Thermal Behavior and Flammability of Epoxy Composites Based on Multi-Walled Carbon Nanotubes and Expanded Graphite: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization
3. Results and Discussion
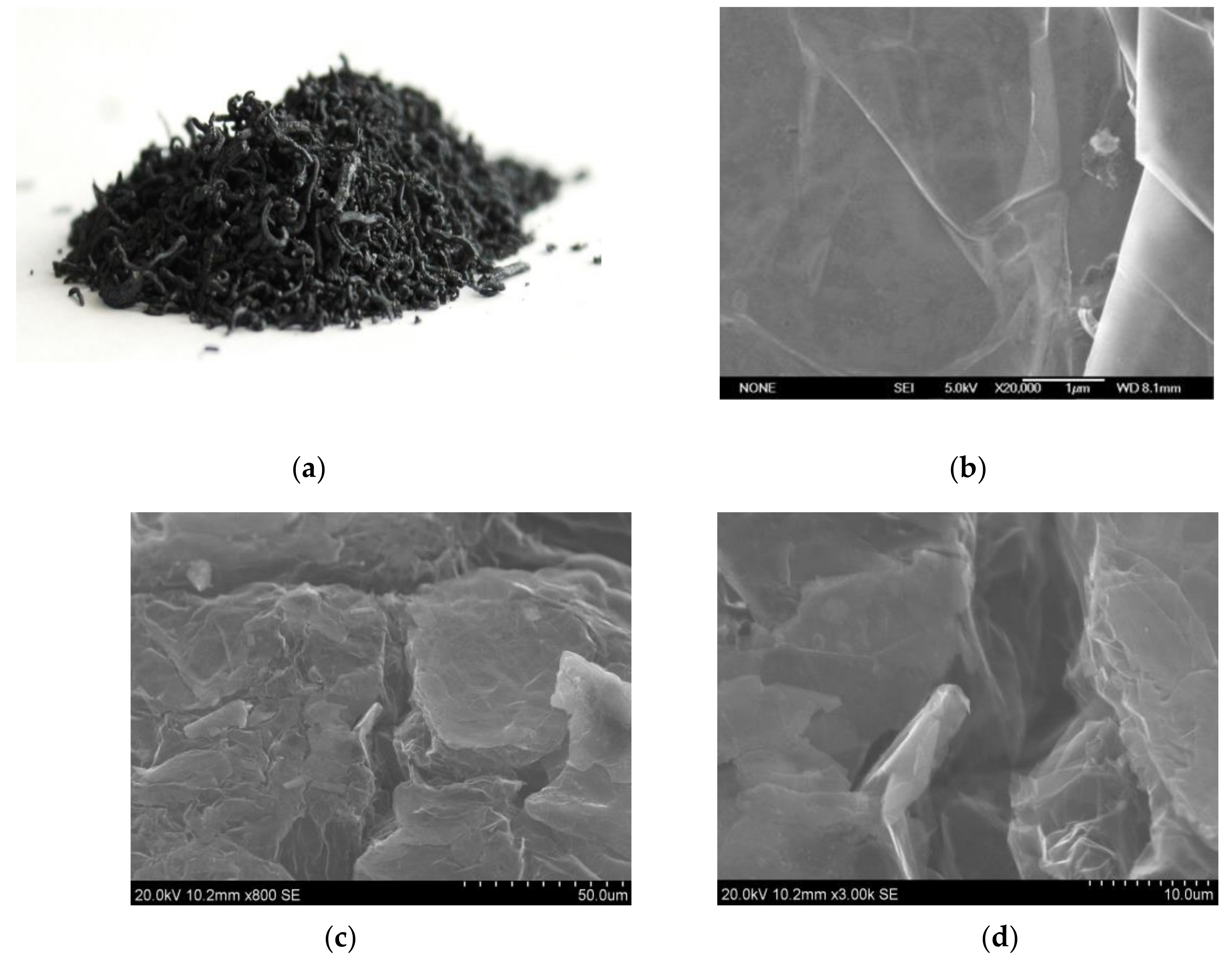
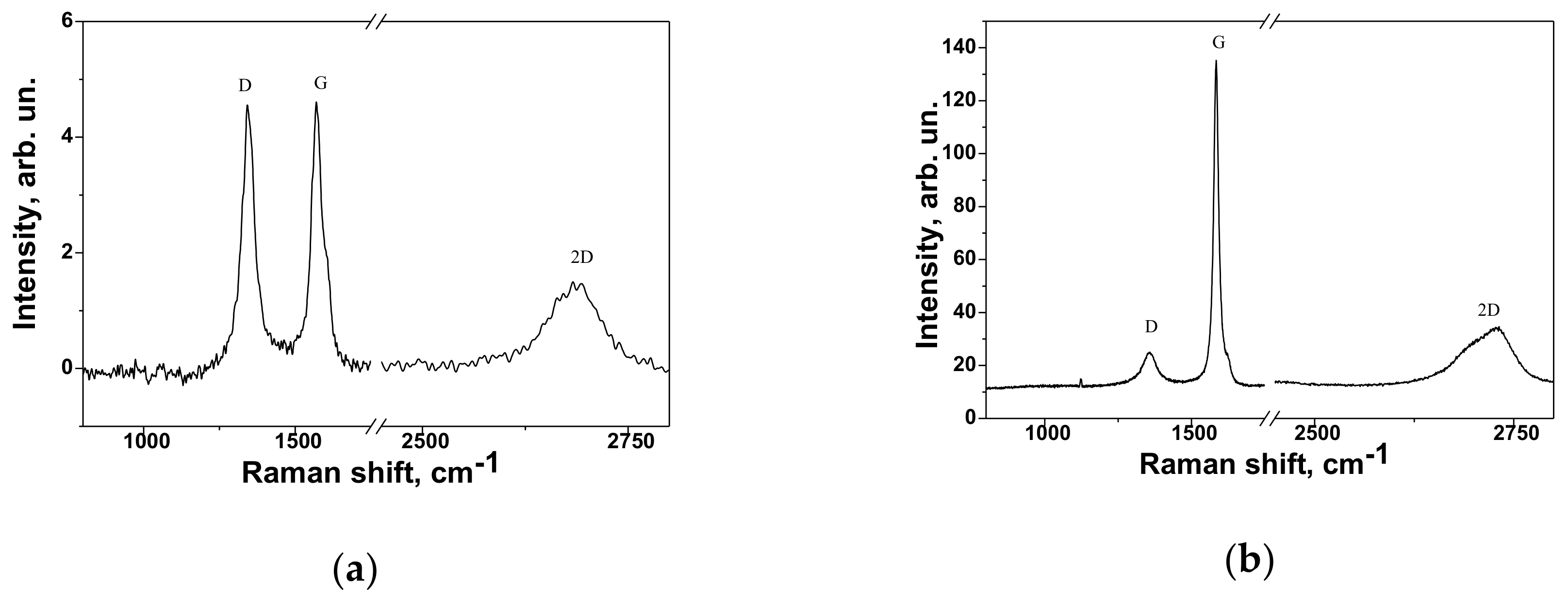
3.1. Characterization of Fillers
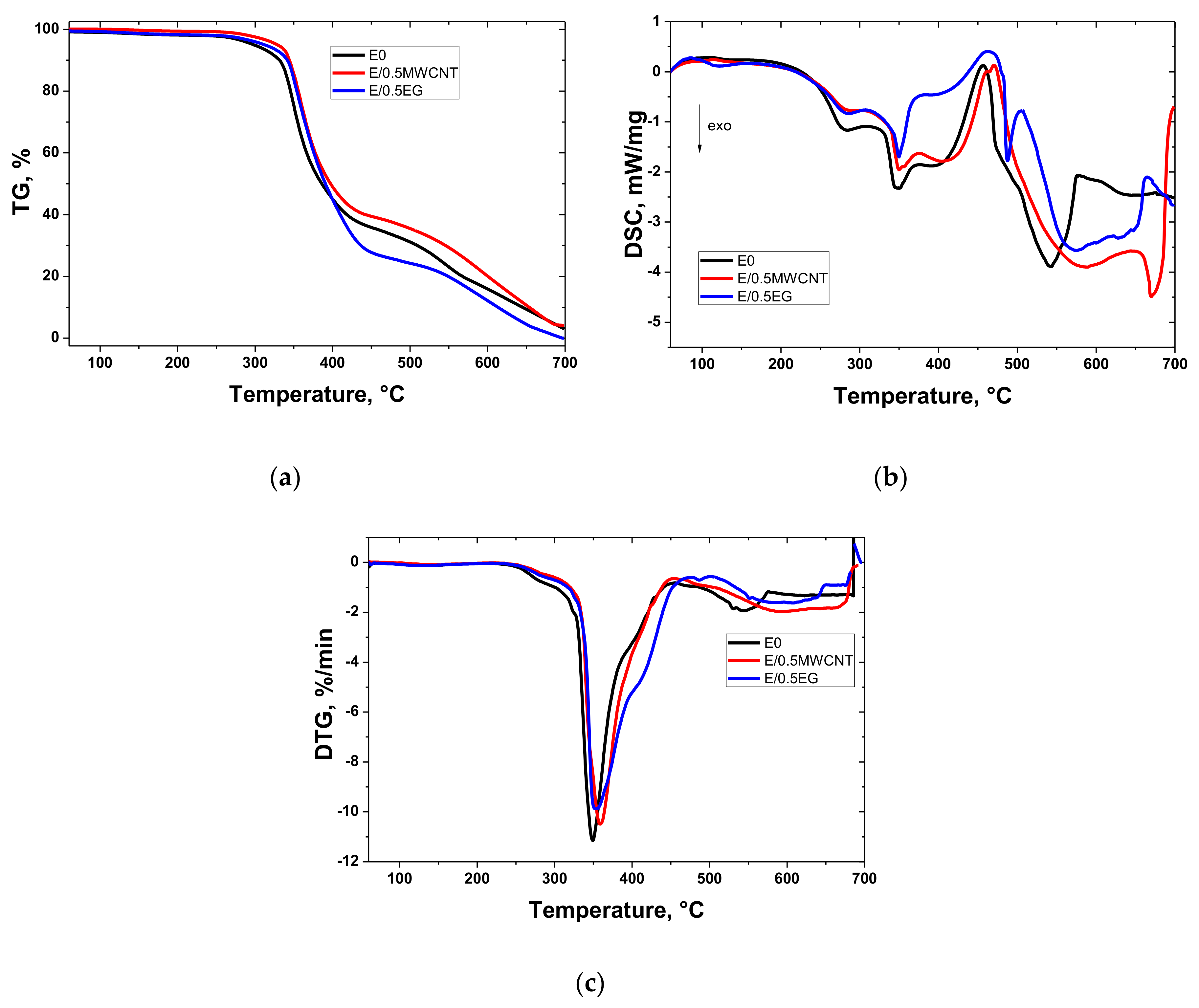
3.2. Thermal Oxidation Behavior
3.3. Hardness
3.4. Flammability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ebewele, R.O. Polymer Science and Technology; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Bower, D.I. An Introduction to Polymer Physics; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Lee, H.; Neville, K. Handbook of Epoxy Resins; McGraw-Hill: New York, NY, USA, 1967. [Google Scholar]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Irvine, D.J.; McCluskey, J.A.; Robinson, I.M. Fire hazards and some common polymers. Polym. Degrad. Stab. 2000, 67, 383–396. [Google Scholar] [CrossRef]
- Vengatesan, M.R.; Varghese, A.M.; Mittal, V. Thermal Properties of Thermoset Polymers. In Thermosets: Structure, Properties, and Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Wilkie, C.A.; Morgan, A.B. Fire Retardancy of Polymeric Materials; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Dasari, A.; Yu, Z.Z.; Cai, G.P.; Mai, Y.W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Shah, A.U.R.; Prabhakar, M.N.; Song, J. Il Current advances in the fire retardancy of natural fiber and bio-based composites—A review. Int. J. Precis. Eng. Manuf. Green Technol. 2017, 4, 247–262. [Google Scholar] [CrossRef]
- Schmidt, C.; Ciesielski, M.; Greiner, L.; Döring, M. Novel organophosphorus flame retardants and their synergistic application in novolac epoxy resin. Polym. Degrad. Stab. 2018, 158, 190–201. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A review of the synthesis and applications of polymer-nanoclay composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. An Overview of Flame Retardancy of Polymeric Materials: Application, Technology, and Future Directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef]
- Gilman, J.W.; Kashiwagi, T.; Lichtenhan, J.D. A Revolutionary New Flame Retardant Approach. SAMPE J. 1997, 33, 40–46. [Google Scholar]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Reports 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Arao, Y. Flame Retardancy of Polymer Nanocomposite. In Flame Retardans; Springer: Cham, Switzerland, 2015; pp. 15–44. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Nguyen, Q.T.; Bach, T.P. Mechanical Properties and Flame Retardancy of Epoxy Resin/Nanoclay/Multiwalled Carbon Nanotube Nanocomposites. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Toldy, A.; Szebényi, G.; Molnár, K.; Tóth, L.F.; Magyar, B.; Hliva, V.; Czigány, T.; Szolnoki, B. The effect of multilevel carbon reinforcements on the fire performance, conductivity, and mechanical properties of epoxy composites. Polymers 2019, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.C.; Soutis, C.; Bailey, C.G.; Hu, Y. Fire Safety Assessment of Epoxy Composites Reinforced by Carbon Fibre and Graphene. Appl. Compos. Mater. 2020, 27, 619–639. [Google Scholar] [CrossRef]
- Kamaraj, M.; Dodson, E.A.; Datta, S. Effect of graphene on the properties of flax fabric reinforced epoxy composites. Adv. Compos. Mater. 2020, 29. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Hu, Y. Functionalization of graphene with grafted polyphosphamide for flame retardant epoxy composites: Synthesis, flammability and mechanism. Polym. Chem. 2014, 5, 1145–1154. [Google Scholar] [CrossRef]
- Zhuo, D.; Wang, R.; Wu, L.; Guo, Y.; Ma, L.; Weng, Z.; Qi, J. Flame retardancy effects of graphene nanoplatelet/carbon nanotube hybrid membranes on carbon fiber reinforced epoxy composites. J. Nanomater. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Feng, Y.; He, C.; Wen, Y.; Ye, Y.; Zhou, X.; Xie, X.; Mai, Y.W. Improving thermal and flame retardant properties of epoxy resin by functionalized graphene containing phosphorous, nitrogen and silicon elements. Compos. Part A Appl. Sci. Manuf. 2017, 103, 74–83. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, H.; Li, M.; Chen, T.; Xu, Y.; Yuan, C.; Zeng, B.; Dai, L. Effect of functionalized graphene oxide with phosphaphenanthrene and isocyanurate on flammability, mechanical properties, and thermal stability of epoxy composites. J. Appl. Polym. Sci. 2020, 137, 1–11. [Google Scholar] [CrossRef]
- Zhi, M.; Liu, Q.; Chen, H.; Chen, X.; Feng, S.; He, Y. Thermal Stability and Flame Retardancy Properties of Epoxy Resin Modified with Functionalized Graphene Oxide Containing Phosphorus and Silicon Elements. ACS Omega 2019, 4, 10975–10984. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Yakovlev, A.V. Reinforcement of Epoxy Composites with Graphite-Graphene Structures. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube-polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Liew, K.M.; Lei, Z.X.; Zhang, L.W. Mechanical analysis of functionally graded carbon nanotube reinforced composites: A review. Compos. Struct. 2015, 120, 90–97. [Google Scholar] [CrossRef]
- Bhattacharya, M. Polymer nanocomposites-A comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chevali, V.S.; Xu, Z.; Hui, D.; Wang, H. A Review of Extending Performance of Epoxy Resins using Carbon Materials. Compos. Part B 2018, 136, 197–213. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, Y.; Ding, Y.; Zhu, G.; Zheng, J. Polymer/carbon nanotubes nanocomposites: Relationship between interfacial adhesion and performance of nanocomposites. J. Mater. Sci. 2018, 53, 10160–10172. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Zeng, L.; Li, R.; Tian, H.; Fu, X.; Wang, Y.; Zhong, W.H. A review of the electrical and mechanical properties of carbon nanofiller-reinforced polymer composites. J. Mater. Sci. 2019, 54, 1036–1076. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.J.; Son, Y.R.; In, I.; An, K.H.; Kim, B.J.; Park, S.J. Recent advanced thermal interfacial materials: A review of conducting mechanisms and parameters of carbon materials. Carbon N. Y. 2019, 142, 445–460. [Google Scholar] [CrossRef]
- Berdyugina, I.S.; Steksova, Y.P.; Shibaev, A.A.; Maksimovskii, E.A.; Bannov, A.G. Thermal degradation of epoxy composites based on thermally expanded graphite and multiwalled carbon nanotubes. Russ. J. Appl. Chem. 2016, 89, 1447–1453. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A Review on Carbon Nanotubes and Graphene as Fillers in Reinforced Polymer Nanocomposites. J. Ind. Chem. Res. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Jen, Y.M.; Huang, J.C. Synergistic effect on the thermomechanical and electrical properties of epoxy composites with the enhancement of carbon nanotubes and graphene nano platelets. Materials 2019, 12, 255. [Google Scholar] [CrossRef]
- Kuan, C.F.; Chen, W.J.; Li, Y.L.; Chen, C.H.; Kuan, H.C.; Chiang, C.L. Flame retardance and thermal stability of carbon nanotube epoxy composite prepared from sol-gel method. J. Phys. Chem. Solids 2010, 71, 539–543. [Google Scholar] [CrossRef]
- Gantayat, S.; Prusty, G.; Rout, D.; Swain, S.K. Expanded graphite as a filler for epoxy matrix composites to improve their thermal, mechanical and electrical properties. Carbon N. Y. 2016, 98, 734. [Google Scholar] [CrossRef]
- Asante, J.; Modiba, F.; Mwakikunga, B. Thermal Measurements on Polymeric Epoxy-Expandable Graphite Material. Int. J. Polym. Sci. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Steksova, Y.P.; Berdyugina, I.S.; Shibaev, A.A.; Ukhina, A.V.; Maksimovskii, E.A.; Popov, M.V.; Bannov, A.G. Effect of synthesis parameters on characteristics of expanded graphite. Russ. J. Appl. Chem. 2016, 89, 1588–1595. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 95–107. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Bannov, A.G.; Uvarov, N.F.; Ukhina, A.V.; Chukanov, I.S.; Dyukova, K.D.; Kuvshinov, G.G. Structural changes in carbon nanofibers induced by ball milling. Carbon N. Y. 2012, 50, 1090–1098. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Bannov, A.G.; Popov, M.V.; Yun, J.-W.; Nguyen, A.D.; Kim, Y.S. High-temperature-treated multiwall carbon nanotubes for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 6526–6531. [Google Scholar] [CrossRef]
- Chairat, A.; Joulia, X.; Floquet, P.; Vergnes, H.; Ablitzer, C.; Fiquet, O.; Brothier, M. Thermal degradation kinetics of a commercial epoxy resin - Comparative analysis of parameter estimation methods. J. Appl. Polym. Sci. 2015, 132, 6–9. [Google Scholar] [CrossRef]
- Kumar, R.; Mohanty, S.; Nayak, S.K. Study on epoxy resin-based thermal adhesive filled with hybrid expanded graphite and graphene nanoplatelet. SN Appl. Sci. 2019, 1, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, R.; Wang, J.; Qi, S. Thermal conductivity improvement of epoxy composite filled with expanded graphite. Ceram. Int. 2015, 41, 13541–13546. [Google Scholar] [CrossRef]
- Laachachi, A.; Burger, N.; Apaydin, K.; Sonnier, R.; Ferriol, M. Is expanded graphite acting as flame retardant in epoxy resin? Polym. Degrad. Stab. 2015, 117, 22–29. [Google Scholar] [CrossRef]
- Bannov, A.G.; Shibaev, A.A.; Mochalova, E.N.; Galikhanov, M.F.; Limarenko, N.A.; Vakhitova, R.N. The application of carbon nanotubes for enhancement of the epoxy thermoelectret properties. In Proceedings of the 2016 11th International Forum on Strategic Technology, Novosibirsk, Russia, 1–3 June 2016. [Google Scholar]
- Kashiwagi, T.; Mu, M.; Winey, K.; Cipriano, B.; Raghavan, S.R.; Pack, S.; Rafailovich, M.; Yang, Y.; Grulke, E.; Shields, J.; et al. Relation between the viscoelastic and flammability properties of polymer nanocomposites. Polymer 2008, 49, 4358–4368. [Google Scholar] [CrossRef]
- Ciecierska, E.; Boczkowska, A.; Kurzydlowski, K.J.; Rosca, I.D.; Van Hoa, S. The effect of carbon nanotubes on epoxy matrix nanocomposites. J. Therm. Anal. Calorim. 2013, 111, 1019–1024. [Google Scholar] [CrossRef]
- Mochane, M.J.; Luyt, A.S. The effect of expanded graphite on the flammability and thermal conductivity properties of phase change material based on PP/wax blends. Polym. Bull. 2015, 72, 2263–2283. [Google Scholar] [CrossRef]



| Sample Code | Epoxy Resin | MWCNTs | EG |
|---|---|---|---|
| E0 | 100 | 0 | 0 |
| E/0.01MWCNT | 100 | 0.01 | 0 |
| E/0.05MWCNT | 100 | 0.05 | 0 |
| E/0.1MWCNT | 100 | 0.1 | 0 |
| E/0.5MWCNT | 100 | 0.5 | 0 |
| E/0.5EG | 10 | 0 | 0.5 |
| E/1.0EG | 100 | 0 | 1.0 |
| E/1.5EG | 100 | 0 | 1.5 |
| E/2.0EG | 100 | 0 | 2.0 |
| E/2.5EG | 100 | 0 | 2.5 |
| Sample | T5% | T20% | T50% | T80% |
|---|---|---|---|---|
| E0 | 300 | 352 | 385 | 568 |
| E/0.01MWCNT | 301 | 352 | 389 | 574 |
| E/0.05MWCNT | 312 | 354 | 389 | 582 |
| E/0.1MWCNT | 319 | 352 | 390 | 563 |
| E/0.5MWCNT | 326 | 353 | 396 | 598 |
| E/0.5EG | 316 | 352 | 392 | 548 |
| E/1.0EG | 338 | 356 | 392 | 552 |
| E/1.5EG | 310 | 352 | 395 | 580 |
| E/2.0EG | 315 | 352 | 400 | 682 |
| E/2.5EG | 305 | 350 | 388 | 656 |
| MWCNTs | 563 | 600 | 625 | 644 |
| EG | 595 | 687 | 741 | 785 |
| Sample | Hardness |
|---|---|
| E0 | 78 ± 2 |
| E/0.01MWCNT | 79 ± 3.2 |
| E/0.05MWCNT | 79 ± 1.7 |
| E/0.1MWCNT | 81 ± 1.1 |
| E/0.5MWCNT | 75 ± 2.3 |
| E/0.5EG | 80 ± 1.1 |
| E/1.0EG | 76 ± 2.5 |
| E/1.5EG | 73 ± 2.2 |
| E/2.0EG | 77 ± 1.4 |
| E/2.5EG | 78 ± 2.1 |
| Sample | Ignition Temperature (°C) | Time-to-Ignition (s) |
|---|---|---|
| E0 | 308 | 437 |
| E/0.01MWCNT | 318 | 346 |
| E/0.05MWCNT | 328 | 356 |
| E/0.1MWCNT | 316 | 381 |
| E/0.5MWCNT | 315 | 446 |
| E/0.5EG | 319 | 371 |
| E/1.0EG | 325 | 334 |
| E/1.5EG | 326 | 367 |
| E/2.0EG | 326 | 348 |
| E/2.5EG | 321 | 346 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bannov, A.G.; Nazarenko, O.B.; Maksimovskii, E.A.; Popov, M.V.; Berdyugina, I.S. Thermal Behavior and Flammability of Epoxy Composites Based on Multi-Walled Carbon Nanotubes and Expanded Graphite: A Comparative Study. Appl. Sci. 2020, 10, 6928. https://doi.org/10.3390/app10196928
Bannov AG, Nazarenko OB, Maksimovskii EA, Popov MV, Berdyugina IS. Thermal Behavior and Flammability of Epoxy Composites Based on Multi-Walled Carbon Nanotubes and Expanded Graphite: A Comparative Study. Applied Sciences. 2020; 10(19):6928. https://doi.org/10.3390/app10196928
Chicago/Turabian StyleBannov, Alexander G., Olga B. Nazarenko, Evgeny A. Maksimovskii, Maxim V. Popov, and Irina S. Berdyugina. 2020. "Thermal Behavior and Flammability of Epoxy Composites Based on Multi-Walled Carbon Nanotubes and Expanded Graphite: A Comparative Study" Applied Sciences 10, no. 19: 6928. https://doi.org/10.3390/app10196928
APA StyleBannov, A. G., Nazarenko, O. B., Maksimovskii, E. A., Popov, M. V., & Berdyugina, I. S. (2020). Thermal Behavior and Flammability of Epoxy Composites Based on Multi-Walled Carbon Nanotubes and Expanded Graphite: A Comparative Study. Applied Sciences, 10(19), 6928. https://doi.org/10.3390/app10196928







