Fontan Hemodynamics Investigation via Modeling and Experimental Characterization of Idealized Pediatric Total Cavopulmonary Connection
Abstract
:1. Introduction
2. Experimental Setup
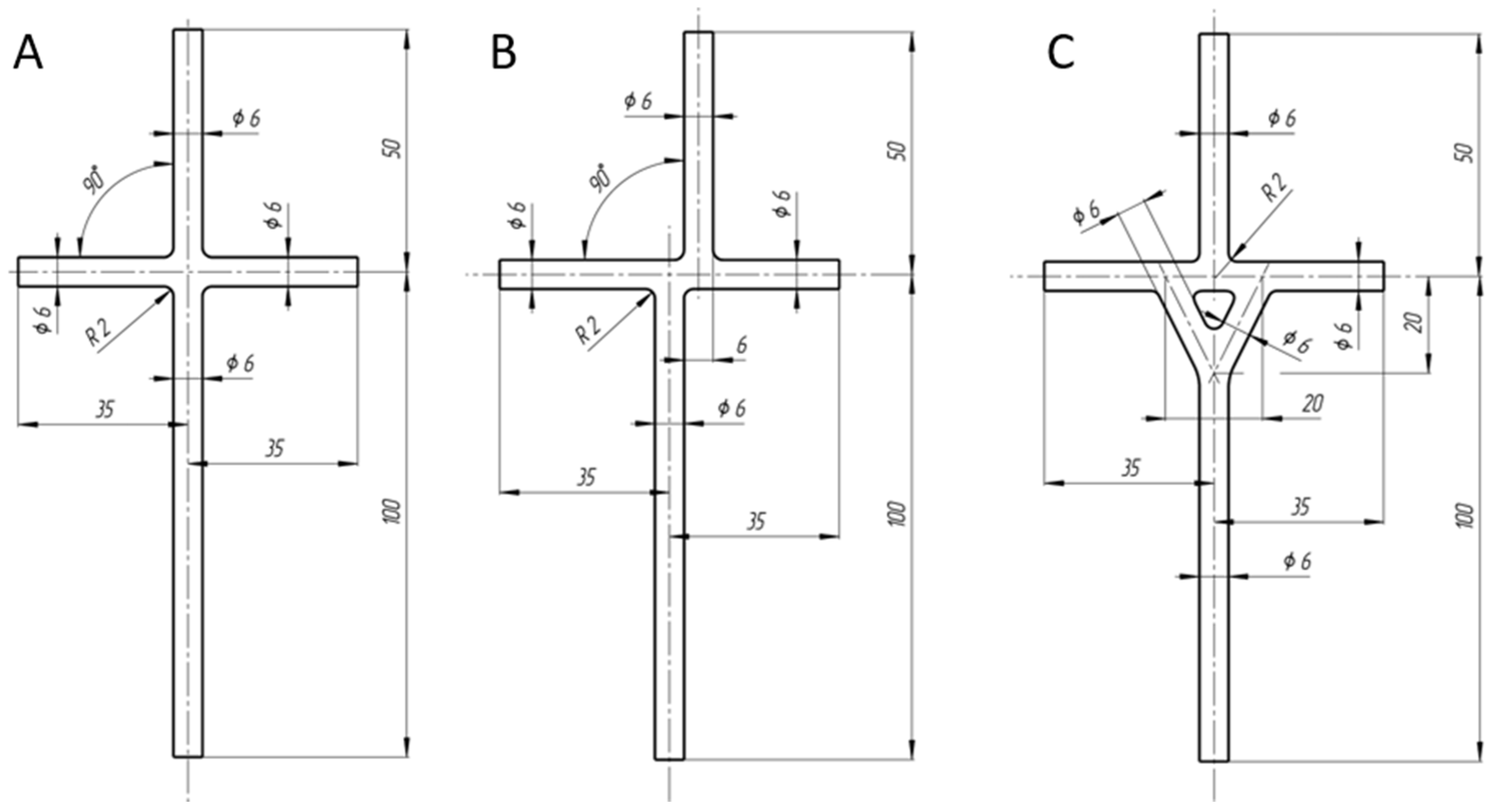
2.1. TCPC Models CFD
2.2. TCPC Models Preparation
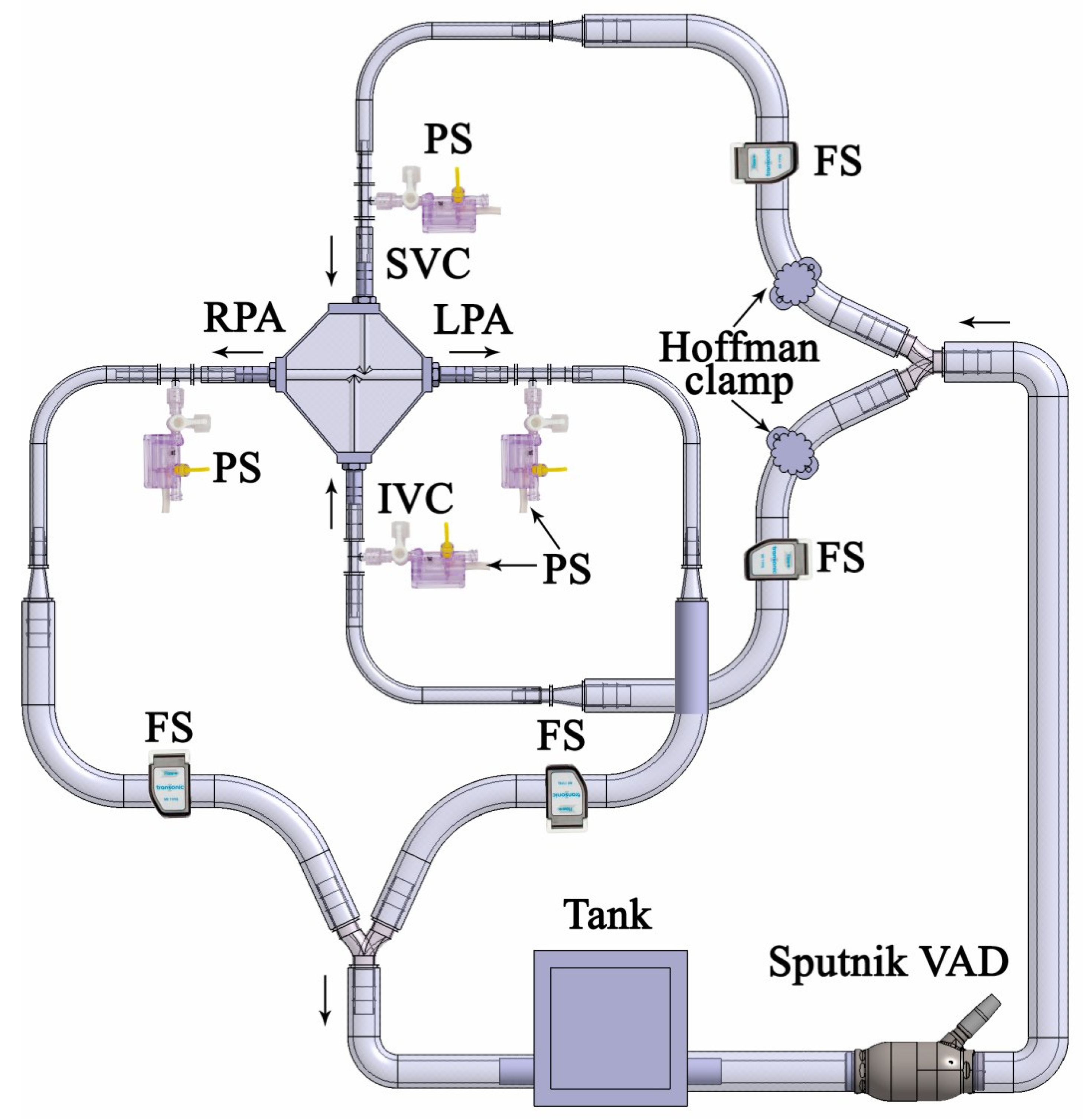
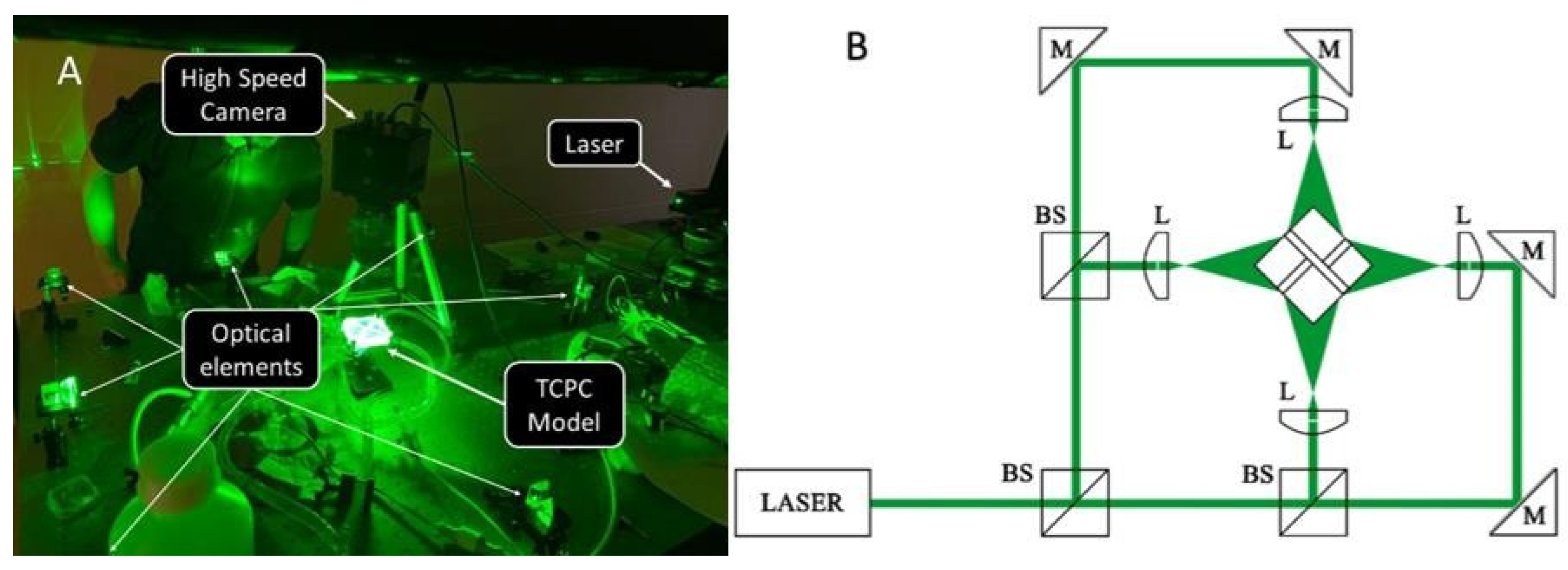
2.3. Mock Circulation Loop and Flow Visualization Setup
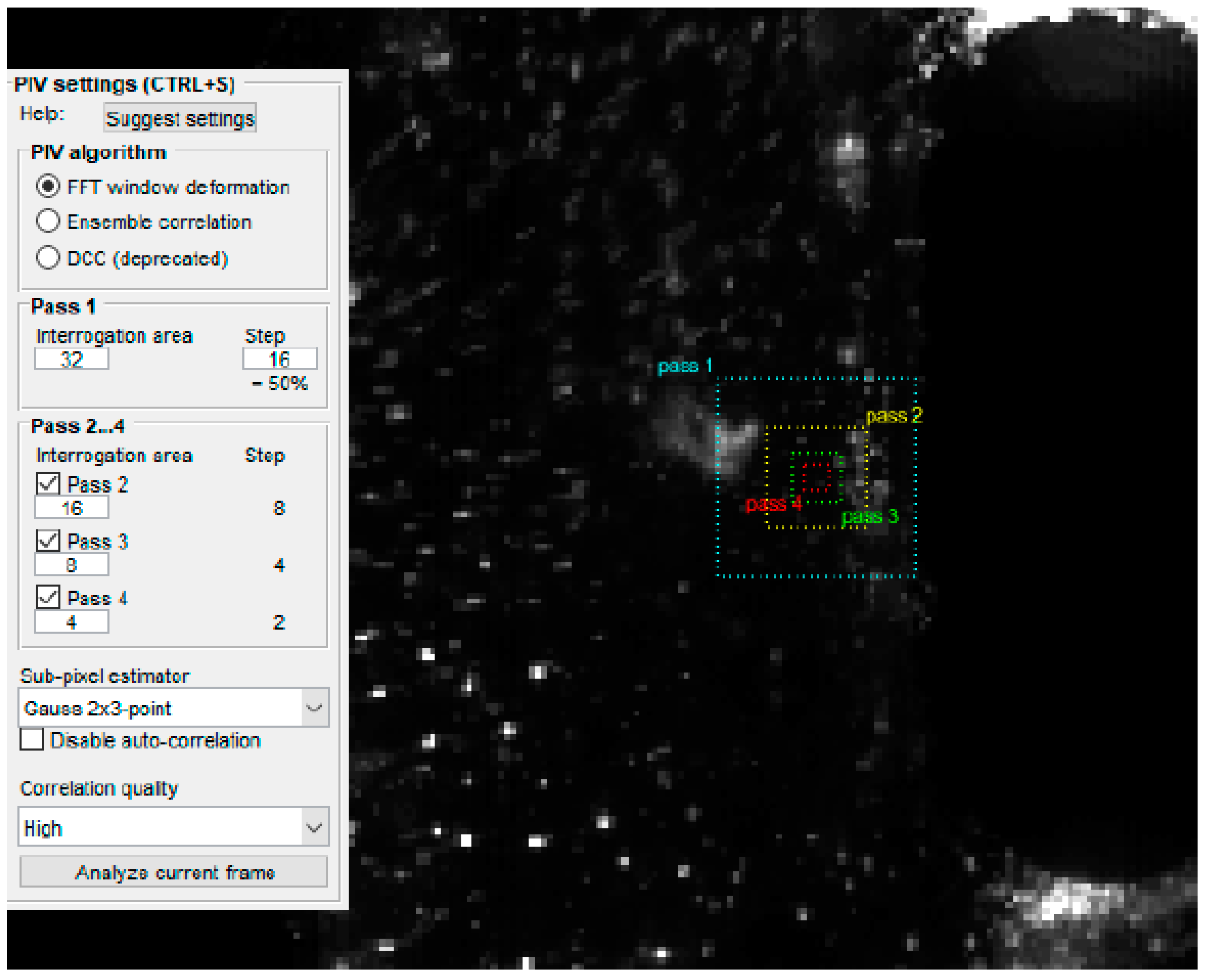
3. Measurement Techniques
CFD Study
4. Characterization of Experimental Conditions
5. Results
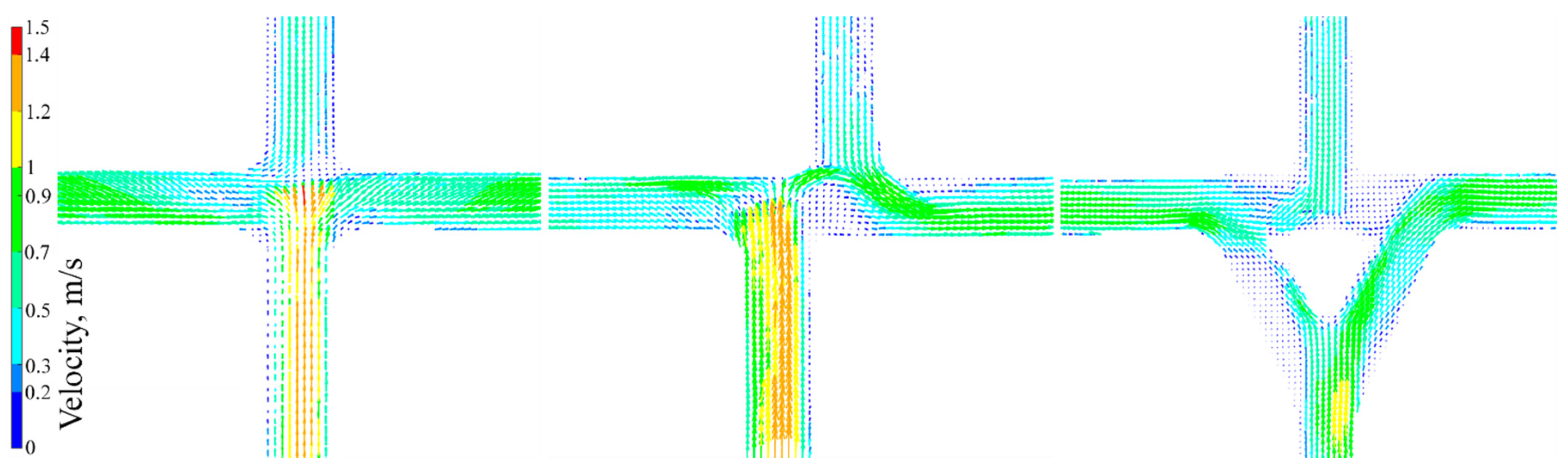
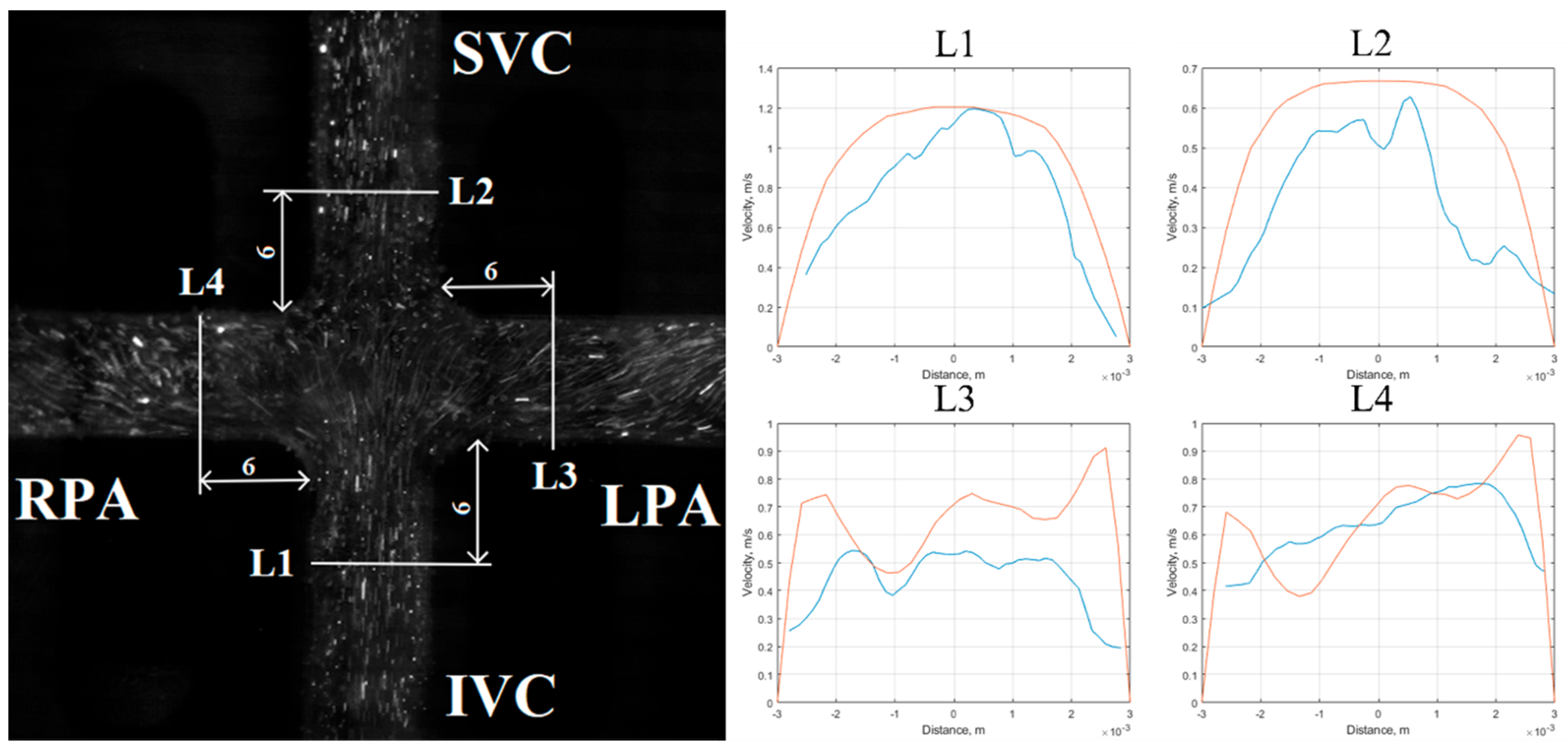
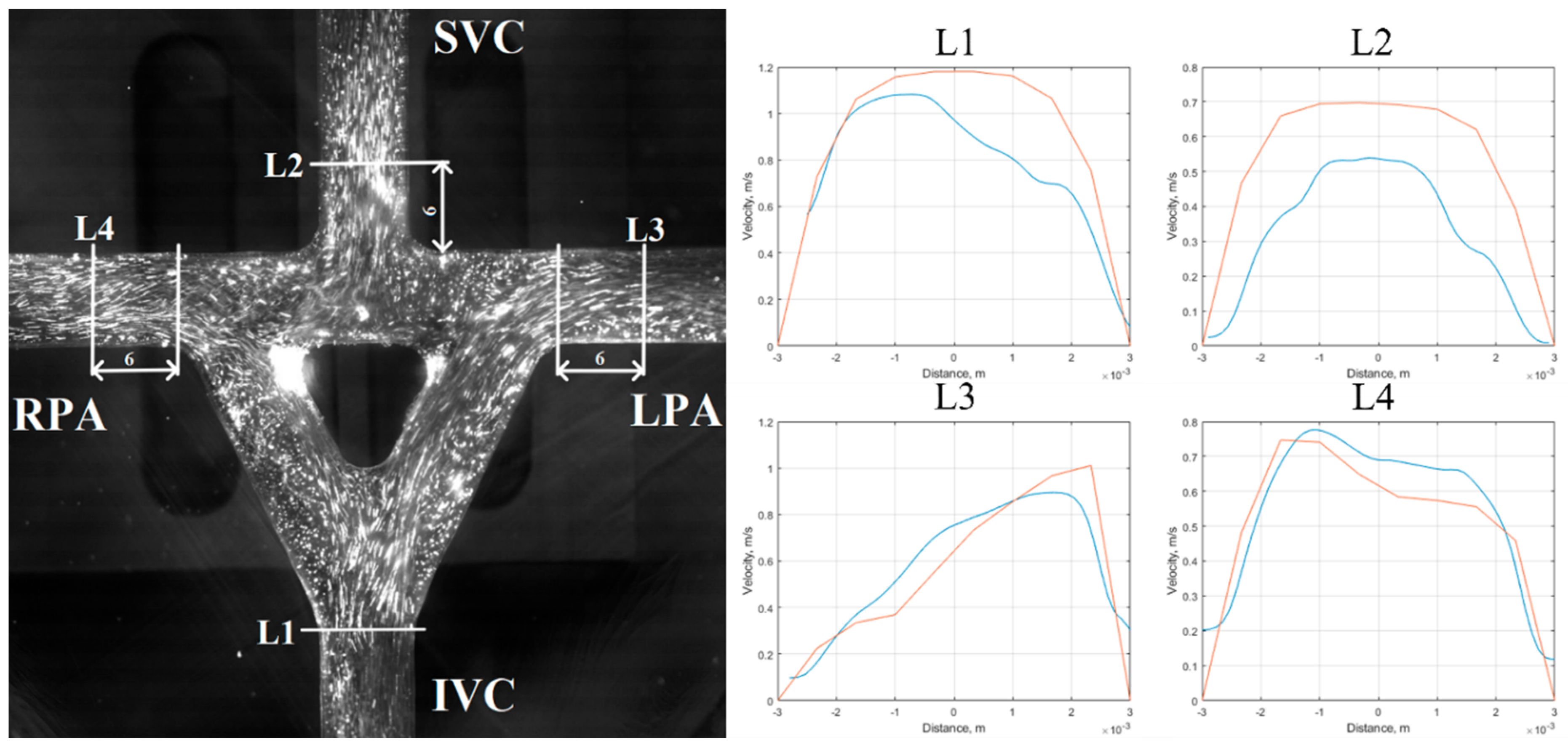
5.1. Velocity Field Results
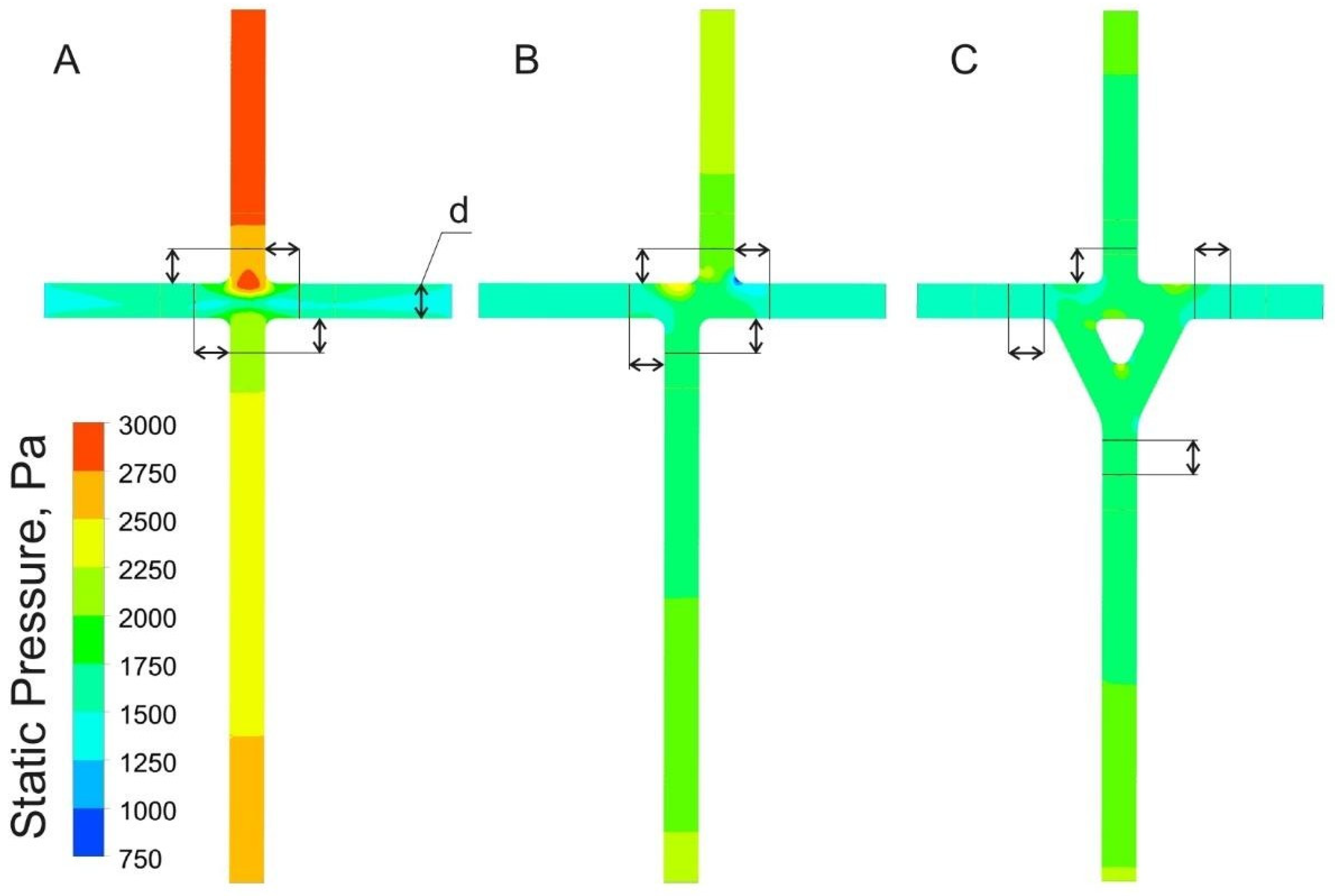
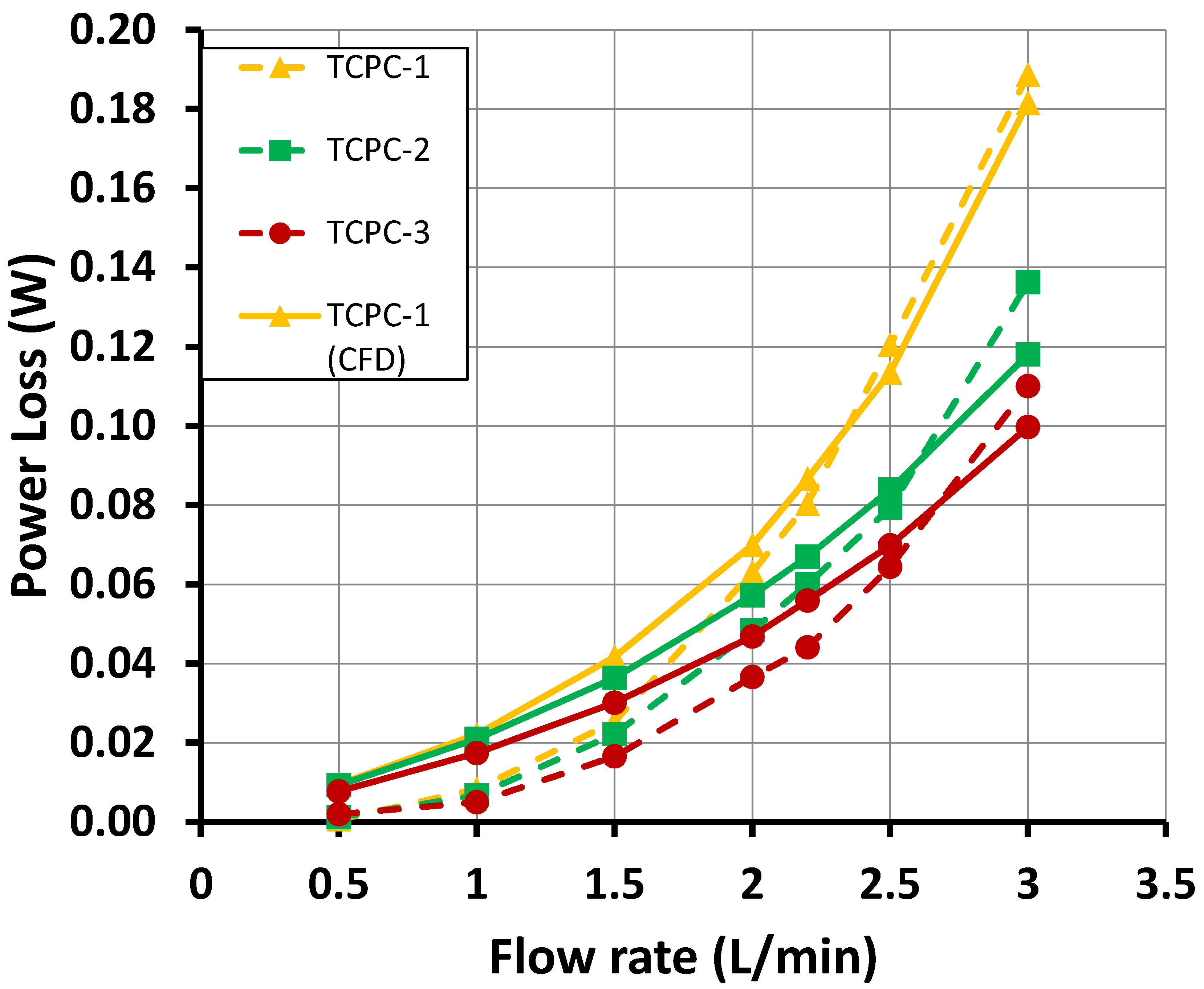
5.2. Hydraulic Results
6. Discussion
Limitation
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TCPC | total cavopulmonary connection |
| SVC | superior vena cava |
| IVC | inferior vena cava |
| LPA | left pulmonary artery |
| RPA | right pulmonary artery |
| CFD | computational fluid dynamics |
| VAD | ventricular assist device |
| CVS | cardiovascular system |
| PIV | particle image velocimetry |
| V | average velocity |
| R | radius |
| P | static pressure |
| H | flow rate |
| Re | Reynolds number |
| ρ | fluid density |
| μ | fluid viscosity |
| Pstatic | static pressure |
| Pdynamic | dynamic pressure |
| F | cross-section area |
| U | velocity magnitude |
| Ptube | hydrodynamic losses |
| PLmagor | major losses |
| PLminor | minor losses |
| fD | Darcy friction factor, |
| L | pipe length |
| D | hydraulic diameter of pipe |
| <υ> | average water velocity |
| K | resistance coefficient |
References
- Khairy, P.; Poirier, N.; Mercier, L.-A. Univentricular Heart. Circulation 2007, 115, 800–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leval, M.R.; Deanfield, J.E. Four decades of Fontan palliation. Nat. Rev. Cardiol. 2010, 7, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Mack, E.; Untaroiu, A. Hemodynamics Characteristics of a Four-Way Right-Atrium Bypass Connector. J. Fluids Eng. 2018, 140, 121106. [Google Scholar] [CrossRef]
- Jagani, J.; Untaroiu, A. A Study of TCPC-Stent Conjunction for Cavopulmonary Assist in Fontan Patients with Right Ventricular Dysfunction. In Proceedings of the American Society of Mechanical Engineers, Phoenix, AZ, USA, 11–17 November; Volume 7, p. V007T09A093.
- Marsden, A.L.; Bernstein, A.J.; Reddy, V.M.; Shadden, S.C.; Spilker, R.L.; Chan, F.P.; Taylor, C.A.; Feinstein, J.A. Evaluation of a novel Y-shaped extracardiac Fontan baffle using computational fluid dynamics. J. Thorac. Cardiovasc. Surg. 2009, 137, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijnberg, F.M.; Hazekamp, M.G.; Wentzel, J.J.; de Koning, P.J.H.; Westenberg, J.J.M.; Jongbloed, M.R.M.; Blom, N.A.; Roest, A.A.W. Energetics of Blood Flow in Cardiovascular Disease. Circulation 2018, 137, 2393–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, M.W.; Prather, R.O.; Rodriguez, G.; Quinn, R.; Divo, E.; Fogel, M.; Kassab, A.J.; DeCampli, W.M. Computational Investigation of a Self-Powered Fontan Circulation. Cardiovasc. Eng. Technol. 2018, 9, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Migliavacca, F.; Dubini, G.; Bove, E.L.; de Leval, M.R. Computational Fluid Dynamics Simulations in Realistic 3-D Geometries of the Total Cavopulmonary Anastomosis: The Influence of the Inferior Caval Anastomosis. J. Biomech. Eng. 2003, 125, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Soerensen, D.D.; Pekkan, K.; de Zélicourt, D.; Sharma, S.; Kanter, K.; Fogel, M.; Yoganathan, A.P. Introduction of a New Optimized Total Cavopulmonary Connection. Ann. Thorac. Surg. 2007, 83, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Telyshev, D.; Denisov, M.; Markov, A.; Fresiello, L.; Verbelen, T.; Selishchev, S. Energetics of blood flow in Fontan circulation under VAD support. Artif. Organs 2020, 44, 50–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutty, S.; Li, L.; Hasan, R.; Peng, Q.; Rangamani, S.; Danford, D.A. Systemic Venous Diameters, Collapsibility Indices, and Right Atrial Measurements in Normal Pediatric Subjects. J. Am. Soc. Echocardiogr. 2014, 27, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Knobel, Z.; Kellenberger, C.J.; Kaiser, T.; Albisetti, M.; Bergsträsser, E.; Valsangiacomo Buechel, E.R. Geometry and dimensions of the pulmonary artery bifurcation in children and adolescents: Assessment in vivo by contrast-enhanced MR-angiography. Int. J. Cardiovasc. Imaging 2011, 27, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Telyshev, D.; Denisov, M.; Pugovkin, A.; Selishchev, S.; Nesterenko, I. The Progress in the Novel Pediatric Rotary Blood Pump Sputnik Development. Artif. Organs 2018, 42, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Thielicke, W.; Stamhuis, E.J. PIVlab—Towards User-friendly, Affordable and Accurate Digital Particle Image Velocimetry in MATLAB. J. Open Res. Softw. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.P.; Herfkens, R.J.; Taylor, C.A.; Feinstein, J.A. Proximal pulmonary artery blood flow characteristics in healthy subjects measured in an upright posture using MRI: The effects of exercise and age. J. Magn. Reson. Imaging 2005, 21, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.; Eden, F.R.C.P. The normal pulmonary arteriel pressures in the first year of life. J. Pediatr. 1957, 51, 1–4. [Google Scholar] [CrossRef]
- Throckmorton, A.L.; Chopski, S.G. Pediatric Circulatory Support: Current Strategies and Future Directions. Biventricular and Univentricular Mechanical Assistance. ASAIO J. 2008, 54, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.; Restrepo, M.; Haggerty, C.M.; Mirabella, L.; Bethel, J.; Whitehead, K.K.; Fogel, M.A.; Yoganathan, A.P. Geometric Characterization of Patient-Specific Total Cavopulmonary Connections and its Relationship to Hemodynamics. JACC Cardiovasc. Imaging 2014, 7, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasi, L.P.; KrishnankuttyRema, R.; Kitajima, H.D.; Pekkan, K.; Sundareswaran, K.S.; Fogel, M.; Sharma, S.; Whitehead, K.; Kanter, K.; Yoganathan, A.P. Fontan hemodynamics: Importance of pulmonary artery diameter. J. Thorac. Cardiovasc. Surg. 2009, 137, 560–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itatani, K.; Miyaji, K.; Tomoyasu, T.; Nakahata, Y.; Ohara, K.; Takamoto, S.; Ishii, M. Optimal Conduit Size of the Extracardiac Fontan Operation Based on Energy Loss and Flow Stagnation. Ann. Thorac. Surg. 2009, 88, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Kilner, P.J. Valveless pump models that laid a false but fortuitous trail on the way towards the total cavopulmonary connection. Cardiol. Young 2005, 15, 74–79. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porfiryev, A.; Markov, A.; Galyastov, A.; Denisov, M.; Burdukova, O.; Gerasimenko, A.Y.; Telyshev, D. Fontan Hemodynamics Investigation via Modeling and Experimental Characterization of Idealized Pediatric Total Cavopulmonary Connection. Appl. Sci. 2020, 10, 6910. https://doi.org/10.3390/app10196910
Porfiryev A, Markov A, Galyastov A, Denisov M, Burdukova O, Gerasimenko AY, Telyshev D. Fontan Hemodynamics Investigation via Modeling and Experimental Characterization of Idealized Pediatric Total Cavopulmonary Connection. Applied Sciences. 2020; 10(19):6910. https://doi.org/10.3390/app10196910
Chicago/Turabian StylePorfiryev, Andrey, Aleksandr Markov, Andrey Galyastov, Maxim Denisov, Olga Burdukova, Alexander Yu. Gerasimenko, and Dmitry Telyshev. 2020. "Fontan Hemodynamics Investigation via Modeling and Experimental Characterization of Idealized Pediatric Total Cavopulmonary Connection" Applied Sciences 10, no. 19: 6910. https://doi.org/10.3390/app10196910









