Evaluation of Non-Conventional Biological and Molecular Parameters as Potential Indicators of Quality and Functionality of Urban Biosolids Used as Organic Amendments of Agricultural Soils
Abstract
:1. Introduction
2. Materials and Methods
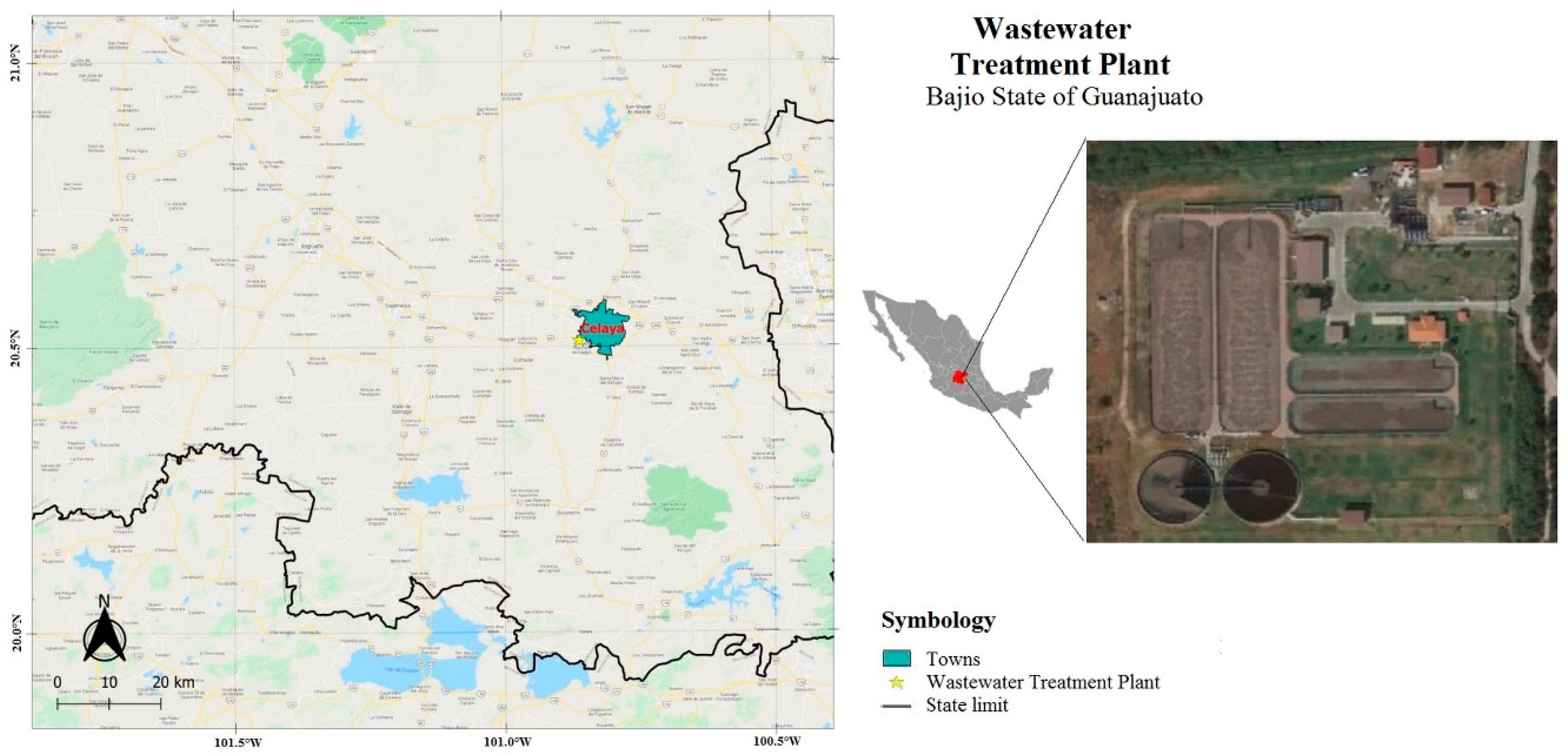
2.1. Production, Sampling and Status of Biosolids
2.2. Physicochemical Characterisation of Biosolids and Obtaining FTIR Spectra
2.3. Biological Characterisation and Enzymatic Profile of Biosolids
2.4. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterisation of Biosolids
3.2. Characteristics of the FTIR Spectrum of Biosolids
3.3. Biological Characterisation of Biosolids
3.4. Extracellular Enzyme Profiles per API ZYM® Kit for Biosolids
3.5. Relationship between Biological and Physicochemical Parameters and Nutrient Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Melo, J.J.; Camara, A.S. Models for the optimization of regional wastewater treatment systems. Eur. J. Oper. Res. 1994, 73, 1–16. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, W.; Gong, Y.; Yu, Q.; Li, Q.; Sun, J.; Yuan, Z. Technologies for reducing sludge production in wastewater treatment plants: State of the art. Sci. Total Environ. 2017, 587, 510–521. [Google Scholar] [CrossRef]
- Rodríguez, N.H.; Granados, R.J.; Blanco-Varela, M.T.; Cortina, J.L.; Martínez-Ramírez, S.; Marsal, M.; Guillem, M.; Puig, J.; Fos, C.; Larrotcha, E.; et al. Evaluation of a lime-mediated sewage sludge stabilisation process. Product characterisation and technological validation for its use in the cement industry. Waste Manag. 2012, 32, 550–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Van Houten, R.T.; Borger, A.R.; Eikelboom, D.H.; Fan, Y. Minimization of excess sludge production for biological wastewater treatment. Water Res. 2003, 37, 4453–4467. [Google Scholar] [CrossRef]
- Reungoat, J.; Escher, B.I.; Macova, M.; Argaud, F.X.; Gernjak, W.; Keller, J. Ozonation and biological activated carbon filtration of wastewater treatment plant effluents. Water Res. 2012, 46, 863–872. [Google Scholar] [CrossRef]
- Pronk, M.; De Kreuk, M.K.; De Bruin, B.; Kamminga, P.; Kleerebezem, R.V.; Van Loosdrecht, M.C.M. Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res. 2015, 84, 207–217. [Google Scholar] [CrossRef]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Samaras, P.; Papadimitriou, C.A.; Haritou, I.; Zouboulis, A.I. Investigation of sewage sludge stabilization potential by the addition of fly ash and lime. J. Hazard. Mater. 2008, 154, 1052–1059. [Google Scholar] [CrossRef]
- Kazimierczak, M. Sewage sludge stabilization indicators in aerobic digestion–a review. Annals of Warsaw University of Life Sciences-SGGW. Land Reclam. 2012, 44, 101–109. [Google Scholar] [CrossRef]
- Conagua. Inventario Nacional de Plantas Municipales de Potabilización y de Tratamiento de Aguas Residuales en Operación; Comisión Nacional del Agua/Secretaría de Medio Ambiente y Recursos Naturales: Ciudad de México, México, 2016; 308p, Available online: https://agua.org.mx/biblioteca/inventario-plantas-potabilizadoras-municipales-2016/ (accessed on 14 November 2019).
- Matos, A.T.; Diniz, I.C.; Matos, M.P.; Borges, A.C.; Pereira, A.A. Degradation rate of anaerobically digested sewage sludge in soil. J. Water Sanit. Hyg. Dev. 2018, 8, 17–26. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.J.; Chang, Y.; Lee, Y.J. Sludge treatment: Current research trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Paramashivam, D.; Dickinson, N.M.; Clough, T.J.; Horswell, J.; Robinson, B.H. Potential environmental benefits from blending biosolids with other organic amendments before application to land. J. Environ. Qual. 2017, 46, 481–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Brown, S.L.; Magesan, G.N.; Slade, A.H.; Quintern, M.; Clinton, P.W.; Payn, T.W. Technological options for the management of biosolids. Environ. Sci. Pollut. Res. 2008, 15, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, F.; Bhogal, A.; Taylor, M.; McGrath, S.; Withers, P. Long-term Effects of Biosolids on Soil Quality and Fertility. Soil Sci. 2018, 183, 89–98. [Google Scholar] [CrossRef]
- Obrador, A.; Rico, M.I.; Mingot, J.I.; Alvarez, J.M. Metal mobility and potential bioavailability in organic matter-rich soil-sludge mixtures: Effect of soil type and contact time. Sci. Total Environ. 1997, 206, 117–126. [Google Scholar] [CrossRef]
- Alvarez, E.A.; Mochon, M.C.; Sánchez, J.J.; Rodríguez, M.T. Heavy metal extractable forms in sludge from wastewater treatment plants. Chemosphere 2002, 47, 765–775. [Google Scholar] [CrossRef]
- Usman, K.; Khan, S.; Ghulam, S.; Khan, M.U.; Khan, N.; Khan, M.A.; Khalil, S.K. Sewage sludge: An important biological resource for sustainable agriculture and its environmental implications. Am. J. Plant Sci. 2012, 3, 1708. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Sarkar, A.; Singh, P.; Singh, R.P. Agricultural utilization of biosolids: A review on potential effects on soil and plant grown. Waste Manag. 2017, 64, 117–132. [Google Scholar] [CrossRef]
- Pepper, I.L.; Brooks, J.P.; Gerba, C.P. Land Application of Organic Residuals: Municipal Biosolids and Animal Manures. In Environmental and Pollution Science, 3rd ed.; Brusseau, M.L., Pepper, I.L., Gerba, C.P., Eds.; Academic Press, Elsevier: London, UK, 2019; Part III; pp. 419–434. [Google Scholar] [CrossRef]
- Secretaría del Medio Ambiente y Recursos Naturales (Semarnat). Norma Oficial Mexicana NOM-004-SEMARNAT-2002. Protección Ambiental. Lodos y Biosólidos. Especificaciones y Límites Máximos Permisibles de Contaminantes Para su Aprovechamiento y Disposición Final. 2002. Diario Oficial de la Federación, México. 15 August 2003. Available online: http://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/libros2009/DO2251.pdf (accessed on 8 January 2020).
- Environmental Protection Agency (EPA). Federal Register. Title 40—Protection of Environment: Part 503—Standards for the Use or Disposal of Sewage Sludge; Environmental Protection Agency: Washington, DC, USA, 2002.
- European Economic Community (ECC). Directiva 86/278/CEE de 12 de junio de 1986, relativa a la protección del medio ambiente y, en particular, de los suelos, en la utilización de lodos de depuradora en agricultura (86/278/CEE). Diario Oficial de las Comunidades Europeas. 1986. Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/PDF/?uri=CELEX:31986L0278&from=ES (accessed on 8 January 2020).
- Anderson, J.P.E.; Domsch, K.H. Quantities of plant nutrients in the microbial biomass of selected soils. Soil Sci. 1980, 130, 211–216. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Sparling, G.P.; West, A.W. Modifications to the fumigation extraction technique to permit simultaneous extraction and estimation of soil microbial C and N. Commun Soil Sci Plant Anal. 1988, 19, 327–344. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Application of eco-physiological quotients (qCO2 and qD) on microbial biomasses from soils of different cropping histories. Soil Biol. Biochem. 1990, 22, 251–255. [Google Scholar] [CrossRef]
- Von Mersi, W.; Schinner, F. An improved and accurate method for determining the dehydrogenase activity of soils with iodonitrotetrazolium chloride. Biol. Fertil. Soils 1991, 11, 216–220. [Google Scholar] [CrossRef]
- Johnston, C.T.; Aochi, Y. Fourier transform infrared and raman spectroscopy. In Methods of Soil Analysis; Bartels, J.M., Bigham, J.M., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 269–321. [Google Scholar] [CrossRef]
- Perucci, P.; Dumontet, S.; Bufo, S.A.; Mazzatura, A.; Casucci, C. Effects of organic amendment and herbicide treatment on soil microbial biomass. Biol. Fertil. Soils 2000, 32, 17–23. [Google Scholar] [CrossRef]
- Dumontet, S.; Mazzatura, A.; Casucci, C.; Perucci, P. Effectiveness of microbial indexes in discriminating interactive effects of tillage and crop rotations in a Vertic Ustorthens. Biol. Fertil. Soils 2001, 34, 411–416. [Google Scholar] [CrossRef]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Martínez, E.J.; Fierro, J.; Sánchez, M.E.; Gómez, X. Anaerobic co-digestion of FOG and sewage sludge: Study of the process by Fourier transform infrared spectroscopy. Int. Biodeterior. Biodegrad. 2012, 75, 1–6. [Google Scholar] [CrossRef]
- Tiquia, S.M. Evolution of extracellular enzyme activities during manure composting. J. Appl. Microbiol. 2002, 92, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Baldrian, P.; Voříšková, J.; Dobiášová, P.; Merhautová, V.; Lisá, L.; Valášková, V. Production of extracellular enzymes and degradation of biopolymers by saprotrophic microfungi from the upper layers of forest soil. Plant Soil 2011, 338, 111–125. [Google Scholar] [CrossRef]
- Boluda, R.; Roca-Pérez, L.; Iranzo, M.; Gil, C.; Mormeneo, S. Determination of enzymatic activities using a miniaturized system as a rapid method to assess soil quality. Eur. J. Soil Sci. 2014, 65, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Shemekite, F.; Gómez-Brandón, M.; Franke-Whittle, I.H.; Praehauser, B.; Insam, H.; Assefa, F. Coffee husk composting: An investigation of the process using molecular and non-molecular tools. Waste Manag. 2014, 34, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Martinez, D.; Molina, M.J.; Sanchez, J.; Moscatelli, M.C.; Marinari, S. API ZYM assay to evaluate enzyme fingerprinting and microbial functional diversity in relation to soil processes. Biol. Fertil. Soils 2016, 52, 77–89. [Google Scholar] [CrossRef]
- Patel, D.; Gismondi, R.; Alsaffar, A.; Tiquia-Arashiro, S.M. Applicability of API ZYM to capture seasonal and spatial variabilities in lake and river sediments. Environ. Technol. 2019, 40, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Wolińska, A.; Frąc, M.; Oszust, K.; Szafranek-Nakonieczna, A.; Zielenkiewicz, U.L.; Stępniewska, Z. Microbial biodiversity of meadows under different modes of land use: Catabolic and genetic fingerprinting. World J. Microbiol. Biotechnol. 2017, 33, 154. [Google Scholar] [CrossRef] [Green Version]
- Environmental Protection Agency (EPA). A Guide to the Biosolids Risk Assessments for the EPA Part 503 Rule; Tech. Rep. EPA/832-B-93-005; Office of Wastewater Management: Washington, DC, USA, 1995.
- Lu, Q.; He, Z.L.; Stoffella, P.J. Land application of biosolids in the USA: A review. Appl. Environ. Soil Sci. 2012, 2012, 201462. [Google Scholar] [CrossRef] [Green Version]
- Coello-Oviedo, M.C.; Barragán-Sánchez, J.B.; Quiroga-Alonso, J.Q. Enzymatic estimation of biosolids stability in aerobic digestion systems. Enzyme Microb. Technol. 2005, 36, 191–197. [Google Scholar] [CrossRef]
- Boczar, B.A.; Begley, W.M.; Larson, R.J. Characterization of enzyme activity in activated sludge using rapid analyses for specific hydrolases. Water Environ. Res. 1992, 64, 792–797. [Google Scholar] [CrossRef]
- Ho, C.P.; Yuan, S.T.; Jien, S.H.; Hseu, Z.Y. Elucidating the process of co-composting of biosolids and spent activated clay. Bioresour. Technol. 2010, 101, 8280–8286. [Google Scholar] [CrossRef]
- Tian, G.; Franzluebbers, A.J.; Granato, T.C.; Cox, A.E.; O’connor, C. Stability of soil organic matter under long-term biosolids application. Appl. Soil Ecol. 2013, 64, 223–227. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and Soil Acidity. In Methods of Soil Analysis Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar] [CrossRef] [Green Version]
- Hendrickx, J.M.H.; Das, B.; Corwin, D.L.; Wraith, J.M.; Kachanoski, R.G. Relationship between soil water solute concentration and apparent soil electrical conductivity. In Methods of Soil Analysis: Part 4. Physical Methods; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 1275–1282. [Google Scholar] [CrossRef]
- Walkley, A.; Black, C.A. An examination of different methods for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995; pp. 60–61. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen—Total. In Methods of Soil Analysis Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar] [CrossRef]
- US-EPA Method 3051A. Microwave Assisted Acid Digestion of Sediment, Sludges, Soils, and Oils; United States Environmental Protection Agency: Washington, DC, USA, 2007. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf (accessed on 14 November 2019).
- Link, D.D.; Walter, P.J.; Kingston, H.M. Development and validation of the new EPA microwave-assisted leach method 3051A. Environ. Sci. Technol. 1998, 32, 3628–3632. [Google Scholar] [CrossRef]
- Bettinelli, M.; Baroni, U. A microwave oven digestion method for the determination of metals in sewage sludges by ICP-AES and GFAAS. Int. J. Environ. Anal. Chem. 1991, 43, 33–40. [Google Scholar] [CrossRef]
- Sparling, G.P.; Williams, B.L. Microbial biomass in organic soils: Estimation of biomass C, and effect of glucose or cellulose amendments on the amounts of N and P released by fumigation. Soil Biol. Biochem. 1986, 18, 507–513. [Google Scholar] [CrossRef]
- Barragán-Sánchez, J.; Quiroga-Alonso, J.M.; Coello-Oviedo, M.D. Use of microbial activity parameters for determination of a biosolid stability index. Bioresour. Technol. 2006, 97, 562–568. [Google Scholar] [CrossRef]
- Anderson, J.P. Soil respiration. In Methods of Soil Analysis Part 2—Chemical and Microbiological Properties; Page, L., Ed.; Soil Science Society of America: Madison, WI, USA, 1982; pp. 831–871. [Google Scholar] [CrossRef]
- API ZYM 25200. Sistema de investigación de actividades enzimáticas; 07883F-es-2014/01; bioMérieux SA: Marcy-l’Étoile, France, 2014; p. 5. [Google Scholar]
- Kennedy, A.C.; Smith, K.L. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 1995, 170, 75–86. [Google Scholar] [CrossRef]
- Minitab, Inc. Minitab Version 18; Minitab, Inc.: State College, PA, USA, 2018. [Google Scholar]
- Withers, P.J.A.; Flynn, N.J.; Warren, G.P.; Taylor, M.; Chambers, B.J. Sustainable management of biosolid phosphorus: A field study. Soil Use Manag. 2016, 32, 54–63. [Google Scholar] [CrossRef]
- Torri, S.I.; Correa, R.S.; Renella, G. Biosolid application to agricultural land—A contribution to global phosphorus recycle: A review. Pedosphere 2017, 27, 1–16. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Mondini, C.; De Nobili, M.; Leita, L.; Roig, A. Land application of biosolids. Soil response to different stabilization degree of the treated organic matter. Waste Manag. 2004, 24, 325–332. [Google Scholar] [CrossRef]
- Torres, D.; Mendoza, B.; Meru Marco, L.; Gómez, C. Riesgos de salinización y sodificación por el uso de abonos orgánicos en la depresión de Quíbor-Venezuela. Multiciencias 2016, 16, 133–142. Available online: https://www.redalyc.org/articulo.oa?id=90452745003 (accessed on 15 July 2019).
- Cytryn, E.; Kautsky, L.; Ofek, M.; Mandelbaum, R.T.; Minz, D. Short-term structure and functional changes in bacterial community composition following amendment with biosolids compost. Appl. Soil Ecol. 2011, 48, 160–167. [Google Scholar] [CrossRef]
- Sciubba, L.; Cavani, L.; Negroni, A.; Zanaroli, G.; Fava, F.; Ciavatta, C.; Marzadori, C. Changes in the functional properties of a sandy loam soil amended with biosolids at different application rates. Geoderma 2014, 221, 40–49. [Google Scholar] [CrossRef]
- Flores-Margez, J.P.; Corral-Díaz, B.; Sapien-Mediano, G. Mineralización de nitrógeno de biosólidos estabilizados con cal en suelos agrícolas. Terra Latinoam. 2007, 25, 409–417. Available online: http://portal.amelica.org/ameli/jatsRepo/57315558009 (accessed on 8 January 2020).
- Potisek-Talavera, M.D.C.; Figueroa-Viramontes, U.; González-Cervantes, G.; Jasso-Ibarra, R.; Orona-Castillo, I. Aplicación de biosólidos al suelo y su efecto sobre contenido de materia orgánica y nutrimentos. Terra Latinoam. 2010, 28, 327–333. Available online: http://www.scielo.org.mx/pdf/tl/v28n4/v28n4a4.pdf (accessed on 8 January 2020).
- González-Flores, E.; Ramos-Barragán, J.E.; Tornero-Campante, M.A.; Murillo-Murillo, M. Evaluación de dosis de biosólidos urbanos en maíz bajo condiciones de invernadero. Rev. Mex. Cienc. Agríc. 2017, 8, 119–132. Available online: http://www.scielo.org.mx/pdf/remexca/v8n1/2007-0934-remexca-8-01-119-en.pdf (accessed on 20 December 2019).
- Singh, R.P.; Agrawal, M. Effect of different sewage sludge applications on growth and yield of Vigna radiata L. field crop: Metal uptake by plant. Ecol. Eng. 2010, 36, 969–972. [Google Scholar] [CrossRef]
- Xue, D.; Huang, X. The impact of sewage sludge compost on tree peony growth and soil microbiological, and biochemical properties. Chemosphere 2013, 93, 583–589. [Google Scholar] [CrossRef]
- Lloret, E.; Pascual, J.A.; Brodie, E.L.; Bouskill, N.J.; Insam, H.; Juárez, M.F.D.; Goberna, M. Sewage sludge addition modifies soil microbial communities and plant performance depending on the sludge stabilization process. Appl. Soil Ecol. 2016, 101, 37–46. [Google Scholar] [CrossRef] [Green Version]
- United States Salinity Laboratory Staff (USSLS). Diagnosis and Improvement of Saline and Alkali Soils; United States Department of Agriculture and Government Printing Office: Washington, DC, USA, 1954. Available online: https://www.ars.usda.gov/ARSUserFiles/20360500/hb60_pdf/hb60complete.pdf (accessed on 14 November 2019).
- Pérez-Sanz, A.; Lucena, J.J.; Graham, M.C. Characterization of Fe–humic complexes in an Fe-enriched biosolid by-product of water treatment. Chemosphere 2006, 65, 2045–2053. [Google Scholar] [CrossRef]
- Rowell, D.M.; Prescott, C.E.; Preston, C.M. Decomposition and nitrogen mineralization from biosolids and other organic materials. J. Environ. Qual. 2001, 30, 1401–1410. [Google Scholar] [CrossRef]
- Gilmour, J.T.; Cogger, C.G.; Jacobs, L.W.; Evanylo, G.K.; Sullivan, D.M. Decomposition and plant-available nitrogen in biosolids. J. Environ. Qual. 2003, 32, 1498–1507. [Google Scholar] [CrossRef]
- Rigby, H.; Clarke, B.O.; Pritchard, D.L.; Meehan, B.; Beshah, F.; Smith, S.R.; Porter, N.A. A critical review of nitrogen mineralization in biosolids-amended soil, the associated fertilizer value for crop production and potential for emissions to the environment. Sci. Total Environ. 2016, 541, 1310–1338. [Google Scholar] [CrossRef] [PubMed]
- Jin, V.L.; Johnson, M.V.V.; Haney, R.L.; Arnold, J.G. Potential carbon and nitrogen mineralization in soils from a perennial forage production system amended with class B biosolids. Agric. Ecosyst. Environ. 2011, 141, 461–465. [Google Scholar] [CrossRef]
- Hseu, Z.Y.; Huang, C.C. Nitrogen mineralization potentials in three tropical soils treated with biosolids. Chemosphere 2005, 59, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2008; pp. 100–109. [Google Scholar]
- Lü, F.; Shao, L.M.; Zhang, H.; Fu, W.D.; Feng, S.J.; Zhan, L.T.; Chen, Y.M.; He, P.J. Application of advanced techniques for the assessment of bio-stability of biowaste-derived residues: A minireview. Bioresour. Technol. 2018, 248, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.M.; Cogger, C.G.; Bary, A.I. Fertilising with Biosolids; A Pacific North West Extension Publication, Oregon State University: Corvallis, OR, USA; Washington State University: Pullman, WA, USA; University of Idaho: Moscow, ID, USA, 2015; Available online: https://catalog.extension.oregonstate.edu/sites/catalog/files/project/pdf/pnw508_0.pdf (accessed on 17 December 2019).
- Sigua, G.C. Recycling biosolids and lake-dredged materials to pasture-based animal agriculture: Alternative nutrient sources for forage productivity and sustainability. A review. Agron. Sustain. Dev. 2009, 29, 143–160. [Google Scholar] [CrossRef]
- Zhang, M.; Heaney, D.; Henriquez, B.; Solberg, E.; Bittner, E. A four-year study on influence of biosolids/MSW cocompost application in less productive soils in Alberta: Nutrient dynamics. Compost Sci. Util. 2006, 14, 68–80. [Google Scholar] [CrossRef]
- Kokkora, M.I.; Antille, D.L.; Tyrrel, S.F. Considerations for recycling of compost and biosolids in agricultural soil. In Soil Engineering; Dedousis, A.P., Bartzanas, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 20, pp. 195–215. [Google Scholar] [CrossRef]
- Bedoya-Urrego, K.; Acevedo-Ruíz, J.M.; Peláez-Jaramillo, C.A.; Agudelo-López, S.D.P. Caracterización de biosólidos generados en la planta de tratamiento de agua residual San Fernando, Itagüí (Antioquia, Colombia). Rev. Salud Pública 2013, 15, 778–790. Available online: https://www.scielosp.org/scielo.php?script=sci_arttext&pid=S0124-00642013000500013 (accessed on 20 April 2019).
- Dede, G.; Özdemir, S.; Dede, Ö.H.; Altundağ, H.; Dündar, M.Ş.; Kızıloğlu, F.T. Effects of biosolid application on soil properties and kiwi fruit nutrient composition on high-pH soil. Int. J. Environ. Sci. Technol. 2017, 14, 1451–1458. [Google Scholar] [CrossRef]
- Burducea, M.; Zheljazkov, V.D.; Lobiuc, A.; Pintilie, C.A.; Virgolici, M.; Silion, M.; Asandulesa, M.; Burducea, I.; Zamfirache, M.M. Biosolids application improves mineral composition and phenolic profile of basil cultivated on eroded soil. Sci. Hortic. 2019, 249, 407–418. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Gong, X.; Li, Y.; Wu, C.; Ren, N. Spectral study of dissolved organic matter in biosolid during the composting process using inorganic bulking agent: UV–vis, GPC, FTIR and EEM. Int. Biodeterior. Biodegrad. 2013, 85, 617–623. [Google Scholar] [CrossRef]
- Zhou, Y.; Selvam, A.; Wong, J.W. Evaluation of humic substances during co-composting of food waste, sawdust and Chinese medicinal herbal residues. Bioresour. Technol. 2014, 168, 229–234. [Google Scholar] [CrossRef]
- Zhang, J.; Lü, F.; Shao, L.; He, P. The use of biochar-amended composting to improve the humification and degradation of sewage sludge. Bioresour. Technol. 2014, 168, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Soobhany, N.; Gunasee, S.; Rago, Y.P.; Joyram, H.; Raghoo, P.; Mohee, R.; Garg, V.K. Spectroscopic, thermogravimetric and structural characterization analyses for comparing Municipal Solid Waste composts and vermicomposts stability and maturity. Bioresour. Technol. 2017, 236, 11–19. [Google Scholar] [CrossRef]
- El Fels, L.; Zamama, M.; El Asli, A.; Hafidi, M. Assessment of biotransformation of organic matter during co-composting of sewage sludge-lignocelullosic waste by chemical, FTIR analyses, and phytotoxicity tests. Int. Biodeterior. Biodegrad. 2014, 87, 128–137. [Google Scholar] [CrossRef]
- Tandy, S.; Healey, J.R.; Nason, M.A.; Williamson, J.C.; Jones, D.L.; Thain, S.C. FT-IR as an alternative method for measuring chemical properties during composting. Bioresour. Technol. 2010, 101, 5431–5436. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, C.; Severcan, F. Role of vibrational spectroscopy in stem cell research. J. Spectrosc. 2012, 27, 167–184. [Google Scholar] [CrossRef]
- Droussi, Z.; D’orazio, V.; Provenzano, M.R.; Hafidi, M.; Ouatmane, A. Study of the biodegradation and transformation of olive-mill residues during composting using FTIR spectroscopy and differential scanning calorimetry. J. Hazard. Mater. 2009, 164, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, present and future of soil quality indices: A biological perspective. Geoderma 2008, 147, 159–171. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S. Biological indices for soil quality evaluation: Perspectives and limitations. Land Degrad. Dev. 2016, 27, 14–25. [Google Scholar] [CrossRef]
- Joniec, J. Enzymatic activity as an indicator of regeneration processes in degraded soil reclaimed with various types of waste. Int. J. Environ. Sci. Technol. 2018, 15, 2241–2252. [Google Scholar] [CrossRef] [Green Version]
- Wardle, D.A. A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol. Rev. 1992, 67, 321–358. [Google Scholar] [CrossRef]
- Rinklebe, J.; Langer, U. Relationship between soil microbial biomass determined by SIR and PLFA analysis in floodplain soils. J. Soil Sediments 2010, 10, 4–8. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Soil microbial biomass: The eco-physiological approach. Soil Biol. Biochem. 2010, 42, 2039–2043. [Google Scholar] [CrossRef]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; McGraw-Hill Series in Water Resources and Environmental Engineering; McGraw-Hill: Boston, MA, USA, 2001. [Google Scholar]
- Nayak, B.S.; Levine, A.D.; Cardoso, A.; Harwood, V.J. Microbial population dynamics in laboratory-scale solid waste bioreactors in the presence or absence of biosolids. J. Appl. Microbiol. 2009, 107, 1330–1339. [Google Scholar] [CrossRef]
- Sciubba, L.; Cavani, L.; Marzadori, C.; Ciavatta, C. Effect of biosolids from municipal sewage sludge composted with rice husk on soil functionality. Biol. Fertil. Soils 2013, 49, 597–608. [Google Scholar] [CrossRef]
- Moore, J.M.; Klose, S.; Tabatabai, M.A. Soil microbial biomass carbon and nitrogen as affected by cropping systems. Biol. Fertil. Soils 2000, 31, 200–210. [Google Scholar] [CrossRef]
- Li, F.M.; Song, Q.H.; Jjemba, P.K.; Shi, Y.C. Dynamics of soil microbial biomass C and soil fertility in cropland mulched with plastic film in a semiarid agro-ecosystem. Soil Biol. Biochem. 2004, 36, 1893–1902. [Google Scholar] [CrossRef]
- Fernandes, S.A.P.; Bettiol, W.; Cerri, C.C. Effect of sewage sludge on microbial biomass, basal respiration, metabolic quotient and soil enzymatic activity. Appl. Soil Ecol. 2005, 30, 65–77. [Google Scholar] [CrossRef]
- Debosz, K.; Petersen, S.O.; Kure, L.K.; Ambus, P. Evaluating effects of sewage sludge and household compost on soil physical, chemical and microbiological properties. Appl. Soil Ecol. 2002, 19, 237–248. [Google Scholar] [CrossRef]
- Masunga, R.H.; Uzokwe, V.N.; Mlay, P.D.; Odeh, I.; Singh, A.; Buchan, D.; De Neve, S. Nitrogen mineralization dynamics of different valuable organic amendments commonly used in agriculture. Appl. Soil Ecol. 2016, 101, 185–193. [Google Scholar] [CrossRef]
- Molina-Herrera, S.; Romanyà, J. Synergistic and antagonistic interactions among organic amendments of contrasted stability, nutrient availability and soil organic matter in the regulation of C mineralisation. Eur. J. Soil Biol. 2015, 70, 118–125. [Google Scholar] [CrossRef]
- Servicio Meteorológico Nacional (SMN). Resúmenes Mensuales de Temperaturas y Lluvia; Servicio Meteorológico Nacional: Ciudad de México, México, 2019. Available online: https://smn.conagua.gob.mx (accessed on 18 August 2019).
- Sautour, M.; Dantigny, P.; Divies, C.; Bensoussan, M. A temperature-type model for describing the relationship between fungal growth and water activity. Int. J. Food Microbiol. 2001, 67, 63–69. [Google Scholar] [CrossRef]
- Ratkowsky, D.A.; Lowry, R.K.; McMeekin, T.A.; Stokes, A.N.; Chandler, R.E. Model for bacterial culture growth rate throughout the entire biokinetic temperature range. J. Bacteriol. 1983, 154, 1222–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Wolińska, A.; Bennicelli, R.P. Dehydrogenase activity response to soil reoxidation process described as varied conditions of water potential, air porosity and oxygen availability. Pol. J. Environ. Stud. 2010, 19, 651–657. [Google Scholar]
- Quilchano, C.; Marañón, T. Dehydrogenase activity in Mediterranean forest soils. Biol. Fertil. Soils 2002, 35, 102–107. [Google Scholar] [CrossRef]
- Kumar, S.; Chaudhuri, S.; Maiti, S.K. Soil dehydrogenase enzyme activity in natural and mine soil—A review. Middle East J. Sci. Res. 2013, 13, 898–906. [Google Scholar] [CrossRef]
- Wolińska, A.; Rekosz-Burlaga, H.; Goryluk-Salmonowicz, A.; Błaszczyk, M.; Stępniewska, Z. Bacterial Abundance and Dehydrogenase Activity in Selected Agricultural Soils from Lublin Region. Pol. J. Environ. Stud. 2015, 24, 2677–2682. [Google Scholar] [CrossRef]
- Pascual, J.A.; Garcia, C.; Hernandez, T.; Moreno, J.L.; Ros, M. Soil microbial activity as a biomarker of degradation and remediation processes. Soil Biol. Biochem. 2000, 32, 1877–1883. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gascó, G.; Gutiérrez, B.; Méndez, A. Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol. Fertil. Soils 2012, 48, 511–517. [Google Scholar] [CrossRef]
- Roig, N.; Sierra, J.; Martí, E.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Long-term amendment of Spanish soils with sewage sludge: Effects on soil functioning. Agric. Ecosyst. Environ. 2012, 158, 41–48. [Google Scholar] [CrossRef]
- Wijesekara, H.; Bolan, N.S.; Thangavel, R.; Seshadri, B.; Surapaneni, A.; Saint, C.; Hetherington, C.; Matthews, P.; Vithanage, M. The impact of biosolids application on organic carbon and carbon dioxide fluxes in soil. Chemosphere 2017, 189, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Gajda, A.M.; Przewłoka, B.; Gawryjołek, K. Changes in soil quality associated with tillage system applied. Int. Agrophys. 2013, 27, 133–141. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Attinà, E. Early warning indicators of changes in soil ecosystem functioning. Ecol. Ind. 2015, 48, 542–549. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Mondini, C.; Cayuela, M.L.; Roig, A.; Contin, M.; De Nobili, M. Fluorescein diacetate hydrolysis, respiration and microbial biomass in freshly amended soils. Biol. Fertil. Soils 2008, 44, 885–890. [Google Scholar] [CrossRef]
- Carlson, J.; Saxena, J.; Basta, N.; Hundal, L.; Busalacchi, D.; Dick, R.P. Application of organic amendments to restore degraded soil: Effects on soil microbial properties. Environ. Monit. Assess. 2015, 187, 109. [Google Scholar] [CrossRef] [PubMed]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. In Sustainable Agriculture; Lichtfouse, E., Hamelin, M., Navarrete, M., Debaeke, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 2, pp. 761–786. [Google Scholar] [CrossRef]
- Kızılkaya, R.; Bayraklı, B. Effects of N-enriched sewage sludge on soil enzyme activities. Appl. Soil Ecol. 2005, 30, 192–202. [Google Scholar] [CrossRef]
- Yakushev, A.V.; Blagodatsky, S.A.; Byzov, B.A. The effect of earthworms on the physiological state of the microbial community at vermicomposting. Microbiology 2009, 78, 510–519. [Google Scholar] [CrossRef]
- Tian, G.; Chiu, C.Y.; Franzluebbers, A.J.; Oladeji, O.O.; Granato, T.C.; Cox, A.E. Biosolids amendment dramatically increases sequestration of crop residue-carbon in agricultural soils in western Illinois. Appl. Soil Ecol. 2015, 85, 86–93. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Dumontet, S.; Mazzatura, A.; Pasquale, V. Toxic effects of four sulphonylureas herbicides on soil microbial biomass. J. Environ. Sci. Health Part B 2012, 47, 653–659. [Google Scholar] [CrossRef]
- Seal, A.; Datta, A.; Saha, S.; Chatterjee, A.K.; Barik, A.K.; Bhattacharyya, S.; Levin, Y.; Nain, A.S.; Asthana, A.; Bera, R. Soil Microbial Rejuvenation through Soil Resource Recycling as a part of Sustainable Management Programme: A Case Study from Lakhipara Tea Estate, Dooars, West Bengal, India. J. Agric. Sci. Technol. 2016, 5, 18–34. Available online: http://orgprints.org/31300/1/1248-4636-1-PB%20%28Lakhipara%20Compost%29.pdf (accessed on 20 December 2019).
- Tian, L.; Dell, E.; Shi, W. Chemical composition of dissolved organic matter in agroecosystems: Correlations with soil enzyme activity and carbon and nitrogen mineralization. Appl. Soil Ecol. 2010, 46, 426–435. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Billings, S.A. Indirect effects of nitrogen amendments on organic substrate quality increase enzymatic activity driving decomposition in a mesic grassland. Ecosystems 2011, 14, 234–247. [Google Scholar] [CrossRef]
- Zhao, S.; Li, K.; Zhou, W.; Qiu, S.; Huang, S.; He, P. Changes in soil microbial community, enzyme activities and organic matter fractions under long-term straw return in north-central China. Agric. Ecosyst. Environ. 2016, 216, 82–88. [Google Scholar] [CrossRef]
- Bending, G.D.; Turner, M.K.; Jones, J.E. Interactions between crop residue and soil organic matter quality and the functional diversity of soil microbial communities. Soil Biol. Biochem. 2002, 34, 1073–1082. [Google Scholar] [CrossRef]
- Kemmitt, S.J.; Lanyon, C.V.; Waite, I.S.; Wen, Q.; Addiscott, T.M.; Bird, N.R.; O’Donnell, A.G.; Brookes, P.C. Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass—A new perspective. Soil Biol. Biochem. 2008, 40, 61–73. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Wang, X.; Song, D.; Liang, G.; Zhang, Q.; Ai, C.; Zhou, W. Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Appl. Soil Ecol. 2015, 96, 265–272. [Google Scholar] [CrossRef]
- Yeshitela, K. Effects of Anthropogenic Disturbance on the Diversity of Foliicolous Lichens in Tropical Rainforests of East Africa: Godere (Ethiopia), Budongo (Uganda) and Kakamega (Kenya). Ph.D. Thesis, Universität Koblenz-Landau, Mainz, Germany, 2008; pp. 13–14. [Google Scholar]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Stone, M.M.; DeForest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]




| Parameter | Value * | MPL of Contaminants in Biosolids | ||
|---|---|---|---|---|
| Mexico | EE. UU. | E.U. | ||
| Fecal coliforms (MPN g−1) | 210,000 | <2,000,000 | <2,000,000 | N.R. |
| Salmonella sp. (MPN g−1) | <3 | <300 | <4 | N.R. |
| Helminth Eggs (eggs g−1) | <1 | <35 | <1 | N.R. |
| As (mg kg−1) | 1.81 | 75 | 41 | N.R. |
| Cd (mg kg−1) | 7.41 | 85 | 39 | 20–40 |
| Cu (mg kg−1) | 107.28 | 4300 | 1500 | 1000–1750 |
| Cr (mg kg−1) | 28.46 | 3000 | N.R. | N.R. |
| Hg (mg kg−1) | 1.03 | 840 | 17 | 16–25 |
| Ni (mg kg−1) | 30.19 | 57 | 420 | 300–400 |
| Pb (mg kg−1) | 38.19 | 420 | 300 | 750–1200 |
| Zn (mg kg−1) | 707.95 | 7500 | 2800 | 2500–4000 |
| Parameters | Biosolids Analysed for Each Season of the Year | |||
|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | |
| pH | 6.10 ± 0.17 a | 6.20 ± 0.40 a | 6.17 ± 0.17 a | 6.41±0.83 a |
| EC (dS m−1) | 5.71 ± 1.93 a | 3.38 ± 1.25 a, b | 1.60 ± 0.01 b | 1.65 ± 0.18 b |
| TOC (%) | 37.25 ± 4.37 c | 57.94 ± 1.25 a | 50.25 ± 0.96 b | 36.90 ± 1.17 c |
| TN (%) | 6.67 ± 0.98 a | 6.78 ± 0.82 a | 6.59 ± 0.47 a | 6.35 ± 0.42 a |
| C/N | 5.64 ± 0.78 b | 8.64 ± 1.18 a | 7.65 ± 0.56 a, b | 5.83 ± 1.38 b |
| P (%) | 2.65 ± 0.17 a | 1.48 ± 0.14 b | 1.76 ± 0.03 b | 2.28 ± 0.13 a |
| K (%) | 1.09 ± 0.12 a | 0.69 ± 0.07 b, c | 0.59 ± 0.03 c | 0.86 ± 0.04 b |
| Fe (g kg−1) ͳ | 16.10 ± 0.40 c | 39.28 ± 1.77 a | 20.43 ± 0.59 b | 17.34 ± 1.91 c |
| B (mg kg−1) ͳ | 143.33 ± 12.01 b | 145.00 ± 8.00 b | 172.62 ± 2.13 a | 122.41 ± 8.41 b |
| Mn (mg kg−1) ͳ | 130.00 ± 8.00 c | 215.62 ± 7.39 a | 166.30 ± 4.59 b | 126.85 ± 7.56 c |
| Zn (mg kg−1) ͳ | 778.67 ± 13.50 b | 635.65 ± 10.55 c | 602.76 ± 2.34 d | 813.51 ± 10.58 b |
| Cu (mg kg−1) ͳ | 133.64 ± 12.01 a | 112.39 ± 5.56 b | 95.22 ± 2.84 b | 132.42 ± 5.64 a |
| Parameters | Biosolids Analysed for Each Season of the Year | |||
|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | |
| MBC (mg Cmic kg−1) ͳ | 966.6 ± 46.4 b | 1251.9 ± 134 a | 1149 ± 115 a,b | 587.2 ± 76.7 c |
| MBN (mg Nmic kg−1) ͳ | 84.25 ± 10.6 b | 109.3 ± 1.4 b | 304.7 ± 32.9 a | 119.0 ±20.1 b |
| Cmic/Nmic | 11.66 ± 2.09 a | 11.46 ± 1.32 a | 3.82 ± 0.77 b | 5.07 ± 1.32 b |
| DSH (mg INF * kg−1 h−1) ͳ | 2631.8 ± 147 a | 2209.5 ± 119 a | 2426.1 ± 230 a | 2277.6 ± 225 a |
| FDA (mg F ** kg−1 h−1) ͳ | 342.7 ± 40.0 b | 279.7 ± 30.0 b | 437.96 ± 44.2 a | 368.6 ± 24 a, b |
| DSH/FDA (mg INF mg−1 F *) | 7.78 ± 1.35 a | 7.99 ± 1.26 a | 5.55 ± 0.22 a | 6.21 ± 0.84 a |
| URS (mg N-NH4+ kg−1 h−1) ͳ | 186.0 ± 17.1 a | 194.5 ± 23.0 a | 109.8 ± 7.7 b | 186.5 ±13.7 a |
| qCO2 (mg C-CO2 g−1 Cmic d−1) | 157.95 ± 7.40 b | 124.31 ± 13.5 b | 115.42 ± 11.6 b | 270.19 ± 32.9 a |
| qFDA (mg F ** g−1 Cmic h−1) | 356.27 ± 56.7 b | 223.27 ± 4.84 b | 381.57 ± 26.9 b | 636.39 ± 106 a |
| Parameters | Biosolids Analysed for Each Season of the Year | |||
|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | |
| H’ | 2.61 ± 0.08 a | 2.78 ± 0.17 a | 2.46 ± 0.12 a | 2.60 ± 0.10 a |
| * SEI | 293.3 ± 63.3 b | 488.3 ± 115.6 a | 311.7 ± 36.9 a, b | 331.7 ± 40.7 a, b |
| Parameters | Correlation Coefficient (r2) | Parameters | Correlation Coefficient (r2) |
|---|---|---|---|
| MBC | FDA Activity | ||
| TOC | 0.798 ** | Fe | −0.585 * |
| C/N | 0.643 * | URS | −0.643 * |
| P | −0.585 * | H’ | −0.832 *** |
| Fe | 0.646 * | ||
| B | 0.693 * | URS Activity | |
| Mn | 0.748 ** | K | 0.578 * |
| Zn | −0.823 *** | B | −0.702 ** |
| Cu | −0.631 * | Cu | 0.637 * |
| qCO2 | −0.957 *** | H’ | 0.714 ** |
| qFDA | −0.899 *** | ||
| qCO2 | |||
| MBN | COT | −0.692 * | |
| K | −0.707 ** | B | −0.798 ** |
| B | 0.753 ** | Mn | −0.624 * |
| Zn | −0.660 * | Zn | 0.810 *** |
| Cu | −0.791 ** | Cu | 0.650 * |
| Cmic/Nmic | −0.729 ** | qFDA | 0.892 *** |
| FDA activity | 0.723 ** | ||
| URS activity | −0.900 *** | qFDA | |
| H’ | −0.701 ** | TOC | 0.712 ** |
| C/N | −0.580 * | ||
| Cmic/Nmic | Fe | −0.655 * | |
| EC | 0.794 ** | Mn | −0.711 ** |
| FDA activity | −0.744 ** | Zn | 0.641 * |
| URS | 0.618 * | ||
| qFDA | −0.647 * | H’ | |
| H’ | 0.721 ** | Fe | 0.69 * |
| SEI | 0.832 *** | ||
| DSH Activity | |||
| SEI | −0.627 * | SEI | |
| TOC | 0.613 * | ||
| Fe | 0.803 ** | ||
| Mn | 0.639 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Herrera, M.d.R.; Negrete-Rodríguez, M.d.l.L.X.; Álvarez-Trejo, J.L.; Samaniego-Hernández, M.; González-Cruz, L.; Bernardino-Nicanor, A.; Conde-Barajas, E. Evaluation of Non-Conventional Biological and Molecular Parameters as Potential Indicators of Quality and Functionality of Urban Biosolids Used as Organic Amendments of Agricultural Soils. Appl. Sci. 2020, 10, 517. https://doi.org/10.3390/app10020517
Medina-Herrera MdR, Negrete-Rodríguez MdlLX, Álvarez-Trejo JL, Samaniego-Hernández M, González-Cruz L, Bernardino-Nicanor A, Conde-Barajas E. Evaluation of Non-Conventional Biological and Molecular Parameters as Potential Indicators of Quality and Functionality of Urban Biosolids Used as Organic Amendments of Agricultural Soils. Applied Sciences. 2020; 10(2):517. https://doi.org/10.3390/app10020517
Chicago/Turabian StyleMedina-Herrera, Miriam del Rocío, María de la Luz Xochilt Negrete-Rodríguez, José Luis Álvarez-Trejo, Midory Samaniego-Hernández, Leopoldo González-Cruz, Aurea Bernardino-Nicanor, and Eloy Conde-Barajas. 2020. "Evaluation of Non-Conventional Biological and Molecular Parameters as Potential Indicators of Quality and Functionality of Urban Biosolids Used as Organic Amendments of Agricultural Soils" Applied Sciences 10, no. 2: 517. https://doi.org/10.3390/app10020517
APA StyleMedina-Herrera, M. d. R., Negrete-Rodríguez, M. d. l. L. X., Álvarez-Trejo, J. L., Samaniego-Hernández, M., González-Cruz, L., Bernardino-Nicanor, A., & Conde-Barajas, E. (2020). Evaluation of Non-Conventional Biological and Molecular Parameters as Potential Indicators of Quality and Functionality of Urban Biosolids Used as Organic Amendments of Agricultural Soils. Applied Sciences, 10(2), 517. https://doi.org/10.3390/app10020517







