Phenolic Compounds in Trees and Shrubs of Central Europe
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Phenolic Compounds
3.1.1. Phenolic Acids
| Trees and Shrubs | Phenolic Acids | Literature |
|---|---|---|
| Scots pine Pinus sylvestris L. | Caffeic acid, salicylic, ferulic, vanillic, gallic, sinapic, p-coumaric, protocatechuic acids | [52,53,54,55,56,57] |
| Norway spruce Picea abies H. Karst in the roots, wood, mature seeds | Shikimic acid, galusic acid, p-coumaric acid, protocatechuic acid, ferulic, vanillic, syringic, sinapic, salicylic, quinic acids, protocatechuic, gallic acids | [52,58,59,60,61,62] |
| Silver fir Abies alba Mill. Wood and bark | Gallic acid, homovanillic acid protocatehuic acid, p-hydroxybenzoic acid, vanillic and p-coumaric acids | [63] |
| European beech Fagus sylvatica L.-leaves | Caffeic acid, ferulic acid, chlorogenic acid syringic, gallic, abscisic and cinnamic acids | [52,58,64] |
| Oak Quercus robus L. | Ellagic acid, gallic acid, gentisic acid, p-hydroxybenzoic acid, protocatechuic acid syringic acid vanillic acid, p-coumaric acid, caffeic acid, ferulic acid sinapic acid | [52,58,65,66,67] |
| Walnut Juglans regia L. | Ellagic acid, caffeic acid, p-coumaric acid, galusic acid | [52,58,68] |
| Willow Salix spp. | Ferulic, caffeic, salicylic, vanillic, syringic, α-resorcylic, m and p-hydroxybenzoic, p-coumaric, cinnamic acids | [58,69] |
| Salix alba L. | Salicylic and p-coumaric acid | [70] |
| Salix babylonica L.-leaves | Caffeic and p-coumaric acids | [71] |
| Salix capitata L.-leaves | Protocatechuic acid | [72,73] |
| Silver birch Betula pendula Roth-leaves | Chlorogenic, p-hydroxybenzoic, caffeic, gallic, coumaric, p-hydroxycinnamic acids | [74] |
| Hawthorn Crataegus L. | Chlorogenic, caffeic acid | [58,75] |
| Rowan Sorbus aucuparia L. | Neochlorogenic, chlorogenic, protocatechuic, caffeic and p-hydroxybenzoic acids | [76] |
| White poplar Populus alba L.-buds | Benzoic, ferulic, caffeic acids, cinnamic, cis-p-coumaric and trans-p-coumaric acids | [58,77] |
| Bird cherry Prunus padus L. fruits | Caffeic acid, ferulic, coumaric, chlorogenic, elagic, gallic acids | [58,78] |
| Prunus serotina Ehrh. | Gallic acid, caffeic and p-hydroxybenzoic acids, p-coumaric, ferulic, cinnamic acids | [79] |
3.1.2. Flavonoids
3.1.3. Tannins
3.1.4. Stilbenes
Author Contributions
Funding
Conflicts of Interest
References
- Kaplan, I.; Halitschke, R.; Kessler, A.; Sardanelli, S.; Denno, R.F. Constitutive and induced defenses to herbivory in above- and belowground plant tissues. Ecology 2008, 89, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Carmona, D.; Lajeunesse, M.J.; Johnson, M.T. Plant traits that predict resistance to herbivores. Funct. Ecol. 2010, 25, 358–367. [Google Scholar] [CrossRef]
- Rayner, A.D.M.; Boddy, L. Fungal Decomposition of Wood—Its Biology and Ecology; John Wiley and Sons: Chichester, UK; New York, NY, USA; Brisbane, Australia; Toronto, ON, Canada; Singapore, 1988; pp. 1–428. [Google Scholar]
- Kozłowska, M. Phenolic composition of red raspberry canes in relation to Didymella applanata (Niessl) Sacc. response. Acta Physiol. Plant 1994, 16, 211–215. [Google Scholar]
- Evensen, P.C.; Solheim, H.; Høiland, K.; Stenersen, J. Induced resistance of Norway spruce, variation of phenolic compounds and their effects on fungal pathogens. For. Pathol. 2000, 30, 97–108. [Google Scholar] [CrossRef]
- Przybył, K.; Karolewski, P.; Oleksyn, J.; Łabędzki, A.; Reich, P. Fungal Diversity of Norway Spruce Litter: Effects of Site Conditions and Premature Leaf Fall Caused by Bark Beetle Outbreak. Microb. Ecol. 2007, 56, 332–340. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, H.; Min, H.; Park, E.; Lee, K.; Ahn, Y.H.; Cho, Y.; Pyee, J. Antibacterial and antifungal activity of pinosylvin, a constituent of pine. Fitoterapia 2005, 76, 258–260. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Shiliang, L.; Rongjie, Y. Regulations of reactive oxygen species in plants abiotic stress: An integrated overview. Plant Life Under Chang. Environ. 2020, 323–353. [Google Scholar]
- Vertuani, S.; Angusti, A.; Manfredini, S. The antioxidants and pro-antioxidants network: An overview. Curr. Pharm. Des. 2004, 10, 1677–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darvill, A.G.; Albersheim, P. Phytoalexins and their elicitors—A defense against microbial infection in plants. Annu. Rev. Plant Physiol. 1984, 35, 243–275. [Google Scholar] [CrossRef]
- Van Loon, L.C. Pathogenesis-related proteins. Plant Mol. Biol. 1985, 4, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.P.; Klessig, D.F. The Pathogenesis-Related Proteins of Plants. In Genetic Engineering: Principles and Methods; Setlow, J.K., Ed.; Plenum Press: New York, NY, USA, 1989; Volume 11, pp. 65–109. [Google Scholar]
- Dixon, R.A.; Harrison, M.J.; Lamb, C.J. Early events in the activation of plant defense responses. Ann. Rev. Phytopathol. 1994, 32, 479–501. [Google Scholar] [CrossRef]
- Agrawal, A.A. Macroevolution of plant defense strategies. Trends Ecol. Evol. 2007, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.E.; Koricheva, J. The Ontogeny of Plant Defense and Herbivory: Characterizing General Patterns Using Meta-Analysis. Am. Nat. 2010, 175, 481–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forkner, R.E.; Marquis, R.J.; Lill, J.T. Feeny revisited: Condensed tannins asanti-herbivore defences in leaf−chewing herbivore communities of Quercus. Ecol. Entomol. 2004, 29, 174–187. [Google Scholar] [CrossRef]
- Manninen, A.-M.; Vuorinen, M.; Holopainen, J.K. Variation in Growth, Chemical Defense, and Herbivore Resistance in Scots Pine Provenances. J. Chem. Ecol. 1998, 24, 1315–1331. [Google Scholar] [CrossRef]
- Harborne, J.B. The comparative biochemistry of phytoalexin induction in plants. Biochem. Syst. Ecol. 1999, 27, 335–367. [Google Scholar] [CrossRef]
- Leszczyński, B. Rola allelozwiązków w oddziaływaniach owady-rośliny. In Biochemiczne Oddziaływania Środowiskowe [Biochemical Environmental Interactions]; Oleszek, W., Głowniak, K., Leszczyński, B., Eds.; Akademia Medyczna: Lublin, Poland, 2001; pp. 61–85. [Google Scholar]
- Malinowski, H. Strategie obronne roślin drzewiastych przed szkodliwymi owadami. Defensive strategies of woody plants against harmful insects. Lesne Pr. Badaw. For. Res. Pap. 2008, 69, 165–173. [Google Scholar]
- Dziedziński, M.; Kobus-Cisowska, J.; Szymanowska, D.; Stuper-Szablewska, K.; Baranowska, M.; Baranowska, M. Identification of Polyphenols from Coniferous Shoots as Natural Antioxidants and Antimicrobial Compounds. Molecules 2020, 25, 3527. [Google Scholar] [CrossRef] [PubMed]
- Hermann, K. Review on nonessential constituents of vegetables. III. Carrots, celery, pars-nips, beets, spinach, lettuce, endives, chicory, rhubarb, and artichokes. Lebensm. Unters. Forsch. 1978, 167, 262–273. [Google Scholar]
- Kolesnikov, M.P.; Gins, V.K. Characteristics of the accumulation of phenolic compounds in amaranth leaves under the effect of growth stimulators Prikl. Biokhim. Mikrobiol. 2001, 37, 616–620. [Google Scholar]
- Rothwell, J.A.; Pérez-Jiménez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Herrmann, K.M. The Shikimate Pathway: Early Steps in the Biosynthesis of Aromatic Compounds. Plant Cell 1995, 7, 907–919. [Google Scholar] [CrossRef]
- Schmid, J.; Amrhein, N. Molecular organization of the shikimate pathway in higher plants. Phytochemistry 1995, 39, 737–749. [Google Scholar] [CrossRef]
- Swanson, P.G. Tannins and Polyphenols. Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2003; pp. 5729–5733. [Google Scholar]
- Robbins, R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Jeszka, M.; Flaczek, E.; Kobus-Cisowska, J.; Dziedzic, K. Związki fenolowe—Charakterystyka i znaczenie w technologii żywności. Nauka Przyr. Technol. 2010, 4, 1–13. [Google Scholar]
- Castellucio, C.; Paganga, G.; Melikan, N.; Bowell, G.P.; Pridham, J.; Sampson, J.; Rice-Evans, C. Antioxidant potential of intermediates in phenylopropanoid metabolism in higher plants. FEBS Lett. 1995, 368, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Quideau, S.; Feldman, K.S. Ellagotannin chemistry: The first synthesis of dehydrohexahydroxydiphenoate (DHHDP) esters from oxidative coupling of unetherified methyl gallate. J. Org. Chem. 1997, 62, 8809–8813. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.H.; Törrönen, A. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000, 33, 517–524. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.-S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Gülcü, M.; Uslu, N.; Özcan, M.M.; Gökmen, F.; Özcan, M.M.; Banjanin, T.; Lemiasheuski, V. The investigation of bioactive compounds of wine, grape juice and boiled grape juice wastes. J. Food Process. Preserv. 2019, 43, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Aguilera-Carbó, A.; Augur, C.; Prado-Barragán, L.A.; Aguilar, C.N.; Favela-Torres, E. Extraction and analysis of ellagic acid from novel complex sources. Chem. Pap. 2008, 62, 440–444. [Google Scholar] [CrossRef]
- Narayanan, B.A.; Geoffroy, O.; Willingham, M.C.; Re, G.G.; Nixon, D. p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999, 136, 215–221. [Google Scholar] [CrossRef]
- Han, D.H.; Lee, M.J.; Kim, J.H. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006, 26, 3601–3606. [Google Scholar]
- Festa, F.; Aglitti, T.; Duranti, G.; Ricordy, R.; Perticone, P.; Cozzi, R. Strong antioxidant activity of ellagic acid in mammalian cells in vitro revealed by the comet assay. Anticancer Res. 2001, 21, 3903. [Google Scholar]
- Corbett, S.; Daniel, J.; Drayton, R.; Field, M.; Steinhardt, R.; Garrett, N. Evaluation of the Anti-inflammatory Effects of Ellagic Acid. J. PeriAnesthesia Nurs. 2010, 25, 214–220. [Google Scholar] [CrossRef]
- Lamer-Zarawska, E.; Oszmiański, J. Rola niektórych substancji roślinnych w profilaktyce przeciwnowotworowej. Wiad. Ziel. 1998, 5, 1–4. [Google Scholar]
- Kwiatkowska, E. Kwas elagowy—Zawartość w żywności i rola prozdrowotna. Post. Fitoter. 2010, 4, 211–214. [Google Scholar]
- Sepúlveda, L.; Ascacio, A.; Rodríguez-Herrera, R.; Aguilera-Carbó, A.; Aguilar, C.N. ChemInform Abstract: Ellagic Acid: Biological Properties and Biotechnological Development for Production Processes. Afr. J. Biotechnol. 2012, 43, 4518–4523. [Google Scholar] [CrossRef]
- Lutomski, J.; Alkiewicz, J. Leki Roślinne w Profilaktyce i Terapii; PZWL: Warszawa, Poland, 1993. [Google Scholar]
- Kohlmunzer, S. Farmakognozja; PZWL: Warszawa, Poland, 1993. [Google Scholar]
- Schmid, B.; Kötter, I.; Heide, L. Pharmacokinetics of salicin after oral administration of a standardised willow bark extract. Eur. J. Clin. Pharmacol. 2001, 57, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Schmid, B.; Lüdtke, R.; Selbmann, H.K.; Kötter, I.; Tschirdewahn, B.; Schaffner, W.; Heide, L. Efficacy and tolerability of a standardized willow bark extract in patients with osteoarthritis: Randomized placebo-controlled, double blind clinical trial. Phytother. Res. 2001, 15, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Międzybrodzki, R. Kierunki poszukiwań i zastosowanie niesteroidowych leków przeciwzapalnych. Postęp. Hig. Med. Dośw. 2004, 58, 438–448. [Google Scholar]
- Zejc, A.; Gorczyca, M. Chemia Leków; PZWL: Warszawa, Poland, 2004. [Google Scholar]
- Ożarowski, A.; Jaroniewski, W. Rośliny Lecznicze i ich Praktyczne Zastosowanie; IWZW: Warszawa, Poland, 1987. [Google Scholar]
- Flos Pini Masculinum—Kwiat Męski Sosny w Fitoterapii. Available online: http://rozanski.li/1750/flos-pini-masculinum-kwiat-meski-sosnyw-fitoterapii/ (accessed on 11 November 2019).
- Pan, H.; Lundgren, L.N. Phenolic extractives from root bark of Picea abies. Phytochemistry 1995, 39, 1423–1428. [Google Scholar] [CrossRef]
- Antonova, G.F.; Chaplygina, I.A.; Varaksina, T.N.; Stasova, V.V. Ascorbic acid and xylem development in trunks of the Siberian larch trees. Russ. J. Plant Physiol. 2005, 52, 83–92. [Google Scholar] [CrossRef]
- Antonova, G.F.; Varaksina, T.N.; Zheleznichenko, T.V.; Stasova, V.V. Changes in phenolic acids during maturation and lignification of Scots pine xylem. Онтoгенез 2012, 43, 199–208. [Google Scholar] [CrossRef]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef] [Green Version]
- Strona Archiwalna. Available online: http://www.rozanski.ch/fitoterapia1.htm (accessed on 11 November 2019).
- Strack, D.; Kottke, I.; Oberwinkler, F. Phenolics of mycorrhizas and non-mycorrhizal roots of Norway spruce. Planta 1990, 182, 142–148. [Google Scholar] [CrossRef]
- Cvikrová, M.; Malá, J.; Hrubcová, M.; Eder, J.; Foretová, S. Induced changes in phenolic acids and stilbenes in embryogenic cell cultures of Norway spruce by culture filtrate of Ascocalyx abietina. J. Plant Dis. Prot. 2008, 115, 57–62. [Google Scholar] [CrossRef]
- Malá, J.; Hrubcová, M.; Máchová, P.; Cvrčková, H.; Martincová, O.; Cvikrová, M. Changes in phenolic acids and stilbenes induced in embryogenic cell cultures of Norway spruce by two fractions of Sirococcus strobilinus mycelian. J. For. Sci. 2011, 57, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Koutaniemi, S.; Warinowski, T.; Kärkönen, A.; Alatalo, E.; Fossdal, C.G.; Saranpää, P.; Laakso, T.; Fagerstedt, K.V.; Simola, L.K.; Paulin, L.; et al. Expression profiling of the lignin biosynthetic pathway in Norway spruce using EST sequencing and real-time RT-PCR. Plant Mol. Biol. 2007, 65, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T. Antioxidant Efficiency of Beech (Fagus sylvatica L.) Bark Polyphenols Assessed by Chemometric Methods. Ind. Crops Prod. 2017, 108, 26–35. [Google Scholar] [CrossRef]
- Pirvu, L.; Grigore, A.; Bubueanu, C.; Draghici, E.M. Comparative analytical and antioxidant activity studies on a series of Fagus sylvatica L. leaves extracts. J. Planar Chromatogr. Mod. TLC 2013, 26, 237–242. [Google Scholar] [CrossRef]
- De Simón, B.F.; Cadahía, E.; Conde, E.; García-Vallejo, M.C.; Simón, B.F. Low Molecular Weight Phenolic Compounds in Spanish Oak Woods. J. Agric. Food Chem. 1996, 44, 1507–1511. [Google Scholar] [CrossRef]
- Cadahía, E.; Muñoz, L.; De Simón, B.F.; García-Vallejo, M.C.; Simón, B.F. Changes in Low Molecular Weight Phenolic Compounds in Spanish, French, and American Oak Woods during Natural Seasoning and Toasting. J. Agric. Food Chem. 2001, 49, 1790–1798. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Sanz, M.; Cadahía, E.; Poveda, P.; Broto, M. Chemical characterization of oak heartwood from Spanish forests of Quercus pyrenaica (wild.). Ellagitannins, low molecular weight phenolic, and volatile compounds. J. Agric. Food Chem. 2006, 54, 8314–8321. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.; Ferreres, F.; Bento, A.A.; Seabra, R.; Estevinho, L.M. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L. Zastosowanie Metod Chromatograficznych w Badaniach Składu Chemicznego Kory Niektórych Gatunków Klonów Wierzby. Ph.D. Thesis, Farmecautical Department, Medicial Academy, Gdańsk, Poland, 2006. [Google Scholar]
- Bisset, N.; Wichtl, M. Herbal Drugs and Phytopharmaceuticals; CRC: London, UK, 2001. [Google Scholar]
- Duke, J.A. Handbook of Phytochemical Constituents of GRAS Herbs and Other Economic Plants; CRC: London, UK, 1992. [Google Scholar]
- Pohjamo, S.P.; Hemming, J.E.; Willför, S.; Reunanen, M.H.; Holmbom, B.R. Phenolic extractives in Salix caprea wood and knots. Phytochemistry 2003, 63, 165–169. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Moreirinha, C.; Silva, S.; Costa, E.M.; Veiga, M.; Coscueta, E.R.; Santos, S.A.O.; Almeida, A.; Pintado, M.M.E.; Freire, C.S.; et al. The Health-Promoting Potential of Salix spp. Bark Polar Extracts: Key Insights on Phenolic Composition and In Vitro Bioactivity and Biocompatibility. Antioxidants 2019, 8, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najda, A.; Bekier, J.; Guleac, E.; Filiks, A. Profil Kwasów Fenolowych Liści Brzozy Brodawkowatej (Betula pendula roth) [Phenolic Acid Profile of Silver Birch (Betula pendula Roth) Leaves]. Episteme 2014, 25, 235–243. [Google Scholar]
- Bobinaitė, R.; Grootaert, C.; Van Camp, J.; Šarkinas, A.; Liaudanskas, M.; Žvikas, V.; Viškelis, P.; Rimantas Venskutonis, P. Chemical composition, antioxidant, antimicrobial and antiproliferative activities of the extracts isolated from the pomace of rowanberry (Sorbus aucuparia L.). Food Res. Int. 2020, 136, 109310. [Google Scholar] [CrossRef] [PubMed]
- Kuchukhidze, J.; Jokhadze, M.; Murtazashvili, T.; Mshvildadze, V. Antioxidant polyphenols from Populus alba growing in Georgia. Georgian Med. News 2011, 199, 94–97. [Google Scholar]
- Dimkić, I.; Ristivojević, P.; Janakiev, T.; Berić, T.; Trifković, J.; Milojković-Opsenica, D.; Stanković, S. Phenolic profiles and antimicrobial activity of various plant resins as potential botanical sources of Serbian propolis. Ind. Crop. Prod. 2016, 94, 856–871. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Ligaj, M.; Stuper-Szablewska, K.; Szymanowska, D.; Tichoniuk, M.; Szulc, P. Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties. Open Chem. 2020, 18, 1125–1135. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; De Biaggi, M.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. New Findings in Prunus padus L. Fruits as a Source of Natural Compounds: Characterization of Metabolite Profiles and Preliminary Evaluation of Antioxidant Activity. Molecules 2018, 23, 725. [Google Scholar] [CrossRef] [Green Version]
- Slavin, J.; Marquart, L.; Jakobs, D. Consumption of whole-grain food and decreased risk of cancer: Proposed mechanisms. Cereal Foods World 2000, 45, 54–58. [Google Scholar]
- Colette, N.; Bernier, J.L.; Catteau, J.P.; Pommery, J.; Wallet, J.C.; Gaydou, E.M. Antioxidant properties of hydroxyflavones. Free Radic. Biol. Med. 1996, 20, 35–43. [Google Scholar]
- Ligor, M. Polifenole. In Badanie Substancji Biologicznie Aktywnych w Surowcach Roślinnych i Produktach Naturalnych z Zastosowaniem Łączonych technik Chromatograficznych; Ligor, M., Ed.; UMK: Toruń, Poland, 2013; pp. 55–63. [Google Scholar]
- Lamer-Zarawska, E. Flawonoidy, Fitoterapia i Leki Roślinne; PZWL: Warszawa, Poland, 2007; pp. 64–67. [Google Scholar]
- Benhamou, N.; Nicole, M. Cell biology of plant immunization against microbial infection: The potential of induced resistance in controlling plant diseases. Plant Physiol. Biochem. 1999, 37, 703–719. [Google Scholar] [CrossRef]
- Skipp, R.A.; Bailey, J.A. The fungitoxicity of isoflavonoid phytoalexins measured using different types of bioassay. Physiol. Plant Pathol. 1977, 11, 101–112. [Google Scholar]
- Phillips, D.; Kapulnik, Y. Plant isoflavonoids, pathogens and symbionts. Trends Microbiol. 1995, 3, 58–64. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol. Mol. Plant Pathol. 1996, 49, 1–20. [Google Scholar] [CrossRef]
- Laracine-Pittet, C.; Lebreton, P. Flavonoid variability within Pinus sylvestris. Phytochemistry 1988, 27, 2663–2666. [Google Scholar] [CrossRef]
- Oleszek, W.; Stochmal, A.; Karolewski, P.; Simonet, A.M.; Macias, F.A.; Tava, A. Flavonoids from Pinus sylvestris needles and their variation in trees of different origin grown for nearly a century at the same area. Biochem. Syst. Ecol. 2002, 30, 1011–1022. [Google Scholar] [CrossRef]
- Viriot, C.; Scalbert, A.; Lapierre, C.; Moutounet, M. Ellagitannins and lignins in aging of spirits in oak barrels. J. Agric. Food Chem. 1993, 41, 1872–1879. [Google Scholar] [CrossRef]
- Vovk, I.; Simonovska, B.; Andrenšek, S.; Vuorela, H. Rotation planar extraction and rotation planar of oak Quercus robus L bark. J. Chromatogr. A 2003, 991, 267–274. [Google Scholar] [CrossRef]
- Benković, E.T.; Grohar, T.; Žigon, D.; Švajger, U.; Janeš, D.; Kreft, S.; Strukelj, B. Chemical composition of the silver fir (Abies alba) bark extract Abigenol® and its antioxidant activity. Ind. Crop. Prod. 2014, 52, 23–28. [Google Scholar] [CrossRef]
- Petrakis, P.V.; Spanos, K.; Feest, A.; Daskalakou, E.N. Phenols in Leaves and Bark of Fagus sylvatica as Determinants of Insect Occurrences. Int. J. Mol. Sci. 2011, 12, 2769–2782. [Google Scholar] [CrossRef]
- Dübeler, A.; Voltmer, G.; Gora, V.; Lunderstädt, J.; Zeeck, A. Phenols from Fagus sylvatica and their role in defence against Cryptococcus fagisuga. Phytochemistry 1997, 45, 51–57. [Google Scholar] [CrossRef]
- Zeneli, G.; Krokene, P.; Christiansen, E.; Krekling, T.; Gershenzon, J. Methyl jasmonate treatment of large Norway spruce (Picea abies) trees increases the accumulation of terpenoid resin components and protects against infection by Ceratocystis polonica, a bark beetle-associated fungus. Tree Physiol. 2006, 26, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, A.U.; Novak, M.; Felicijan, M.; Kraševec, N.; Lešnik, M.; Zupanec, N.; Komel, R. Antioxidative response patterns of Norway spruce bark to low-density Ceratocystis polonica inoculation. Trees 2014, 28, 1145–1160. [Google Scholar] [CrossRef]
- Broda, B.; Mowszowicz, J. Przewodnik do Oznaczania Roślin Leczniczych, Trujących i Użytkowych. [Guide for the Determination of Medicinal, Poisonous and Usable Plants]; PZWL: Warszawa, Poland, 2000. [Google Scholar]
- Sulima, P.; Krauze-Baranowska, M.; Przyborowski, J.A. Variations in the chemical composition and content of salicylic glycosides in the bark of Salix purpurea from natural locations and their significance for breeding. Fitoterapia 2017, 118, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Salix in Practical Phytotherapy. Available online: http://rozanski.li/438/wierzba-salix-w-praktycznej-fitoterapii/ (accessed on 13 June 2019).
- Krauze-Baranowska, M.; Pobłocka-Olech, L.; Głód, D.; Wiwart, M.; Zieliński, J.; Migas, P. HPLC of flavanones and chalcones in different species and clones of Salix. Acta Pol. Pharm. 2013, 70, 27–34. [Google Scholar] [PubMed]
- Harborne, J.B.; Baxter, H. The Handbook of Natural Flavonoids; Willey: Chichester, UK, 1999. [Google Scholar]
- Thapliyal, R.P.; Bahugana, R.P. Fatty acids and flavonoids of Salix Lindlevana. Int. J. Pharmacog. 1993, 31, 165–166. [Google Scholar] [CrossRef]
- Gorobets, A.B.; Bandyukova, V.A.; Shapirom, D.K. Flavonoid composition of pollen of Salix caprea and S. alba. Khim. Prir. Soedin. 1982, 6, 781–782. [Google Scholar]
- Fong, H.H.S.; Bauman, J.L. Hawthorn. J. Cardiovasc. Nurs. 2002, 16, 1–8. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Christensen, L.P.; Kaack, K.; Fretté, X. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. Eur. Food Res. Technol. 2007, 227, 293–305. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Presler, A.; Michel, P. Profiling of Phenolic Compounds and Antioxidant Activity of Dry Extracts from the Selected Sorbus Species. Molecules 2012, 17, 3093–3113. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M. Flavonoids from the Genus Taxus. Z. Nat. C 2004, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž.P. proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Čukanović, J.; Tešević, V.V.; Jadranin, M.B.; Ljubojević, M.; MladenoviĆ, E.; Kostic, S. Horse chestnut (Aesculus hippocastanum L.) seed fatty acids, flavonoids and heavy metals plasticity to different urban environments. Biochem. Syst. Ecol. 2020, 89, 103980. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czeczot, H. Biological activities of flavonoids—A review. Pol. J. Food Nutr. Sci. 2000, 50, 3–13. [Google Scholar]
- Martínez-Flórez, S.; González-Gallego, J.; Culebras, J.M.; Tuñón, M.J. Flavonoids: Properties and anti-oxidizing action. Nutr. Hosp. 2003, 17, 271–278. [Google Scholar]
- Olszewska, M. Flawonoidy i ich zastosowanie w lecznictwie. Farm. Pol. 2003, 59, 391–401. [Google Scholar]
- Yao, L.H.; Jiang, Y.; Shi, J.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in Food and Their Health Benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Zaprometov, M.N. Tannins, Lignans, and Lignins. In Phytochemicals in Plant Cell Cultures; Academic Press: Cambridge, MA, USA, 1988; pp. 89–97. [Google Scholar]
- Nonaka, G. Isolation and structure elucidation of tannins. Pure Appl. Chem. 1989, 61, 357–360. [Google Scholar] [CrossRef]
- Porter, L.J. Flavans and Proanthocyanidins; Harbone, J.B., Ed.; The Flavonoids 23–53; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Bahorun, T.; Aumjaud, E.; Ramphul, H.; Rycha, M.; Luximon-Ramma, A.; Trotin, F.; Aruoma, O.I. Phenolic constituents and antioxidant capacities of Crataegus monogyna (Hawthorn) callus extracts. Nahrung 2003, 47, 191–198. [Google Scholar] [CrossRef]
- Rohdewald, P. Pycnogenol®, a Plant Extract for Women’s Health. Int. J. Women’s Health Care 2017, 2, 10–33140. [Google Scholar]
- Zhang, Z.; Chang, Q.; Zhu, M.; Huang, Y.; Ho, W.K.; Chen, Z. Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem. 2001, 12, 144–152. [Google Scholar] [CrossRef]
- Cisár, P.; Jány, R.; Waczulíková, I.; Sumegová, K.; Muchová, J.; Vojtassák, J.; Duraćková, Z.; Lisý, M.; Rohdewald, P. Effect of pine bark extract (Pycnogenol) on symptoms of knee osteoarthritis. Phytother. Res. 2008, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Grimm, T.; Schäfer, A.; Högger, P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (pycnogenol). Free Radic. Biol. Med. 2004, 36, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Oliff, H. Scientific and Clinical Monograph for Pycnogenol, 2019 Update. The American Botanical Council. Available online: http://abc.herbalgram.org/site/PageServer?pagename=Pycnogenol (accessed on 14 January 2020).
- Gupta, R.K.; Hasla, E. Plant proanthocyanidins. Part 7. Prodelphinidins from Pinus sylvestris. Chem. Soc. Perkin Trans. 1981, 1, 1148–1150. [Google Scholar] [CrossRef]
- Karapandzova, M.; Stefkov, G.; Cvetkovikj, I.; Stanoeva, J.P.; Stefova, M.; Kulevanova, S. Flavonoids and Other Phenolic Compounds in Needles of Pinus peuce and Other Pine Species from the Macedonian Flora. Nat. Prod. Commun. 2015, 10, 987–990. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.-Y.; Jang, M.-K.; Lee, D.-G.; Yu, K.H.; Jang, H.; Kim, M.; Kim, S.G.; Yoo, B.H.; Lee, S.-H. Comparison of methods for proanthocyanidin extraction from pine (Pinus densiflora) needles and biological activities of the extracts. Nutr. Res. Pract. 2010, 4, 16–22. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P.; Jang, J.P. Effects of Water Extraction Temperatures on the Yield, Molecular Weight, and Antioxidant Activity of Proanthocyanidins Extracted from Pinus radiata Bark. For. Prod. J. 2011, 61, 321–325. [Google Scholar] [CrossRef]
- Vivas, N.; Nonier, M.-F.; Pianet, I.; De Gaulejac, N.V.; Fouquet, E. Proanthocyanidins from Quercus petraea and Q. robur heartwood: Quantification and structures. Comptes Rendus Chim. 2006, 9, 120–126. [Google Scholar] [CrossRef]
- Pallenbach, E.; Scholz, E.; König, M.; Rimpler, H. Proanthocyanidins from Quercus petraea Bark. Planta Med. 1993, 59, 264–268. [Google Scholar] [CrossRef]
- Oak in Phytotherapy. Available online: http://rozanski.li/1193/quercus-dab-w-fitoterapii/ (accessed on 13 June 2019).
- Yang, B.; Liu, P. Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. J. Sci. Food Agric. 2012, 92, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Arya, V.; Bhat, Z.A.; Khan, N.A.; Prasad, D.N. The genus Crataegus: Chemical and pharmacological perspectives. Rev. Bras. Farm. 2012, 22, 1187–1200. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Oszmiański, J. Anthocyanins in fruits of Prunus padus (bird cherry). J. Sci. Food Agric. 2002, 82, 1483–1486. [Google Scholar] [CrossRef]
- Bridle, P.; Stott, K.; Timberlake, C. Anthocyanins in Salix species: A new anthocyanin in Salix purpurea bark. Phytochemistry 1973, 12, 1103–1106. [Google Scholar] [CrossRef]
- Wilska-Jeszka, J. Polifenole, Glukozynolany i inne Związki Prozdrowotne i Antyżywieniowe. Chemia Żywności. T. I. Składniki Żywności; Sikorski, Z.E., Ed.; WN-T: Warszawa, Poland, 2007; pp. 206–226. [Google Scholar]
- Lila, M.A. Anthocyanins and Human Health: An In Vitro Investigative Approach. J. Biomed. Biotechnol. 2004, 2004, 306–313. [Google Scholar] [CrossRef]
- Cos, P.; Bruyne, T.D.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A. Proanthocyanidins in health care: Current and new trends Curr. Med. Chem. 2000, 11, 1345–1359. [Google Scholar] [CrossRef]
- Karonen, M.; Loponen, J.; Ossipov, V.; Pihlaja, K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography–electrospray ionization mass spectrometry. Anal. Chim. Acta 2004, 522, 105–112. [Google Scholar] [CrossRef]
- Muszyński, S.; Guzewski, W. Antocyjany Roślin Wyższych; PTB: Kraków, Poland, 1976; Volume 20, p. 4. [Google Scholar]
- Morales, M.; Ros Barcelo, A.; Pedreno, M.A. Plant stilbenes: Recent advances in their chemistry and biology. Adv. Plant Physiol. 2000, 3, 39–70. [Google Scholar]
- Bavaresco, L.; Fregoni, C.; Cantù, E.; Trevisan, M. Stilbene compounds: From the grapevine to wine. Drugs Under Exp. Clin. Res. 1999, 25, 57–63. [Google Scholar]
- Roupe, K.; Remsberg, C.M.; Yáñez, J.A.; Davies, N. Pharmacometrics of Stilbenes: Seguing Towards the Clinic. Curr. Clin. Pharmacol. 2006, 1, 81–101. [Google Scholar] [CrossRef]
- Gu, X.; Chu, Q.; O’Dwyer, M.; Zeece, M. Analysis of resveratrol in wine by capillary electrophoresis. J. Chromatogr. A 2000, 881, 471–481. [Google Scholar] [CrossRef]
- Shakibaei, M.; Harikumar, K.B.; Aggarwal, B.B. Resveratrol addiction: To die or not to die. Mol. Nutr. Food Res. 2009, 53, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Arichi, H.; Kimura, Y.; Okuda, H.; Baba, K.; Kozawa, M.; Arichi, S. Effects of stilbene components of the roots of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem. Pharm. Bull. 1982, 30, 1766–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, B.M.; Trost, M.; Heller, W.; Langebartels, C.; Sandermann, H. Elicitor-induced formation of free and cell-wall-bound stilbenes in cell-suspension cultures of Scots pine (Pinus sylvestris L.). Planta 1994, 194, 143–148. [Google Scholar] [CrossRef]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.-M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317. [Google Scholar] [CrossRef] [PubMed]
- Fliegmann, J.; Schanz, S.; Britsch, L. Molecular analysis of chalcone and dihydropinosylvin synthase from Scots pine (Pinus sylvestris), and differential regulation of these and related enzyme activities in stressed plants. Plant Mol. Biol. 1992, 18, 489–503. [Google Scholar] [CrossRef]
- Erdtman, H.; Misiorny, A. Constituents of pine heartwood. XXXI. The content of pinosylvin phenols in Swedish pines. Sven. Pap. 1952, 55, 605–608. [Google Scholar]
- Kiselev, K.V.; Grigorchuk, V.P.; Ogneva, Z.V.; Suprun, A.R.; Dubrovina, A.S. Stilbene biosynthesis in the needles of spruce. Picea Jezoensis Phytochem. 2016, 131, 57–67. [Google Scholar] [CrossRef]
- Sheng-Hong, L.; Xue-Mei, N.; Zahn, S.; Gershenzon, J.; Weston, J.; Schneider, B. Diastereomeric stilbene glucoside dimers from the bark of Norway spruce (Picea abies L.). Phytochemistry 2008, 69, 772–782. [Google Scholar]
- Ganthaler, A.; Stöggl, W.; Mayr, S.; Kranner, I.; Schüler, S.; Wischnitzki, E.; Sehr, E.M.; Fluch, S.; Trujillo-Moya, C. Association genetics of phenolic needle compounds in Norway spruce with variable susceptibility to needle bladder rust. Plant Mol. Biol. 2017, 94, 229–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergent, T.; Kohnen, S.; Jourez, B.; Beauve, C.; Schneider, Y.-J.; Vincke, C. Characterization of black locust (Robinia pseudoacacia L.) heartwood extractives: Identification of resveratrol and piceatannol. Wood Sci. Technol. 2014, 48, 1005–1017. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.-J.; Bucheli, P.; Zhang, P.-F.; Wei, N.-Z.; Lu, Y.-H. Phytochemical Profiles of Different Mulberry (Morussp.) Species from China. J. Agric. Food Chem. 2009, 57, 9133–9140. [Google Scholar] [CrossRef] [PubMed]
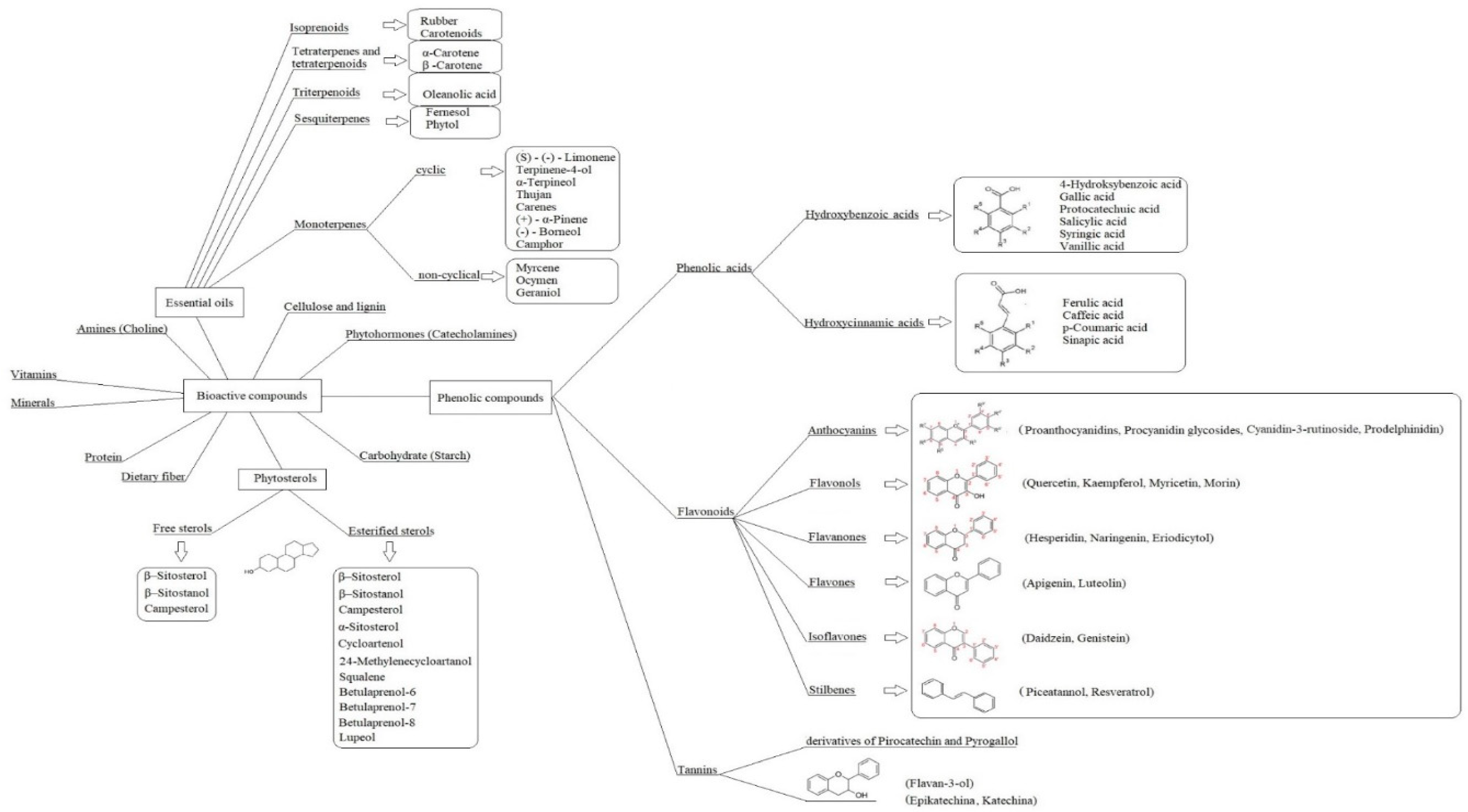

| (a) hydroxybenzoic acids | ||||
| Acids | R1 | R2 | R3 | R4 |
| 3-hydroxybenzoic acid | H | OH | H | H |
| 4-hydroxybenzoic acid | H | H | OH | H |
| Salicylic acid | OH | H | H | H |
| Pyrocatechuic acid | OH | OH | H | H |
| Genistic acid | OH | H | H | OH |
| Procatechuic acid | H | OH | OH | H |
| Vanillic acid | H | OCH3 | OH | H |
| Isovanillic acid | H | OH | OCH3 | H |
| Gallic acid | H | OH | OH | OH |
| Syringic acid | H | OCH3 | OH | OCH3 |
| α-Resorcylic acid | H | OH | H | OH |
| β-Resorcylic acid | OH | H | OH | H |
 | ||||
| (b) hydroxycinnamic acids | ||||
| Acids | R1 | R2 | R3 | R4 |
| Cinnamic acid | H | H | H | H |
| 2-cumaric acid | OH | H | H | H |
| 3-cumaric acid | H | OH | H | H |
| 4-cumaric acid | H | H | OH | H |
| Caffeic acid | H | OH | OH | H |
| 4-metoxycinnamic acid | H | H | OCH3 | H |
| 3,4-dimetoxycinnamic | H | OCH3 | OCH3 | H |
| Ferulic acid | H | H | OH | OCH3 |
| Isoferulic acid | H | H | OCH3 | OH |
| Synapic acid | H | OCH3 | OH | OCH3 |
 | ||||
| Phenolic Acids | Fir | Larch | Pine | Spruce | ||||
|---|---|---|---|---|---|---|---|---|
| Range | Range | Range | Range | |||||
| Gallic | 4.5 | 5.4 | 5.5 | 7.3 | 12.5 | 14.1 | 36.6 | 41.3 |
| 4-hydroxybenzoic | 31.5 | 38.8 | 42.3 | 48.6 | 40.2 | 55.8 | 199.5 | 227.0 |
| Vanilic | 183.6 | 194.4 | 11.2 | 20.4 | 4.6 | 7.1 | 5.7 | 7.6 |
| Caffeic | 27.4 | 30.8 | 161.0 | 177.5 | 87.6 | 107.0 | 204.6 | 287.9 |
| Syringic | 3.6 | 4.3 | 15.9 | 16.3 | 5.3 | 8.9 | 18.3 | 20.1 |
| Vanilin | 10.2 | 11.2 | 16.2 | 24.4 | 0.1 | 0.4 | 0.2 | 1.5 |
| p-Cumaric | 10.0 | 15.3 | 225.2 | 249.0 | 14.6 | 19.9 | 6.3 | 9.0 |
| Benzoic | 216.4 | 239.3 | 48.5 | 60.5 | 8.5 | 12.5 | 0.7 | 6.9 |
| Ferulic | 0.5 | 1.1 | 6.4 | 7.4 | 88.6 | 109.9 | 52.4 | 70.2 |
| Sinapic | 5.3 | 6.9 | 3.6 | 5.2 | 3.1 | 4.3 | 50.5 | 68.4 |
| t-Cinnamic | 27.4 | 29.8 | 55.0 | 63.7 | 5.2 | 8.7 | 36.5 | 45.1 |
| Chlorogenic | 3.4 | 10.9 | 28.7 | 32.4 | 24.2 | 29.1 | 201.7 | 246.2 |
| Protocatechuic | 0.3 | 1.2 | 13.5 | 20.1 | 0.3 | 0.7 | 11.8 | 20.5 |
| Rozmaric | 0.3 | 1.1 | 147.9 | 163.7 | 1.6 | 3.6 | 0.3 | 0.8 |
| Salicylic | 0.09 | 0.1 | 0.1 | 0.9 | 0.2 | 0.5 | 5.6 | 6.8 |
| Total | 524.5 | 590.6 | 781 | 897.4 | 296.6 | 382.5 | 830.7 | 1059.3 |
| Mean | 557.55 | 839.2 | 339.55 | 945 | ||||
| Phenolic Acids | Elderberry Fruit | Bird Cherry Leaves | Bird Cherry Fruit | Bird Cherry Bark | Dogwood Fruit | Dogwood Leaves | ||||||
| Range | Range | Range | Range | Range | Range | |||||||
| Gallic | 2.54 | 14.16 | 115.80 | 209.58 | 37.25 | 38.52 | 14.25 | 19.36 | 110.65 | 130.52 | 89.69 | 112.36 |
| 4-hydroxybenzoic | 0.81 | 12.16 | 1.29 | 3.83 | 13.25 | 19.85 | 98.63 | 124.54 | 6.52 | 864.87 | 6.56 | 8.45 |
| Vanilic | 0.19 | 0.53 | 10.80 | 26.33 | 11.07 | 27.41 | 78.95 | 89.63 | 7.89 | 14.25 | 8.56 | 10.23 |
| Caffeic | 1.69 | 13.57 | 17.25 | 77.47 | 78.51 | 88.52 | 2.44 | 3.88 | 1.40 | 13.52 | 2.55 | 3.46 |
| Syringic | 2.92 | 6.37 | 0.17 | 0.24 | 10.45 | 11.57 | 45.12 | 58.95 | 3.56 | 2.58 | 1.16 | 2.12 |
| Vanilin | 5.39 | 12.74 | 11.07 | 18.70 | 131.54 | 185.70 | 172.45 | 198.63 | 4.58 | 4.32 | 5.69 | 7.56 |
| p-Cumaric | 1.31 | 2.46 | 183.85 | 187.66 | 89.69 | 106.74 | 344.20 | 375.25 | 2.55 | 6.6 | 4.35 | 6.56 |
| Benzoic | 16.05 | 57.80 | 28.04 | 35.56 | 102.54 | 127.40 | 14.95 | 16.98 | 498.52 | 655.23 | 377.51 | 430.56 |
| Ferulic | 58.16 | 169.44 | 3.81 | 9.56 | 3.04 | 5.25 | 77.52 | 89.52 | 2.12 | 3,10 | 1.56 | 3.56 |
| Sinapic | 163.28 | 321.03 | 0.32 | 0.64 | 11.21 | 20.74 | 1.20 | 3.23 | 0.50 | 1.6 | 0.59 | 2.04 |
| t-Cinnamic | 101.40 | 214.89 | 0.11 | 0.74 | 0.10 | 0.60 | 0.60 | 1.10 | 1.10 | 1.8 | 0.22 | 1.23 |
| Chlorogenic | 205.26 | 546.61 | 3.81 | 18.05 | 1.40 | 1.80 | 99.50 | 147.84 | 0.30 | 1.4 | 0.96 | 1.6 |
| Protocatechuic | 1.63 | 3.56 | 0.11 | 0.21 | 0.50 | 0.90 | 13.45 | 17.36 | Nd | 0.2 | 16.74 | 26.56 |
| Rozmaric | 1.32 | 4.37 | 0.21 | 0.64 | 1.70 | 1.90 | 4.74 | 9.69 | Nd | 0.1 | 5.59 | 10.44 |
| Salicylic | 6.90 | 9.99 | 11.18 | 38.00 | 30.50 | 39.84 | 5.85 | 6.46 | 10.32 | 21.54 | 105.45 | 130.55 |
| Total | 568.85 | 1389.6 | 387.82 | 627.22 | 522.75 | 676.74 | 973.85 | 1162.42 | 650.01 | 864.87 | 627.18 | 757.28 |
| Mean | 979.27 | 507.52 | 599.75 | 1068.14 | 754.44 | 692.23 | ||||||
| Flavonoids | Representatives |
|---|---|
| Flavonols | quercetin, kaempferol, myricetin, morin, rutin and others |
| Flavones | luteolin, apigenin, chrysin, acacetin |
| Flavanones | hesperidin, naringenin, eriodictyol, sakuranin |
| Flavan-3-ol | catechin, epicatechin, theaflavin and derivatives |
| flavanonoles | taxifolin, aromadendrin |
| Isoflavones | daidzein, genistein, glycitein |
| Anthocyanidin | anthocyanins: cyanidin, delphinidin, malvidin, pelargonidin, peonidin, petunidin, neoflavonoids (4-phenylcoumarin or 4-phenylchromen-2-one): dalbergin, dalbergichromene, nivetin |
| Tree and Shrub Species | Flavonoids | Literature |
|---|---|---|
| Scots pine Pinus sylvestris L. needles | Prodelphinidin and lacks taxifolin, quercetin and taxifolin taxifolin, taxifolin 3′-O-glucoside, quercetin as well as quercetin 3-O-glucoside and 3′-O-glucoside | [58,89,90] |
| Oak Quercus robus L. | Tannin derivatives of pirocatechin and pyrogallol, epicatechin, flavan-3-ol, catechin, quercetin | [91,92] |
| Silver fir Abies alba Mill. Wood and bark | Catechin, epicatechin and catechin tetramethyl ether | [93] |
| European beech Fagus sylvatica L.-leaves wood Bark | Catechin, kempferol, gallocatechin, kaempferol 3-glucoside, naringenin, quercetin, quercetin-3-glucoside, mirycetyna, quercetin, taxifolin quercetin, kaempferol, chrysin Catechin, cis-coniferin, cis-isoconiferin, cis-syringin, R-glucodistylin, S-glucodistylin, taxifolin-xylopyranoside quercetin, kaempferol, chrysin, taxifolin, and (epi)catechin | [52,58,94] |
| Spruce Picea abies H. Karst-needle | Catechin, kaempferol 3-glucoside, naringenin, quercetin, quercetin 3-glucoside, quercitrin Catechin, epicatechin astringin, piceidin Isorhapotin, piceatannol; prodelphinidins | [52,58,95,96,97,98] |
| Salix purpurea L. | Salipuroside, isosalipuroside. naringenin, naringenin 5-O-glucoside, naringenin 7-O-glucoside, chalcone isosalipurposide, flavan-3-ol, catechin | [97,98] |
| Salix spp: S. alba S. viminalis | Amentoflavone, isoquercetin, quercetin, rutin, quercimeritrin, apigenin, 3-O-glucoside rhamnasine, isosalipurpuroside, hyperoside, catechin naringenin, luteolin, eriodycerol, naringin, kaempferol, apigenin-7-O-glucoside, astralgin, quercimeritrin and quercetin-3,7-di-O-glucoside | [52,58,99,100,101,102,103,104] |
| Hawthorn Crataegus L. | Catechin, epicatechin, hyperoside, quercetin, vitexin, isovitexin, apigenin, rutin and kaempferol | [52,58,105] |
| Black elder Sambucus nigra L.-flowers | rutin, quercetin, astragalin and isoquercetin | [52,58,106,107] |
| Birch-leaves | hyperoside, myricetin and luteolin, catechin tannins | [52,58] |
| Rowan Sorbus aucuparia L. | quercetin, rutin, hyperoside, isoquercetin and quercetin-3-O-sophoroside | [108] |
| Common yew Taxus baccata L. | 3-O-rutinosides quercetin, myricetin, kaempferol, 7-O-glucosides | [109] |
| Black locust Robinia pseudoacacia L. | dihydrorobinetin robinetin, butein dihydromyricetin, fisetin, fustin, isoliquiritigenin, myricetin liquiritigenin | [110] |
| Prunus serotina Ehrh. | Catechin, naringenin, quercetin, | [79] |
| Horse chestnut | quercetin, kaempferol, rutin | [111] |
| Bird cherry Prunus padus L. fruits | Catechin, epicatechin, hyperoside, quercitrin quercetin, rutin | [112] |
| Flavonoids | Fir | Larch | Pine | Spruce | ||||
|---|---|---|---|---|---|---|---|---|
| Range | Range | Range | Range | |||||
| Apigenin | 0.0 | 0.5 | 0.2 | 0.6 | 0.1 | 0.4 | Nd | 0.2 |
| Katechin | 248.3 | 264.2 | 24.6 | 28.9 | 23.7 | 32.5 | 3.6 | 4.9 |
| Kempferol | 0.1 | 0.2 | 0.1 | 1.1 | 0.1 | 0.1 | 4.7 | 7.3 |
| Luteolin | 0.0 | 0.1 | 0.1 | 1.4 | 0.0 | 0.2 | 0.0 | 0.7 |
| Naringenin | 27.5 | 32.8 | 92.0 | 105.6 | 87.4 | 115.8 | 73.6 | 99.8 |
| Quercetin | 0.2 | 0.4 | 9.4 | 11.5 | 3.2 | 6.6 | 0.2 | 0.6 |
| Rutin | 0.9 | 1.6 | 4.5 | 6.6 | 1,7 | 4.1 | 0.6 | 0.7 |
| Vitexin | 0.8 | 1.9 | 6.2 | 10.7 | 0.3 | 1.1 | 2.1 | 3.9 |
| Total | 277.8 | 301.6 | 136.94 | 166.22 | 116.53 | 160.78 | 84.72 | 118.11 |
| Mean | 289.72 | 151.58 | 138.66 | 101.42 | ||||
| Flavonoids | Elderberry Fruit | Bird Cherry Leaves | Bird Cherry Fruit | Bird Cherry Bark | Dogwood Fruit | Dogwood Leaves | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Range | Range | Range | Range | Range | |||||||
| Apigenin | 86.26 | 269.99 | 0.59 | 0.82 | 2.28 | 3.80 | 11.40 | 13.45 | 1.06 | 2.60 | 8.33 | 10.12 |
| Katechin | 0.17 | 1.59 | 0.32 | 0.86 | 566.3 | 687.25 | 858.25 | 904.52 | 284.60 | 314.30 | 0.51 | 0.98 |
| Kempferol | 1.05 | 5.00 | 0.86 | 1.69 | 5.18 | 6.08 | 3.04 | 4.88 | 0.50 | 1.40 | 0.84 | 1.60 |
| Luteolin | 17.07 | 105.69 | 0.11 | 0.64 | Nd | 0.20 | 9.85 | 12.45 | 0.10 | 0.50 | 1.04 | 2.60 |
| Naringenin | 74.67 | 131.38 | 116.01 | 164.08 | 0.80 | 2.01 | 1.52 | 2.30 | 344.65 | 430.50 | 300.55 | 345.62 |
| Quercetin | 671.45 | 1095.18 | 109.55 | 274 | 110.5 | 147.54 | 608.41 | 641.11 | 189.69 | 246.66 | 179.98 | 200.45 |
| Rutin | 1018.19 | 4571.75 | 30.53 | 34.25 | 15.32 | 19.85 | 22.52 | 26.56 | 29.69 | 44.32 | 95.52 | 125.65 |
| Vitexin | 1.12 | 5.28 | 0.59 | 0.85 | 5.84 | 9.85 | 3.15 | 5.50 | 1.56 | 2.56 | 11.51 | 23.56 |
| Total | 1869.9 | 6185.87 | 258.54 | 477.2 | 706.24 | 876.58 | 1518.1 | 1610.77 | 851.85 | 1042.84 | 598.28 | 710.58 |
| Mean | 4027.93 | 367.88 | 791.4 | 1564.46 | 947.35 | 654.43 | ||||||
| Tree and Shrub Species | Proanthocyanidins | Literature |
|---|---|---|
| Scots pine Pinus sylvestris L. Pinus densiflora L. needles | Proanthocyanidins (OPC) procyanidins, prodelphinidins and propelargonidins catechin derivatives, both dimers and trimers. | [124,125,126,127,128] |
| Quercus petraea L. and Q. robur | Proanthocyanidins | [129,130,131,132] |
| Hawthorn Crataegus oxyacantha L. | Procyanidin glycosides | [121,133,134] |
| Bird cherry Prunus padus | Cyanidin-3-rutinoside and cyaniding-3-glucoside | [135] |
| Salix purpurea L. | Cyanidin-3-glucoside, myrtillin (delphinidin-3-glucoside) | [136] |
| Tree and Shrub Species | Stilbenes | Literature |
|---|---|---|
| Scots pine-Pinus sylvestris L. Eastern white pine-Pinus strobus Japanese red pine-Pinus densiflora | Pinosylvin, pinosylvin 3-o-methyl ether | [149,150,151] |
| Spruce Picea abies L. H. Karst | Cis and trans-astringin, trans-piceatannol, cis- and trans-piceid, trans-resveratrol | [140,152,153,154,155] |
| Black locust Robinia pseudoacacia L. | Piceatannol and resveratrol | [156] |
| Morus spp. Mulberry | Resveratrol | [157] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwajkowska-Michałek, L.; Przybylska-Balcerek, A.; Rogoziński, T.; Stuper-Szablewska, K. Phenolic Compounds in Trees and Shrubs of Central Europe. Appl. Sci. 2020, 10, 6907. https://doi.org/10.3390/app10196907
Szwajkowska-Michałek L, Przybylska-Balcerek A, Rogoziński T, Stuper-Szablewska K. Phenolic Compounds in Trees and Shrubs of Central Europe. Applied Sciences. 2020; 10(19):6907. https://doi.org/10.3390/app10196907
Chicago/Turabian StyleSzwajkowska-Michałek, Lidia, Anna Przybylska-Balcerek, Tomasz Rogoziński, and Kinga Stuper-Szablewska. 2020. "Phenolic Compounds in Trees and Shrubs of Central Europe" Applied Sciences 10, no. 19: 6907. https://doi.org/10.3390/app10196907
APA StyleSzwajkowska-Michałek, L., Przybylska-Balcerek, A., Rogoziński, T., & Stuper-Szablewska, K. (2020). Phenolic Compounds in Trees and Shrubs of Central Europe. Applied Sciences, 10(19), 6907. https://doi.org/10.3390/app10196907









