Analysis of Carbon Tetrachloride-Extractable Species from Daxing Bituminous Coal
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparations
2.2. Extraction Procedure
2.3. Analytical Methods
3. Results and Discussion
3.1. Calculation of Different Extraction Yields
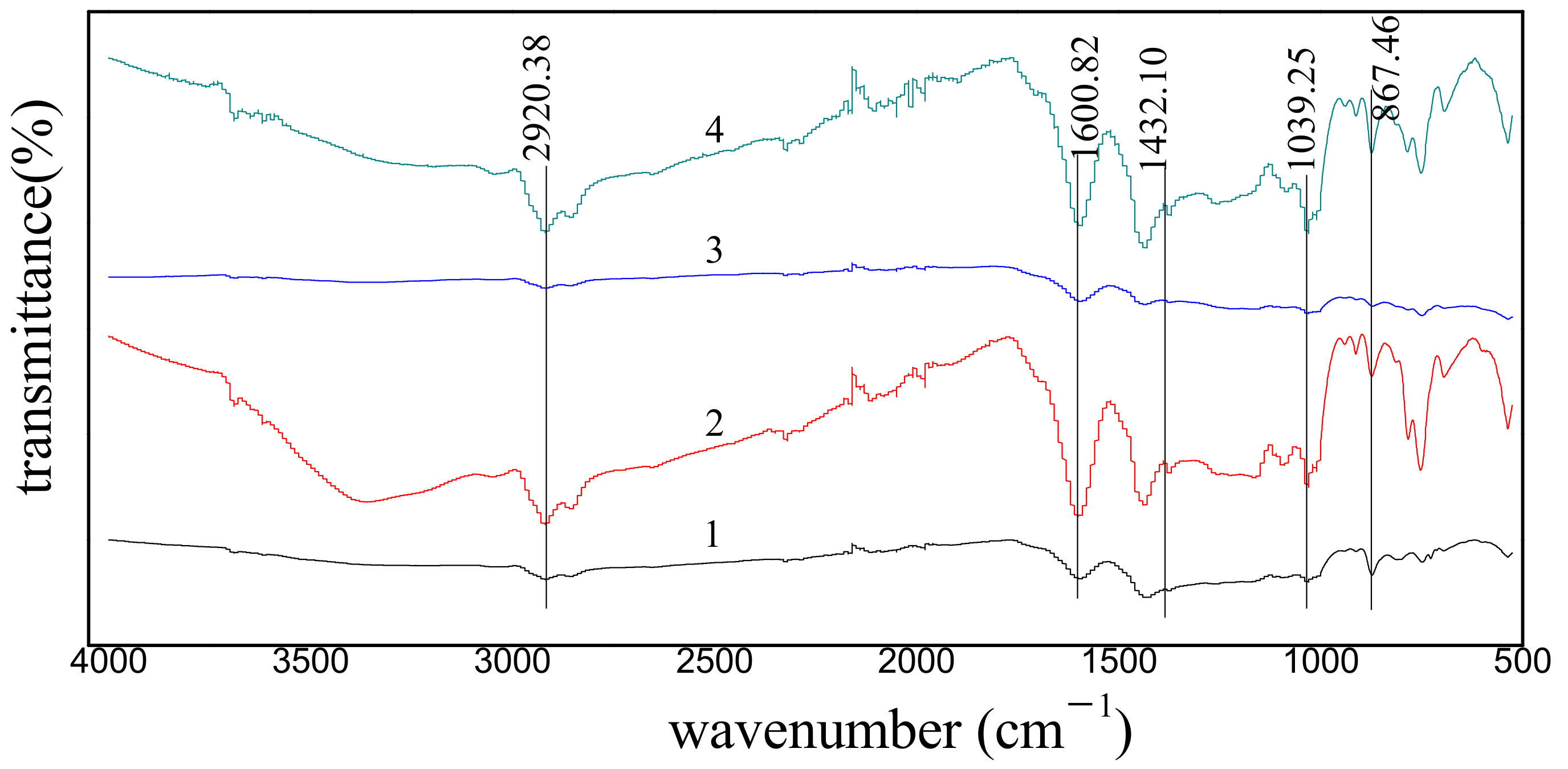
3.2. FT-IR Spectroscopic Analysis
3.3. XRD Analysis
3.4. GC-MS Analysis of Extracts
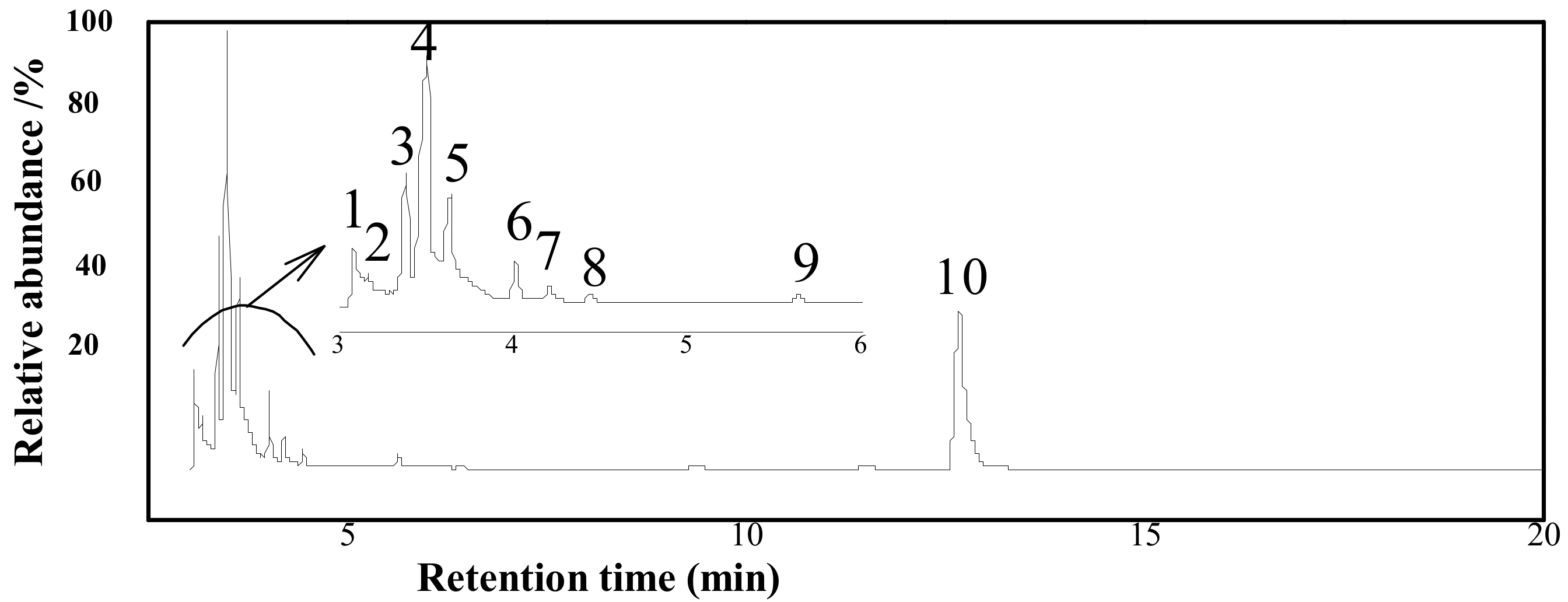
3.4.1. The Extract by SE Process
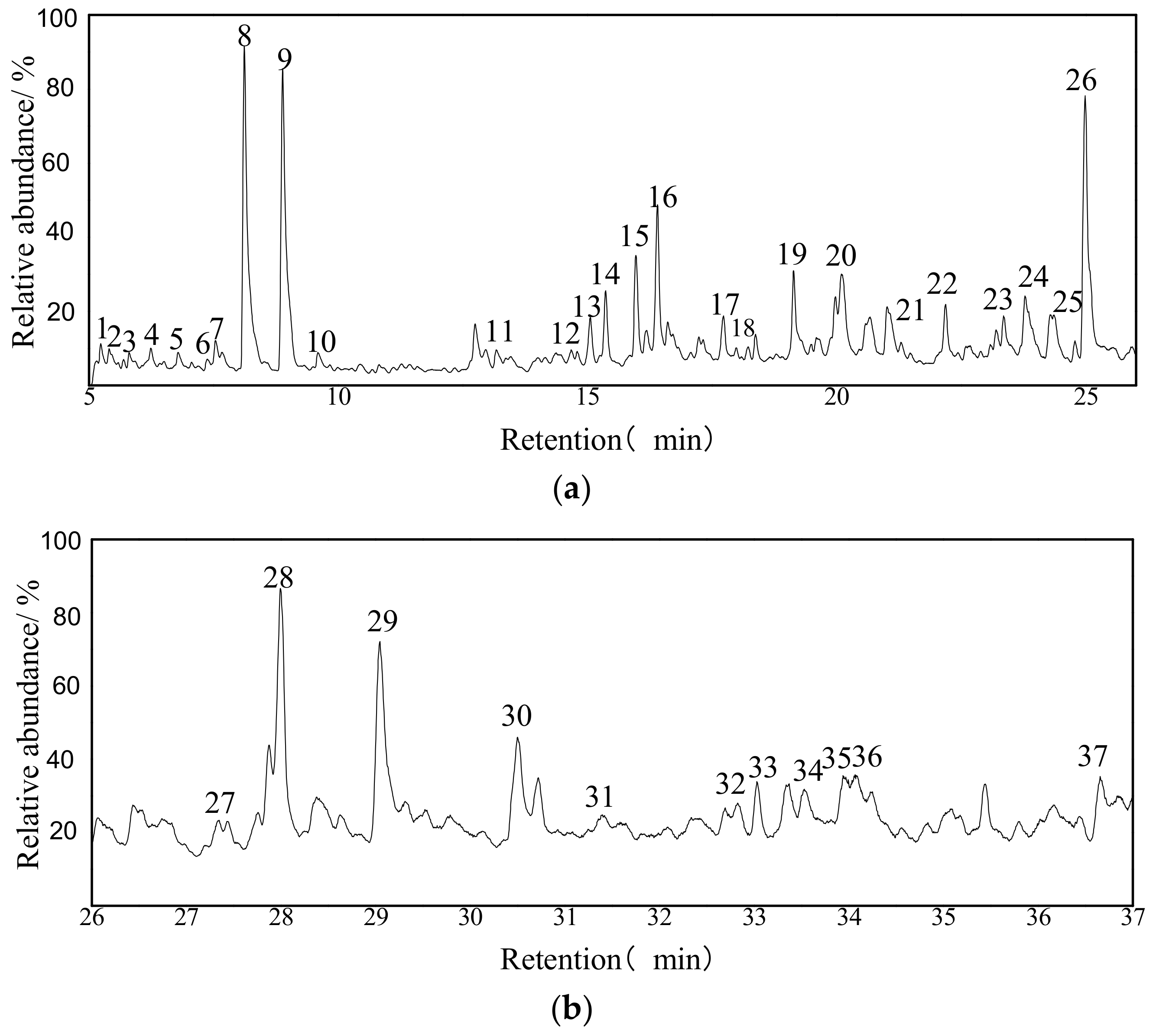
3.4.2. GC/MS Analysis of the Extract from the UAE
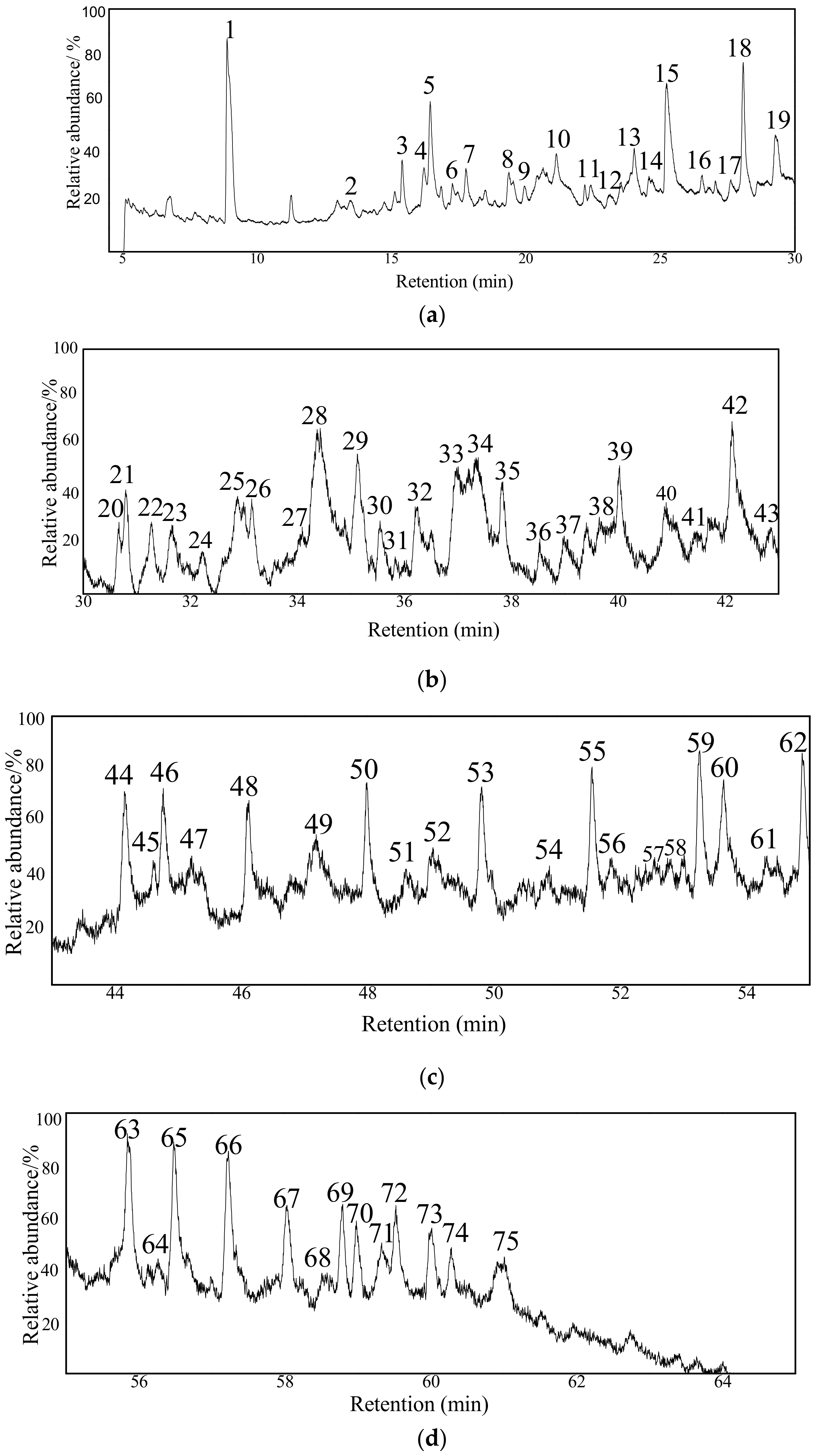
3.4.3. GC/MS Analysis of the Extract from the MAE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lino, M. Network structure of coals and association behavior of coal-derived erials. Fuel Process Technol. 2000, 62, 89–101. [Google Scholar]
- Miura, K. Mild conversion of coal for producing valuable chemicals. Fuel Process Technol. 2000, 62, 19–35. [Google Scholar] [CrossRef]
- Song, C.; Schobert, H.H. Non-fuel uses of coals and synthesis of chemicals and materials. Fuel 1996, 75, 724–736. [Google Scholar] [CrossRef]
- Wei, X.Y.; Wang, X.H.; Zong, Z.M. Extraction of organonitrogen compounds from five Chinese coals with methanol. Energ. Fuel 2009, 23, 4848–4851. [Google Scholar] [CrossRef]
- Wei, X.Y.; Wang, X.H.; Zong, Z.M.; Ni, Z.H.; Zhang, L.F.; Jia, Y.F.; Xie, K.C.; Lee, C.W.; Liu, Z.X.; Chu, N.B.; et al. Identification of organochlorines and organobromines in coals. Fuel 2004, 83, 2435–2438. [Google Scholar] [CrossRef]
- Schobert, H.H.; Song, C.S. Chemicals and materials from coal in the 21st century. Fuel 2002, 81, 15–32. [Google Scholar] [CrossRef]
- Schulten, H.R.; Marzec, A. Time- and temperature-resolved field ionization mass spectrometry of coals and their extracts. Fuel 1986, 65, 855–860. [Google Scholar] [CrossRef]
- Chen, B.; Wei, X.Y.; Zong, Z.M.; Yang, Z.S.; Qing, Y.; Liu, C. Difference in chemical composition of supercritical methanolysis products between two lignites. Appl. Energy 2011, 88, 4570–4576. [Google Scholar] [CrossRef]
- Luis, A.A.; Eduardo, E.W. Supercritical toluene and ethanol extraction of an Illinois No. 6 coal. Fuel 1984, 63, 227–238. [Google Scholar]
- Feng, L.; Liu, X.C.; Song, L.L.; Wang, X.H.; Zhang, Y.; Cui, T.W.; Tang, H.Y. The effect of alkali treatment on some physico-chemical properties of Xilinhaote lignite. Powder Technol. 2013, 247, 19–23. [Google Scholar] [CrossRef]
- Bergins, C. Mechanical/thermal dewatering of lignite. Part 2: A rheological model for consolidation and creep process. Fuel 2004, 83, 267–276. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zong, Z.M.; Cao, J.P.; Ma, Y.M.; Han, L.; Liu, G.F.; Zhao, W.; Li, W.Y.; Xie, K.C.; Bai, X.F.; et al. Difference in chemical composition of carbon disulfide extractable fraction between vitrinite and inertinite from Shenfu-Dongsheng and Pingshuo coal. Fuel 2008, 87, 565–567. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, J.; Miao, Z.; Hu, X.; Li, X.; Shi, X.; Cai, Z.; Shang, Y. Effects of process parameters on pore structure of semi-coke prepared by solid heat carrier with dry destination. Int. J. Min. Sci. Technol. 2013, 23, 423–427. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.W.; Zhao, L.; Ge, L.-M. Microwave-assisted extraction of Shenfu coal and its macromolecule structure. Mining Sci. Technol. 2009, 19, 19–24. [Google Scholar] [CrossRef]
- Qin, Z.H.; Jiang, C.; Hou, C.L.; Li, X.S.; Zhang, L.Y.; Chen, J.; Jiang, B. Solubilization of small molecules from coal and the resulting effects on the pore structure distribution. Min. Sci. Technol. 2009, 19, 761–768. [Google Scholar] [CrossRef]
- Xia, J.L.; Fan, X.; You, C.Y.; Wei, X.Y.; Zhao, Y.P.; Cao, J.P. Sequential ultrasonic extraction of a Chinese coal and characterization of nitrogen-containing compounds in the extracts using high-performance liquid chromatography with mass spectrometry. J. Sep. Sci. 2016, 39, 2491–2498. [Google Scholar] [CrossRef]
- Huang, Y.R.; Han, X.X.; Jiang, X.M. Comparison of fast pyrolysis characteristics of Huadian oil shales from different mines using Curie-point pyrolysis-GC/MS. Fuel Process Technol. 2014, 128, 456–460. [Google Scholar] [CrossRef]
- Li, Z.K.; Wei, X.Y.; Yan, H.L.; Zong, Z.M. Insight into the structural features of Zhaotong lignite using multiple techniques. Fuel 2015, 153, 176–182. [Google Scholar] [CrossRef]
- Sonibare, O.O.; Haeger, T.; Foley, S.F. Structural characterization of Nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy. Energy 2010, 35, 5347–5353. [Google Scholar] [CrossRef]
- Chen, Y.; Mastalerz, M.; Schimmelmann, A. Characterization of chemical functional groups in macerals across different coal ranks via micro-FTIR spectroscopy. Int. J. Coal Geol. 2012, 104, 22–33. [Google Scholar] [CrossRef]
- Takagi, H.; Maruyama, K.; Yoshizawa, N.; Yamada, Y.; Sato, Y. XRD analysis of carbon stacking structure in coal during heat treatment. Fuel 2004, 83, 2427–2433. [Google Scholar] [CrossRef]
- Watanabe, I.; Sakanishi, K.; Mochida, I. Changes in coal aggregate structure by heat treatment and their coal rank dependency. Energy Fuels 2002, 16, 18–22. [Google Scholar] [CrossRef]
- Luque-Gareia, J.L.; Luque de Castro, M.D. Focused microwave-assisted Soxhlet extraction: devices and applications. Talanta 2004, 64, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Parera, J.; Santos, F.J.; Galeeran, M.T. Microwave-assisted extraction versus Soxhlet extraction for the analysis of short-chain chlorinated alkanes in sediments. J. Chromatogr. A 2004, 1046, 19–26. [Google Scholar]
- Rostovshchikova, T.N.; Smirnov, V.V.; Kozhevin, V.M.; Yavsin, D.A.; Gurevich, S.A. Structurally organized nanocomposites in the catalysis of chlorohydrocarbon reactions. Kinet. Catal. 2003, 44, 555–561. [Google Scholar] [CrossRef]
- Liu, C.; Xue, H.; Shen, L.; Liu, C.; Zheng, X.; Shi, J.; Xue, S. Improvement of anthocyanins rate of blueberry powder under variable power of microwave extraction. Sep. Purif. Technol. 2019, 226, 286–298. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, X.; Liu, C.; Sun, Y.; Lin, Z.; Liu, H. Extraction characteristics and optimal parameters of anthocyanin from blueberry powder under microwave-assisted extraction conditions. Sep. Purif. Technol. 2013, 104, 17–25. [Google Scholar] [CrossRef]
- Zuo, H.; Long, S.; Wang, C.; Zhang, P. A review of microwave treatment on coal. In 7th International Symposium on High-Temperature Metallurgical Processing; Springer: Cham, Switzerland, 2016; pp. 617–624. [Google Scholar]

| Proximate Analysis | Ultimate Analysis (daf) | ||||||
|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | C | H | S | N | O a |
| 1.31 | 15.52 | 30.85 | 85.3 | 5.53 | 1.03 | 1.15 | 7.09 |
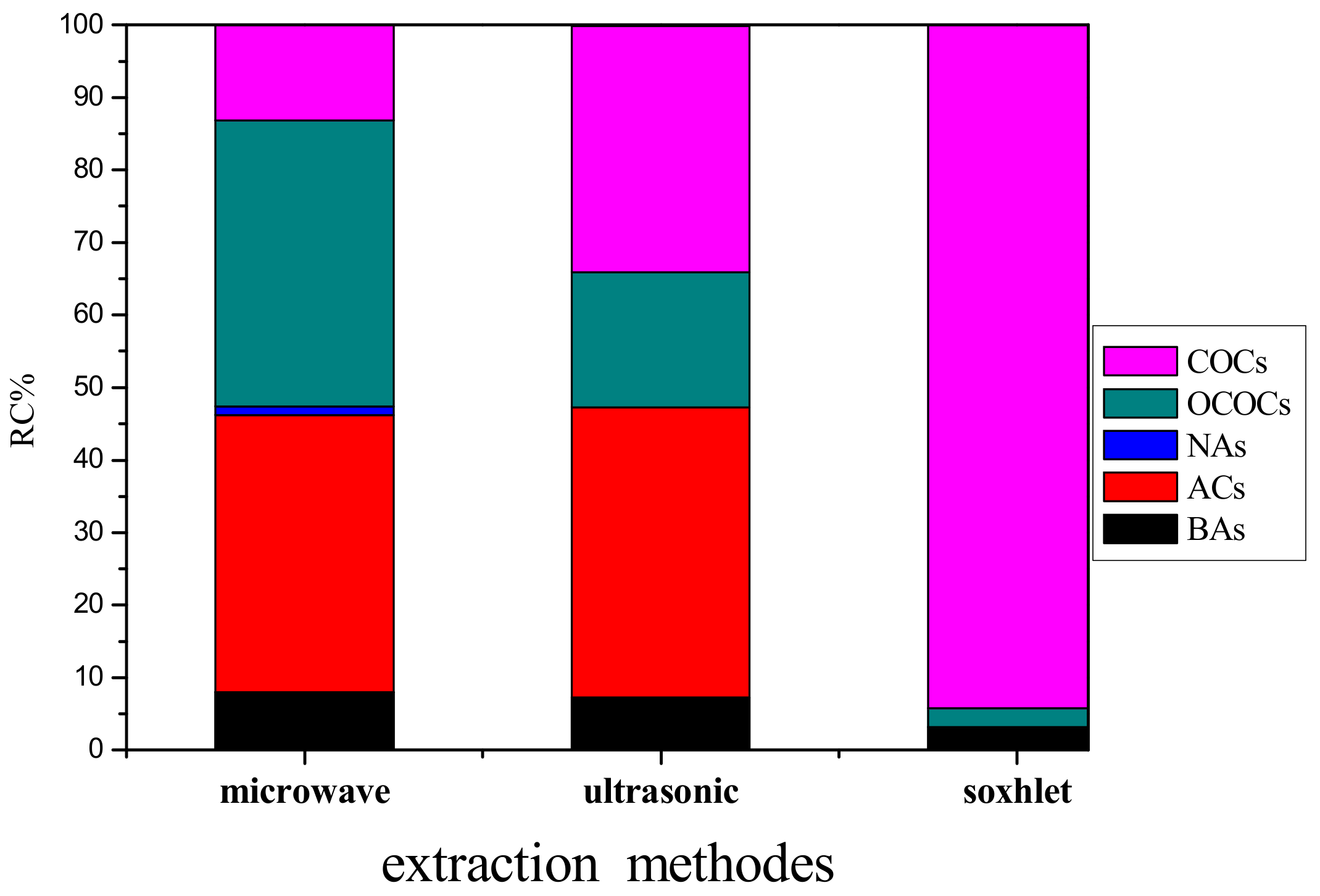
| Number of Organic Species (Abbreviation) and Relative Content in % | Extraction Method | ||
|---|---|---|---|
| SE | MAE | UAE | |
| n-Alkanes (NAs) | 0 | 1 (1.19) | 0 |
| Branched paraffin (BAs) | 1 (3.20) | 5 (7.99) | 3 (7.22) |
| Chlorinated organic compounds (COCs) | 8 (94.21) | 3 (13.12) | 5 (33.97) |
| Oxygen-containing organic compounds (OCOCs) | 1 (2.59) | 53 (39.50) | 13 (18.72) |
| Aromatic compounds (ACs) | 0 | 13 (38.19) | 16 (39.97) |
| Types of organic compounds | 10 | 75 | 37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jv, C.; Li, F.; Yan, P.; Fan, M.; Jia, L.; Zhang, H.; Wei, X.; Zong, Z. Analysis of Carbon Tetrachloride-Extractable Species from Daxing Bituminous Coal. Appl. Sci. 2020, 10, 494. https://doi.org/10.3390/app10020494
Jv C, Li F, Yan P, Fan M, Jia L, Zhang H, Wei X, Zong Z. Analysis of Carbon Tetrachloride-Extractable Species from Daxing Bituminous Coal. Applied Sciences. 2020; 10(2):494. https://doi.org/10.3390/app10020494
Chicago/Turabian StyleJv, Caixia, Fenggang Li, Peng Yan, Mengmeng Fan, Linlin Jia, Hong Zhang, Xianyong Wei, and Zhimin Zong. 2020. "Analysis of Carbon Tetrachloride-Extractable Species from Daxing Bituminous Coal" Applied Sciences 10, no. 2: 494. https://doi.org/10.3390/app10020494




