna–Matthews–Olson Complex with an Efficient Method for Generating a Potential Energy Surface. J. Chem. Theory Comput. 2016, 12, 4128–4137. [Google Scholar] [CrossRef] [PubMed]
- Jurinovich, S.; Curutchet, C.; Mennucci, B. The Fenna–Matthews–Olson Protein Revisited: A Fully Polarizable (TD)DFT/MM Description. ChemPhysChem 2014, 15, 3194–3204. [Google Scholar] [CrossRef] [PubMed]
- König, C.; Neugebauer, J. Protein Effects on the Optical Spectrum of the Fenna–Matthews–Olson Complex from Fully Quantum Chemical Calculations. J. Chem. Theory Comput. 2013, 9, 1808–1820. [Google Scholar] [CrossRef]
- Thyrhaug, E.; Žídek, K.; Dostál, J.; Bína, D.; Zigmantas, D. Exciton Structure and Energy Transfer in the Fenna–Matthews–Olson Complex. J. Phys. Chem. Lett. 2016, 7, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Smyth, C. Measuring Quantum Effects in Photosynthetic Light-Harvesting Complexes with Multipartite Entanglement. Ph.D. Thesis, University of Toronto (Canada), Toronto, ON, Canada, 2015. [Google Scholar]
- Aghtar, M.; Strümpfer, J.; Olbrich, C.; Schulten, K.; Kleinekathöfer, U. Different Types of Vibrations Interacting with Electronic Excitations in Phycoerythrin 545 and Fenna–Matthews–Olson Antenna Systems. J. Phys. Chem. Lett. 2014, 5, 3131–3137. [Google Scholar] [CrossRef] [PubMed]
- Goez, A.; Jacob, C.R.; Neugebauer, J. Modeling environment effects on pigment site energies: Frozen density embedding with fully quantum-chemical protein densities. Comput. Theor. Chem. 2014, 1040, 347–359. [Google Scholar] [CrossRef]
- Cao, J.; Cogdell, R.J.; Coker, D.F.; Duan, H.G.; Hauer, J.; Kleinekathöfer, U.; Jansen, T.L.; Mančal, T.; Miller, R.D.; Ogilvie, J.P.; et al. Quantum biology revisited. Sci. Adv. 2020, 6, eaaz4888. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Allodi, M.A.; Engel, G.S. Quantum coherences reveal excited-state dynamics in biophysical systems. Nat. Rev. Chem. 2019, 3, 477–490. [Google Scholar] [CrossRef]
- Marcus, M.; Knee, G.C.; Datta, A. Towards a spectroscopic protocol for unambiguous detection of quantum coherence in excitonic energy transport. Faraday Discuss. 2019, 221, 110–132. [Google Scholar] [CrossRef] [Green Version]
- Alvertis, A.M.; Barford, W.; Worster, S.B.; Burghardt, I.; Datta, A.; Dijkstra, A.; Fay, T.; Ghosh, S.; Grünbaum, T.; Habershon, S.; et al. Quantum coherence in complex environments: General discussion. Faraday Discuss. 2019, 221, 168–201. [Google Scholar] [CrossRef]
- Mančal, T. A Decade with Quantum Coherence: How our Past Became Classical and the Future Turned Quantum. Chem. Phys. 2020, 110663. [Google Scholar]
- Irgen-Gioro, S.; Gururangan, K.; Saer, R.G.; Blankenship, R.E.; Harel, E. Electronic coherence lifetimes of the Fenna–Matthews–Olson complex and light harvesting complex II. Chem. Sci. 2019, 10, 10503–10509. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Flores, J. Evolution of the Fenna–Matthews–Olson Complex and Its Quantum Coherence Features. Which Led the Way? ACS Cent. Sci. 2017, 3, 1061–1062. [Google Scholar] [CrossRef] [PubMed]
- Scholes, G.D.; Fleming, G.R.; Chen, L.X.; Aspuru-Guzik, A.; Buchleitner, A.; Coker, D.F.; Engel, G.S.; Van Grondelle, R.; Ishizaki, A.; Jonas, D.M.; et al. Using coherence to enhance function in chemical and biophysical systems. Nature 2017, 543, 647–656. [Google Scholar] [CrossRef]
- Bai, S.; Song, K.; Shi, Q. Effects of Different Quantum Coherence on the Pump-Probe Polarization Anisotropy of Photosynthetic Light-Harvesting Complexes: A Computational Study. J. Phys. Chem. Lett. 2015, 6, 1954–1960. [Google Scholar] [CrossRef]
- Song, K.; Bai, S.; Shi, Q. Effect of Pulse Shaping on Observing Coherent Energy Transfer in Single Light-Harvesting Complexes. J. Phys. Chem. B 2016, 120, 11637–11643. [Google Scholar] [CrossRef]
- Chen, G.Y.; Lambert, N.; Shih, Y.A.; Liu, M.H.; Chen, Y.N.; Nori, F. Plasmonic bio-sensing for the Fenna-Matthews-Olson complex. Sci. Rep. 2017, 7, 39720. [Google Scholar] [CrossRef]
- Lee, M.K.; Coker, D.F. Modeling Electronic-Nuclear Interactions for Excitation Energy Transfer Processes in Light-Harvesting Complexes. J. Phys. Chem. Lett. 2016, 7, 3171–3178. [Google Scholar] [CrossRef]
- Olbrich, C.; Kleinekathöfer, U. Time-Dependent Atomistic View on the Electronic Relaxation in Light-Harvesting System II. J. Phys. Chem. B 2010, 114, 12427–12437. [Google Scholar] [CrossRef]
- Olbrich, C.; Jansen, T.L.C.; Liebers, J.; Aghtar, M.; Strümpfer, J.; Schulten, K.; Knoester, J.; Kleinekathöfer, U. From Atomistic Modeling to Excitation Transfer and Two-Dimensional Spectra of the FMO Light-Harvesting Complex. J. Phys. Chem. B 2011, 115, 8609–8621. [Google Scholar] [CrossRef] [Green Version]
- Reimers, J.R.; Biczysko, M.; Bruce, D.; Coker, D.F.; Frankcombe, T.J.; Hashimoto, H.; Hauer, J.; Jankowiak, R.; Kramer, T.; Linnanto, J.; et al. Challenges facing an understanding of the nature of low-energy excited states in photosynthesis. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016, 1857, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, S.E.; Sneddon, S. New methods and applications in solid-state NMR spectroscopy of quadrupolar nuclei. J. Am. Chem. Soc. 2014, 136, 15440–15456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandit, A.; de Groot, H.J. Solid-state NMR applied to photosynthetic light-harvesting complexes. Photosynth. Res. 2012, 111, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facelli, J.C. Chemical shift tensors: Theory and application to molecular structural problems. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 58, 176–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, P.P. Quadrupolar Interactions. In Encyclopedia of Magnetic Resonance; Grant, D.M., Harris, R.K., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 3838–3846. [Google Scholar]
- Sarovar, M.; Ishizaki, A.; Fleming, G.R.; Whaley, K.B. Quantum entanglement in photosynthetic light-harvesting complexes. Nat. Phys. 2010, 6, 462–467. [Google Scholar] [CrossRef]
- Zhu, J.; Kais, S.; Aspuru-Guzik, A.; Rodriques, S.; Brock, B.; Love, P.J. Multipartite quantum entanglement evolution in photosynthetic complexes. J. Chem. Phys. 2012, 137, 074112. [Google Scholar] [CrossRef] [Green Version]
- Bardeen, C.J. Time dependent correlations of entangled states with nondegenerate branches and possible experimental realization using singlet fission. J. Chem. Phys. 2019, 151, 124503. [Google Scholar] [CrossRef]
- Bengtsson, I.; Zyczkowski, K. Geometry of Quantum States: An Introduction to Quantum Entanglement; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Singh, D.; Dasgupta, S. Coherence and Its Role in Excitation Energy Transfer in Fenna–Matthews–Olson Complex. J. Phys. Chem. B 2017, 121, 1290–1294. [Google Scholar] [CrossRef] [Green Version]
- Panitchayangkoon, G.; Voronine, D.V.; Abramavicius, D.; Caram, J.R.; Lewis, N.H.; Mukamel, S.; Engel, G.S. Direct evidence of quantum transport in photosynthetic light-harvesting complexes. Proc. Natl. Acad. Sci. USA 2011, 108, 20908–20912. [Google Scholar] [CrossRef] [Green Version]
- Kell, A.; Khmelnitskiy, A.Y.; Reinot, T.; Jankowiak, R. On uncorrelated inter-monomer Förster energy transfer in Fenna–Matthews–Olson complexes. J. R. Soc. Interface 2019, 16, 20180882. [Google Scholar] [CrossRef] [Green Version]
- Streltsov, A.; Adesso, G.; Plenio, M.B. Colloquium: Quantum coherence as a resource. Rev. Mod. Phys. 2017, 89, 041003. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, D.M.; Dattani, N.S. Why Quantum Coherence Is Not Important in the Fenna–Matthews–Olsen Complex. J. Chem. Theory Comput. 2015, 11, 3411–3419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keren, N.; Paltiel, Y. Photosynthetic energy transfer at the quantum/classical border. Trends Plant Sci. 2018, 23, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Fleming, G.R. On the adequacy of the Redfield equation and related approaches to the study of quantum dynamics in electronic energy transfer. J. Chem. Phys. 2009, 130, 234110. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Fleming, G.R. Unified treatment of quantum coherent and incoherent hopping dynamics in electronic energy transfer: Reduced hierarchy equation approach. J. Chem. Phys. 2009, 130, 234111. [Google Scholar] [CrossRef] [Green Version]
- Tanimura, Y. Numerically “exact” approach to open quantum dynamics: The hierarchical equations of motion (HEOM). J. Chem. Phys. 2020, 153, 020901. [Google Scholar] [CrossRef]
- Hein, B.; Kreisbeck, C.; Kramer, T.; Rodríguez, M. Modelling of oscillations in two-dimensional echo-spectra of the Fenna–Matthews–Olson complex. New J. Phys. 2012, 14, 023018. [Google Scholar] [CrossRef]
- Chen, H.B.; Lambert, N.; Cheng, Y.C.; Chen, Y.N.; Nori, F. Using non-Markovian measures to evaluate quantum master equations for photosynthesis. Sci. Rep. 2015, 5, 12753. [Google Scholar] [CrossRef] [Green Version]
- Kell, A.; Blankenship, R.E.; Jankowiak, R. Effect of Spectral Density Shapes on the Excitonic Structure and Dynamics of the Fenna–Matthews–Olson Trimer from Chlorobaculum tepidum. J. Phys. Chem. A 2016, 120, 6146–6154. [Google Scholar] [CrossRef]
- Zhu, J.; Kais, S.; Rebentrost, P.; Aspuru-Guzik, A. Modified scaled hierarchical equation of motion approach for the study of quantum coherence in photosynthetic complexes. J. Phys. Chem. B 2011, 115, 1531–1537. [Google Scholar] [CrossRef] [Green Version]
- Vulto, S.I.; Neerken, S.; Louwe, R.J.; de Baat, M.A.; Amesz, J.; Aartsma, T.J. Excited-state structure and dynamics in FMO antenna complexes from photosynthetic green sulfur bacteria. J. Phys. Chem. B 1998, 102, 10630–10635. [Google Scholar] [CrossRef]
- Vulto, S.I.; de Baat, M.A.; Louwe, R.J.; Permentier, H.P.; Neef, T.; Miller, M.; van Amerongen, H.; Aartsma, T.J. Exciton simulations of optical spectra of the FMO complex from the green sulfur bacterium Chlorobium tepidum at 6 K. J. Phys. Chem. B 1998, 102, 9577–9582. [Google Scholar] [CrossRef] [Green Version]
- Vulto, S.I.E.; de Baat, M.A.; Neerken, S.; Nowak, F.R.; van Amerongen, H.; Amesz, J.; Aartsma, T.J. Excited State Dynamics in FMO Antenna Complexes from Photosynthetic Green Sulfur Bacteria: A Kinetic Model. J. Phys. Chem. B 1999, 103, 8153–8161. [Google Scholar] [CrossRef]
- Adolphs, J.; Müh, F.; Madjet, M.E.A.; Renger, T. Calculation of pigment transition energies in the FMO protein. Photosynth. Res. 2008, 95, 197. [Google Scholar] [CrossRef]
- Gao, J.; Shi, W.J.; Ye, J.; Wang, X.; Hirao, H.; Zhao, Y. QM/MM Modeling of Environmental Effects on Electronic Transitions of the FMO Complex. J. Phys. Chem. B 2013, 117, 3488–3495. [Google Scholar] [CrossRef]
- Tian, B.L.; Ding, J.J.; Xu, R.X.; Yan, Y. Biexponential theory of Drude dissipation via hierarchical quantum master equation. J. Chem. Phys. 2010, 133, 114112. [Google Scholar] [CrossRef] [Green Version]
- Renger, T.; Müh, F. Theory of excitonic couplings in dielectric media. Photosynth. Res. 2012, 111, 47–52. [Google Scholar] [CrossRef]
- Madjet, M.E.; Abdurahman, A.; Renger, T. Intermolecular Coulomb Couplings from Ab Initio Electrostatic Potentials: Application to Optical Transitions of Strongly Coupled Pigments in Photosynthetic Antennae and Reaction Centers. J. Phys. Chem. B 2006, 110, 17268–17281. [Google Scholar] [CrossRef]
- Cupellini, L.; Jurinovich, S.; Campetella, M.; Caprasecca, S.; Guido, C.A.; Kelly, S.M.; Gardiner, A.T.; Cogdell, R.; Mennucci, B. An Ab Initio Description of the Excitonic Properties of LH2 and Their Temperature Dependence. J. Phys. Chem. B 2016, 120, 11348–11359. [Google Scholar] [CrossRef]
- Aghtar, M.; Kleinekathöfer, U.; Curutchet, C.; Mennucci, B. Impact of Electronic Fluctuations and Their Description on the Exciton Dynamics in the Light-Harvesting Complex PE545. J. Phys. Chem. B 2017, 121, 1330–1339. [Google Scholar] [CrossRef] [Green Version]
- Cole, D.J.; Hine, N.D.M. Applications of large-scale density functional theory in biology. J. Phys. Condens. Matter 2016, 28, 393001. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.; Curutchet, C. Can Förster Theory Describe Stereoselective Energy Transfer Dynamics in a Protein–Ligand Complex? J. Phys. Chem. B 2017, 121, 2265–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, N.H.C.; Gruenke, N.L.; Oliver, T.A.A.; Ballottari, M.; Bassi, R.; Fleming, G.R. Observation of Electronic Excitation Transfer Through Light Harvesting Complex II Using Two-Dimensional Electronic-Vibrational Spectroscopy. J. Phys. Chem. Lett. 2016, 7, 4197–4206. [Google Scholar] [CrossRef]
- Stratmann, R.E.; Scuseria, G.E. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Petersilka, M.; Gossmann, U.J.; Gross, E.K.U. Excitation Energies from Time-Dependent Density-Functional Theory. Phys. Rev. Lett. 1996, 76, 1212–1215. [Google Scholar] [CrossRef] [Green Version]
- Dreuw, A.; Weisman, J.L.; Head-Gordon, M. Long-range charge-transfer excited states in time-dependent density functional theory require non-local exchange. J. Chem. Phys. 2003, 119, 2943–2946. [Google Scholar] [CrossRef]
- Tawada, Y.; Tsuneda, T.; Yanagisawa, S.; Yanai, T.; Hirao, K. A long-range-corrected time-dependent density functional theory. J. Chem. Phys. 2004, 120, 8425–8433. [Google Scholar] [CrossRef]
- Chong, D.P. Recent Advances in Density Functional Methods: (Part I); World Scientific: Singapore, 1995. [Google Scholar]
- Gross, E.K.U.; Kohn, W. Time-Dependent Density-Functional Theory. In Advances in Quantum Chemistry; Löwdin, P.O., Ed.; Academic Press: Cambridge, MA, USA, 1990; Volume 21, pp. 255–291. [Google Scholar]
- Badu, S.; Melnik, R. NMR properties of Fenna–Matthews–Olson light harvesting complex: Photosynthesis and its biomedical applications. In Proceedings of the 2017 IEEE First, Ukraine Conference on Electrical and Computer Engineering (UKRCON), Kyiv, Ukraine, 29 May–2 June 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 318–321. [Google Scholar]
- Badu, S.; Prabhakar, S.; Melnik, R. Component spectroscopic properties of light-harvesting complexes with DFT calculations. Biocell 2020, 44, 279–291. [Google Scholar] [CrossRef]
- Badu, S.; Melnik, R.; Prabhakar, S. Photosynthesis and electronic properties of Fenna-Mathhews-Olson light harvesting complexes. Proceedings and Extended Abstracts. In Proceedings of the IWBBIO 2016 International Work-Conference on Bioinformatics and Biomedical Engineering, IWBBIO 2016, Granada, Spain, 20–22 April 2016; pp. 202–203. [Google Scholar]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.E.; et al. Gaussian 09 Revision D. 01; Inc.: Wallingford, CT, USA, 2014. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Reply to Comment on generalized gradient approximation made simple. Phys. Rev. Lett. 1998, 80, 891. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Khmelnitskiy, A.; Reinot, T.; Jankowiak, R. Impact of Single-Point Mutations on the Excitonic Structure and Dynamics in a Fenna–Matthews–Olson Complex. J. Phys. Chem. Lett. 2018, 9, 3378–3386. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Aghtar, M.; Valleau, S.; Aspuru-Guzik, A.; Kleinekathöfer, U. Influence of Force Fields and Quantum Chemistry Approach on Spectral Densities of BChl a in Solution and in FMO Proteins. J. Phys. Chem. B 2015, 119, 9995–10004. [Google Scholar] [CrossRef] [PubMed]
- Jurinovich, S.; Viani, L.; Curutchet, C.; Mennucci, B. Limits and potentials of quantum chemical methods in modelling photosynthetic antennae. Phys. Chem. Chem. Phys. 2015, 17, 30783–30792. [Google Scholar] [CrossRef]
- Aghtar, M.; Kleinekathöfer, U. Environmental coupling and population dynamics in the PE545 light-harvesting complex. J. Lumin. 2016, 169, Part B, 406–409. [Google Scholar] [CrossRef]
- Zheng, F.; Jin, M.; Mancal, T.; Zhao, Y. Study of Electronic Structures and Pigment-Protein Interactions in the Reaction Center of Thermochromatium tepidum with a Dynamic Environment. J. Phys. Chem. B 2016, 120, 10046–10058. [Google Scholar] [CrossRef]
- Jankowiak, R.; Reppert, M.; Zazubovich, V.; Pieper, J.; Reinot, T. Site selective and single complex laser-based spectroscopies: A window on excited state electronic structure, excitation energy transfer, and electron–phonon coupling of selected photosynthetic complexes. Chem. Rev. 2011, 111, 4546–4598. [Google Scholar] [CrossRef]
- Kramer, T.; Rodríguez, M. Effect of disorder and polarization sequences on two-dimensional spectra of light-harvesting complexes. Photosynth. Res. 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Engel, G.S.; Calhoun, T.R.; Read, E.L.; Ahn, T.K.; Mančal, T.; Cheng, Y.C.; Blankenship, R.E.; Fleming, G.R. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 2007, 446, 782–786. [Google Scholar] [CrossRef]
- Lambrev, P.H.; Akhtar, P.; Tan, H.S. Insights into the mechanisms and dynamics of energy transfer in plant light-harvesting complexes from two-dimensional electronic spectroscopy. Biochim. Biophys. Acta (BBA)-Bioenerg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Claridge, K.; Padula, D.; Troisi, A. On the arrangement of chromophores in light harvesting complexes: chance versus design. Faraday Discuss. 2019, 221, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Gururangan, K.; Harel, E. Coherent and dissipative quantum process tensor reconstructions in two-dimensional electronic spectroscopy. J. Chem. Phys. 2019, 150, 164127. [Google Scholar] [CrossRef] [PubMed]
- Badu, S.; Melnik, R. Fundamental molecular complexes of photosynthesis and their biomedical applications. Proceedings and Extended Abstracts. In Proceedings of the IWBBIO 2017 International Work-Conference on Bioinformatics and Biomedical Engineering, IWBBIO 2017, Granada, Spain, 26–28 April 2017; pp. 94–96. [Google Scholar]
- Croce, R.; van Grondelle, R.; van Amerongen, H.; van Stokkum, I. Light Harvesting in Photosynthesis; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Halder, P.; Azad, A. Recent trends and challenges of algal biofuel conversion technologies. In Advanced Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 167–179. [Google Scholar]
- Syrpas, M.; Venskutonis, P.R. Algae for the production of bio-based products. In Biobased Products and Industries; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–243. [Google Scholar]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total. Environ. 2020, 716, 137116. [Google Scholar] [CrossRef] [PubMed]
- Veeramuthu, A.; Ngamcharussrivichai, C. Potential of microalgal biodiesel: Challenges and applications. In Renewable Energy; IntechOpen: London, UK, 2020. [Google Scholar]
- Hitchcock, A.; Hunter, C.N.; Canniffe, D.P. Progress and challenges in engineering cyanobacteria as chassis for light-driven biotechnology. Microb. Biotechnol. 2020, 13, 363–367. [Google Scholar] [CrossRef]
- Khan, A.Z.; Bilal, M.; Mehmood, S.; Sharma, A.; Iqbal, H. State-of-the-Art Genetic Modalities to Engineer Cyanobacteria for Sustainable Biosynthesis of Biofuel and Fine-Chemicals to Meet Bio–Economy Challenges. Life 2019, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, M.; Zhou, M.; Yang, H.; Liang, L.; Gu, T. Microbial fuel cell hybrid systems for wastewater treatment and bioenergy production: Synergistic effects, mechanisms and challenges. Renew. Sustain. Energy Rev. 2019, 103, 13–29. [Google Scholar] [CrossRef]
- Gul, M.M.; Ahmad, K.S. Bioelectrochemical systems: Sustainable bio-energy powerhouses. Biosens. Bioelectron. 2019, 142, 111576. [Google Scholar] [CrossRef]
- Mouhib, M.; Antonucci, A.; Reggente, M.; Amirjani, A.; Gillen, A.J.; Boghossian, A.A. Enhancing bioelectricity generation in microbial fuel cells and biophotovoltaics using nanomaterials. Nano Res. 2019, 12, 2184–2199. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Vanbroekhoven, K.; Pant, D. Techno-productive potential of photosynthetic microbial fuel cells through different configurations. Renew. Sustain. Energy Rev. 2014, 39, 617–627. [Google Scholar] [CrossRef]
- Qi, X.; Ren, Y.; Liang, P.; Wang, X. New insights in photosynthetic microbial fuel cell using anoxygenic phototrophic bacteria. Bioresour. Technol. 2018, 258, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, L.; Wen, Q.; Chen, Y.; Qi, L.; Huang, J.; Tang, Z. A 3D porous NCNT sponge anode modified with chitosan and Polyaniline for high-performance microbial fuel cell. Bioelectrochemistry 2019, 129, 144–153. [Google Scholar] [CrossRef]
- Milano, F.; Punzi, A.; Ragni, R.; Trotta, M.; Farinola, G.M. Photonics and optoelectronics with bacteria: Making materials from photosynthetic microorganisms. Adv. Funct. Mater. 2019, 29, 1805521. [Google Scholar] [CrossRef]
- Di Lauro, M.; la Gatta, S.; Bortolotti, C.A.; Beni, V.; Parkula, V.; Drakopoulou, S.; Giordani, M.; Berto, M.; Milano, F.; Cramer, T.; et al. A Bacterial Photosynthetic Enzymatic Unit Modulating Organic Transistors with Light. Adv. Electron. Mater. 2020, 6, 1900888. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Yang, P.; Li, N.; Zhao, M.; Zhang, X.; Zhang, Y.; Yuan, Y.; Lu, X.; Lu, X. Extraction of photosynthetic electron from mixed photosynthetic consortium of bacteria and algae towards sustainable bioelectrical energy harvesting. Electrochim. Acta 2020, 336, 135710. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Cruz, J.A.; Avenson, T.J.; Kanazawa, A.; Takizawa, K.; Edwards, G.E.; Kramer, D.M. Plasticity in light reactions of photosynthesis for energy production and photoprotection. J. Exp. Bot. 2005, 56, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.J.; Kramer, D.M.; Fisher, N.; Fu, X. Flexibility in the Energy Balancing Network of Photosynthesis Enables Safe Operation under Changing Environmental Conditions. Plants 2020, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Dimitriev, O.; Yoshida, T.; Sun, H. Principles of solar energy storage. Energy Storage 2020, 2, e96. [Google Scholar] [CrossRef] [Green Version]
- Ravi, S.K.; Rawding, P.; Elshahawy, A.M.; Huang, K.; Sun, W.; Zhao, F.; Wang, J.; Jones, M.R.; Tan, S.C. Photosynthetic apparatus of Rhodobacter sphaeroides exhibits prolonged charge storage. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, Z.; Sun, Z.; Zhang, Y.; Dong, C.; Zhang, M.; Li, Z.; Chen, Y. A high-performance energy storage system from sphagnum uptake waste LIBs with negative greenhouse-gas emission. Nano Energy 2020, 67, 104216. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, P.; Zhang, G. Biomass and pigments production in photosynthetic bacteria wastewater treatment: Effects of light sources. Bioresour. Technol. 2015, 179, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Abinandan, S.; Shanthakumar, S. Challenges and opportunities in application of microalgae (Chlorophyta) for wastewater treatment: A review. Renew. Sustain. Energy Rev. 2015, 52, 123–132. [Google Scholar] [CrossRef]
- Liu, J.; Pemberton, B.; Lewis, J.; Scales, P.J.; Martin, G.J. Wastewater treatment using filamentous algae— A review. Bioresour. Technol. 2019, 122556. [Google Scholar] [CrossRef] [PubMed]
- Umar, L.; Alexander, F.A.; Wiest, J. Application of algae-biosensor for environmental monitoring. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; pp. 7099–7102. [Google Scholar]
- Wati, A.; Rusva, R.; Umar, L. Effect of LED Wavelengths and Light-Dark Cycle on Photosynthetic Production of Chlorella Kessleri for Algae-Based Biosensor Optimization. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1351, p. 012003. [Google Scholar]
- Umar, L.; Hamzah, Y.; Setiadi, R.N. Biosensor signal improvement using current mirror topology for dissolved oxygen measurement. Meas. Sci. Technol. 2019, 30, 065102. [Google Scholar] [CrossRef]
- Xu, M.; Melnik, R.V.; Borup, U. Modeling anti-islanding protection devices for photovoltaic systems. Renew. Energy 2004, 29, 2195–2216. [Google Scholar] [CrossRef]
- Alharbi, F.H.; Kais, S. Theoretical limits of photovoltaics efficiency and possible improvements by intuitive approaches learned from photosynthesis and quantum coherence. Renew. Sustain. Energy Rev. 2015, 43, 1073–1089. [Google Scholar] [CrossRef] [Green Version]
- Brédas, J.L.; Sargent, E.H.; Scholes, G.D. Photovoltaic concepts inspired by coherence effects in photosynthetic systems. Nat. Mater. 2017, 16, 35–44. [Google Scholar] [CrossRef]
- Romero, E.; Novoderezhkin, V.I.; van Grondelle, R. Quantum design of photosynthesis for bio-inspired solar-energy conversion. Nature 2017, 543, 355–365. [Google Scholar] [CrossRef]
- Dong, Y.; Cha, H.; Zhang, J.; Pastor, E.; Tuladhar, P.S.; McCulloch, I.; Durrant, J.R.; Bakulin, A.A. The binding energy and dynamics of charge-transfer states in organic photovoltaics with low driving force for charge separation. J. Chem. Phys. 2019, 150, 104704. [Google Scholar] [CrossRef]
- Gasparini, N.; Salleo, A.; McCulloch, I.; Baran, D. The role of the third component in ternary organic solar cells. Nat. Rev. Mater. 2019, 4, 229–242. [Google Scholar] [CrossRef]
- Segev, G.; Beeman, J.W.; Greenblatt, J.B.; Sharp, I.D. Hybrid photoelectrochemical and photovoltaic cells for simultaneous production of chemical fuels and electrical power. Nat. Mater. 2018, 17, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Gelbwaser-Klimovsky, D.; Aspuru-Guzik, A. On thermodynamic inconsistencies in several photosynthetic and solar cell models and how to fix them. Chem. Sci. 2017, 8, 1008–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.; Yi, Y.; Shuai, Z. From molecular packing structures to electronic processes: theoretical simulations for organic solar cells. Adv. Energy Mater. 2018, 8, 1702743. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Pan, Q.Q.; Geng, Y.; Wu, S.X.; Zhang, M.; Zhao, L.; Su, Z.M. A theorectical design of performant chlorinated benzothiadiazole-based polymers as donor for organic photovoltaic devices. Org. Electron. 2018, 61, 46–55. [Google Scholar] [CrossRef]
- Nelson, T.R.; White, A.J.; Bjorgaard, J.A.; Sifain, A.E.; Zhang, Y.; Nebgen, B.; Fernandez-Alberti, S.; Mozyrsky, D.; Roitberg, A.E.; Tretiak, S. Non-adiabatic Excited-State Molecular Dynamics: Theory and Applications for Modeling Photophysics in Extended Molecular Materials. Chem. Rev. 2020, 120, 2215–2287. [Google Scholar] [CrossRef]
- Schröder, F.A.; Turban, D.H.; Musser, A.J.; Hine, N.D.; Chin, A.W. Tensor network simulation of multi-environmental open quantum dynamics via machine learning and entanglement renormalisation. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.W.; Pan, Q.Q.; Geng, Y.; Wu, Y.; Zhao, L.; Zhang, M.; Su, Z.M. Theoretical Insight into Multiple Charge-Transfer Mechanisms at the P3HT/Nonfullerenes Interface in Organic Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 19699–19707. [Google Scholar] [CrossRef]
- Marmolejo-Valencia, A.F.; Mata-Pinzón, Z.; Dominguez, L.; Amador-Bedolla, C. Atomistic simulations of bulk heterojunctions to evaluate the structural and packing properties of new predicted donors in OPVs. Phys. Chem. Chem. Phys. 2019, 21, 20315–20326. [Google Scholar] [CrossRef]
- Bonke, S.A.; Wiechen, M.; MacFarlane, D.R.; Spiccia, L. Renewable fuels from concentrated solar power: Towards practical artificial photosynthesis. Energy Environ. Sci. 2015, 8, 2791–2796. [Google Scholar] [CrossRef]
- Fukuzumi, S. Artificial photosynthesis for production of hydrogen peroxide and its fuel cells. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016, 1857, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Gamba, I. Biomimetic Approach to CO2 Reduction. Bioinorg. Chem. Appl. 2018, 2379141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Li, P.; Liu, J.; Chen, Z.; Liu, L.; Dontsova, D.; Yan, R.; Fan, T.; Zhang, D.; Ye, J. Biomimetic polymeric semiconductor based hybrid nanosystems for artificial photosynthesis towards solar fuels generation via CO2 reduction. Nano Energy 2016, 25, 128–135. [Google Scholar] [CrossRef]
- Quader, M.; Ahmed, S. Bioenergy with carbon capture and storage (BECCS): Future prospects of carbon-negative technologies. In Clean Energy for Sustainable Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 91–140. [Google Scholar]
- Barber, J. Photosynthetic energy conversion: Natural and artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.; Graetzel, M. Artificial photosynthesis. 1. Photosensitization of titania solar cells with chlorophyll derivatives and related natural porphyrins. J. Phys. Chem. 1993, 97, 6272–6277. [Google Scholar] [CrossRef]
- Berardi, S.; Drouet, S.; Francas, L.; Gimbert-Suriñach, C.; Guttentag, M.; Richmond, C.; Stoll, T.; Llobet, A. Molecular artificial photosynthesis. Chem. Soc. Rev. 2014, 43, 7501–7519. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K.; Suenobu, T. Long-Lived Charge Separation and Applications in Artificial Photosynthesis. Acc. Chem. Res. 2014, 47, 1455–1464. [Google Scholar] [CrossRef]
- Kim, D.; Sakimoto, K.K.; Hong, D.; Yang, P. Artificial Photosynthesis for Sustainable Fuel and Chemical Production. Angew. Chem. Int. Ed. 2015, 54, 3259–3266. [Google Scholar] [CrossRef]
- Faunce, T.; Styring, S.; Wasielewski, M.R.; Brudvig, G.W.; Rutherford, A.W.; Messinger, J.; Lee, A.F.; Hill, C.L.; Degroot, H.; Fontecave, M.; et al. Artificial photosynthesis as a frontier technology for energy sustainability. Energy Environ. Sci. 2013, 6, 1074–1076. [Google Scholar] [CrossRef] [Green Version]
- Mora, S.J.; Odella, E.; Moore, G.F.; Gust, D.; Moore, T.A.; Moore, A.L. Proton-Coupled Electron Transfer in Artificial Photosynthetic Systems. Accounts Chem. Res. 2018, 51, 445–453. [Google Scholar] [CrossRef]
- Odella, E.; Mora, S.J.; Wadsworth, B.L.; Huynh, M.T.; Goings, J.J.; Liddell, P.A.; Groy, T.L.; Gervaldo, M.; Sereno, L.E.; Gust, D.; et al. Controlling proton-coupled electron transfer in bioinspired artificial photosynthetic relays. J. Am. Chem. Soc. 2018, 140, 15450–15460. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; King, P.W. Coupling biology to synthetic nanomaterials for semi-artificial photosynthesis. Photosynth. Res. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, S.; Ueda, T.; Kuruma, Y. Artificial photosynthetic cell producing energy for protein synthesis. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Lee, K.Y.; Park, S.J.; Lee, K.A.; Kim, S.H.; Kim, H.; Meroz, Y.; Mahadevan, L.; Jung, K.H.; Ahn, T.K.; Parker, K.K.; et al. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system. Nat. Biotechnol. 2018, 36, 530–535. [Google Scholar] [CrossRef]
- Lee, Y.V.; Tian, B. Learning from Solar Energy Conversion: Biointerfaces for Artificial Photosynthesis and Biological Modulation. Nano Lett. 2019, 19, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.; Faunce, T. Sustainable fuel, food, fertilizer and ecosystems through a global artificial photosynthetic system: Overcoming anticompetitive barriers. Interface Focus 2015, 5, 20150011. [Google Scholar] [CrossRef]
- Long, S.P.; Zhu, X.G.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef]
- Borg, O.A.; Godinho, S.S.; Lundqvist, M.J.; Lunell, S.; Persson, P. Computational study of the lowest triplet state of ruthenium polypyridyl complexes used in artificial photosynthesis. J. Phys. Chem. A 2008, 112, 4470–4476. [Google Scholar] [CrossRef]
- Barber, J.; Tran, P.D. From natural to artificial photosynthesis. J. R. Soc. Interface 2013, 10, 20120984. [Google Scholar] [CrossRef] [Green Version]
- Asahi, R.; Jinnouchi, R. Atomistic modeling of photoelectric cells for artificial photosynthesis. Multiscale Simul. Electrochem. Devices 2020, 107. [Google Scholar] [CrossRef]
- Aitchison, C.M.; Andrei, V.; Antón-García, D.; Apfel, U.P.; Badiani, V.; Beller, M.; Bocarsly, A.B.; Bonnet, S.; Brueggeller, P.; Caputo, C.A.; et al. Synthetic approaches to artificial photosynthesis: General discussion. Faraday Discuss. 2019, 215, 242–281. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, M.Y.; Llobet, A. Preface for Small Molecule Activation: From Biological Principles to Energy Applications. Part 3: Small Molecules Related to (Artificial) Photosynthesis. Inorg. Chem. 2016, 55, 371–377. [Google Scholar] [CrossRef]
- Guiglion, P.; Berardo, E.; Butchosa, C.; Wobbe, M.C.C.; Zwijnenburg, M.A. Modelling materials for solar fuel synthesis by artificial photosynthesis; predicting the optical, electronic and redox properties of photocatalysts. J. Phys. Condens. Matter 2016, 28, 074001. [Google Scholar] [CrossRef] [PubMed]
- Pann, J.; Roithmeyer, H.; Viertl, W.; Pehn, R.; Bendig, M.; Dutzler, J.; Kriesche, B.; Brüggeller, P. Phosphines in artificial photosynthesis: Considering different aspects such as chromophores, water reduction catalysts (WRCs), water oxidation catalysts (WOCs), and dyads. Sustain. Energy Fuels 2019, 3, 2926–2953. [Google Scholar] [CrossRef]
- Guiglion, P.; Monti, A.; Zwijnenburg, M.A. Validating a density functional theory approach for predicting the redox potentials associated with charge carriers and excitons in polymeric photocatalysts. J. Phys. Chem. C 2017, 121, 1498–1506. [Google Scholar] [CrossRef] [Green Version]
- Shtarev, D.S.; Shtareva, A.V.; Ryabchuk, V.K.; Rudakova, A.V.; Serpone, N. Considerations of Trends in Heterogeneous Photocatalysis. Correlations between conduction and valence band energies with bandgap energies of various photocatalysts. ChemCatChem 2019, 11, 3534–3541. [Google Scholar] [CrossRef]
- Wilbraham, L.; Sprick, R.S.; Jelfs, K.E.; Zwijnenburg, M.A. Mapping binary copolymer property space with neural networks. Chem. Sci. 2019, 10, 4973–4984. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G. Porphyrin Nanotechnology: Discovery, Clinical Translation and Beyond. In Proceedings of the 2016 Asia Communications and Photonics Conference (ACP); IEEE; Piscataway, NJ, USA, 2016; pp. 1–3. [Google Scholar]
- Ng, K.K.; Takada, M.; Harmatys, K.; Chen, J.; Zheng, G. Chlorosome-Inspired Synthesis of Templated Metallochlorin-Lipid Nanoassemblies for Biomedical Applications. ACS Nano 2016, 10, 4092–4101. [Google Scholar] [CrossRef]
- Shao, S.; Rajendiran, V.; Lovell, J.F. Metalloporphyrin nanoparticles: Coordinating diverse theranostic functions. Coord. Chem. Rev. 2019, 379, 99–120. [Google Scholar] [CrossRef]
- Mironov, A.F.; Zhdanova, K.A.; Natal’ya, A.B. Nanosized vehicles for delivery of photosensitizers in photodynamic diagnosis and therapy of cancer. Russ. Chem. Rev. 2018, 87, 859. [Google Scholar] [CrossRef]
- MacLaughlin, J.A.; Anderson, R.R.; Holick, M.F. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science 1982, 216, 1001–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronikis, A.J.; Cevik, M.B.; Allen, R.H.; Shirvani, A.; Sun, A.; Persons, K.S.; Holick, M.F. Evaluation of a Ultraviolet B Light Emitting Diode (LED) for Producing Vitamin D3 in Human Skin. Anticancer Res. 2020, 40, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.; Chen, Y.N.; Cheng, Y.C.; Li, C.M.; Chen, G.Y.; Nori, F. Quantum biology. Nat. Phys. 2013, 9, 10–18. [Google Scholar] [CrossRef]
- Cupellini, L.; Bondanza, M.; Nottoli, M.; Mennucci, B. Successes & challenges in the atomistic modeling of light-harvesting and its photoregulation. Biochim. Biophys. Acta (BBA)-Bioenerg. 2020, 1861, 148049. [Google Scholar]
- Lishchuk, A.; Vasilev, C.; Johnson, M.P.; Hunter, C.N.; Törmä, P.; Leggett, G.J. Turning the challenge of quantum biology on its head: Biological control of quantum optical systems. Faraday Discuss. 2019, 216, 57–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forn-Díaz, P.; Lamata, L.; Rico, E.; Kono, J.; Solano, E. Ultrastrong coupling regimes of light-matter interaction. Rev. Mod. Phys. 2019, 91, 025005. [Google Scholar] [CrossRef] [Green Version]
- Wientjes, E.; Lambrev, P. Ultrafast processes in photosynthetic light-harvesting. Photosynth. Res. 2020, 144, 123–125. [Google Scholar] [CrossRef]
- Duan, H.G.; Nalbach, P.; Miller, R.D.; Thorwart, M. Intramolecular vibrations enhance the quantum efficiency of excitonic energy transfer. Photosynth. Res. 2020, 1–9. [Google Scholar] [CrossRef]





| Type | HOMO (eV) | LUMO (eV) | Gap |
|---|---|---|---|
| BChl 1 | −5.286 | −3.202 | 2.084 |
| BChl 2 | −4.669 | −3.1 | 1.569 |
| BChl 3 | −4.394 | −2.1 | 2.294 |
| BChl 4 | −5.142 | −3.102 | 2.04 |
| BChl 5 | −5.499 | −4.361 | 1.138 |
| BChl 6 | −5.247 | −3.091 | 2.156 |
| BChl 7 | −5.331 | −3.245 | 2.086 |
| Excitation | BChl 6 | BChl 7 | ||||
|---|---|---|---|---|---|---|
| Energy (eV) | Wavelength (nm) | f | Energy (eV) | Wavelength (nm) | f | |
| S0-S1 | 1.9035 | 651.35 | 0.3489 | 0.7744 | 1601.08 | 0.0025 |
| S0-S2 | 2.2434 | 552.67 | 0.1275 | 1.0929 | 1134.41 | 0.0078 |
| S0-S3 | 2.9733 | 416.99 | 0.0007 | 1.1909 | 1041.14 | 0.0172 |
| S0-S4 | 3.0891 | 401.36 | 0.000 | 1.7776 | 697.48 | 0.148 |
| S0-S5 | 3.1408 | 394.75 | 0.0026 | 1.9835 | 625.07 | 0.0146 |
| S0-S6 | 3.3296 | 372.37 | 0.0066 | 2.1139 | 586.51 | 0.357 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badu, S.; Melnik, R.; Singh, S. Analysis of Photosynthetic Systems and Their Applications with Mathematical and Computational Models. Appl. Sci. 2020, 10, 6821. https://doi.org/10.3390/app10196821
Badu S, Melnik R, Singh S. Analysis of Photosynthetic Systems and Their Applications with Mathematical and Computational Models. Applied Sciences. 2020; 10(19):6821. https://doi.org/10.3390/app10196821
Chicago/Turabian StyleBadu, Shyam, Roderick Melnik, and Sundeep Singh. 2020. "Analysis of Photosynthetic Systems and Their Applications with Mathematical and Computational Models" Applied Sciences 10, no. 19: 6821. https://doi.org/10.3390/app10196821
APA StyleBadu, S., Melnik, R., & Singh, S. (2020). Analysis of Photosynthetic Systems and Their Applications with Mathematical and Computational Models. Applied Sciences, 10(19), 6821. https://doi.org/10.3390/app10196821







