Biotreatment of Winery Wastewater Using a Hybrid System Combining Biological Trickling Filters and Constructed Wetlands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Winery Wastewater (WW) Composition and Analytical Procedures
2.2. Biological Treatment Using the Pilot-Scale Trickling Filter
2.3. Post-Treatment Using Horizontal Subsurface Flow (HSF) Constructed Wetlands (CWs)
3. Results and Discussion
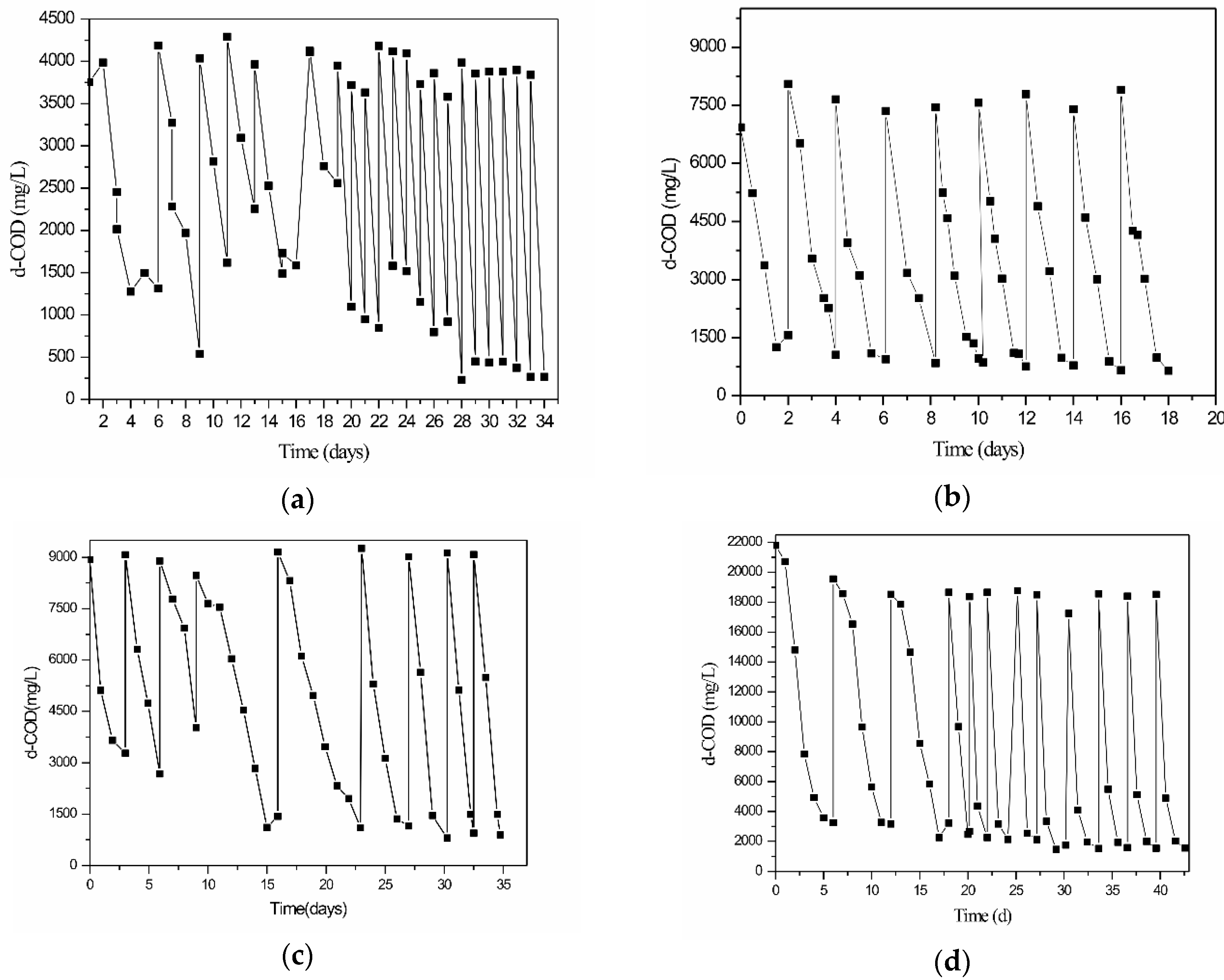
3.1. Pilot-Scale Trickling Filter Experiments
3.2. Post-Treatment Using CW
3.2.1. Effect of HRT
3.2.2. Effects of Vegetation
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Karaouzas, I. Agro-Industrial Wastewater Pollution in Greek River Ecosystems. In The Rivers of Greece Evolution, Current Status and Perspectives; Springer: Berlin/Heidelberg, Germany, 2016; pp. 169–204. ISBN 978-3-662-55367-1. [Google Scholar]
- Bolzonella, D.; Papa, M.; Da Ros, C.; Anga Muthukumar, L.; Rosso, D. Winery wastewater treatment: A critical overview of advanced biological processes. Crit. Rev. Biotechnol. 2019, 39, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Lofrano, G.; Meric, S. A comprehensive approach to winery wastewater treatment: A review of the state-of the-art. Desalin. Water Treat. 2016, 57, 3011–3028. [Google Scholar] [CrossRef]
- Oliveira, M.; Duarte, E. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176. [Google Scholar] [CrossRef]
- Puškaš, V.; Miljić, U.; Bajić, B.; Rončević, Z.; Dodić, J. Characterization of wastewaters from different stages of wine production. J. Process. Energy Agric. 2015, 19, 136–138. [Google Scholar]
- Kyzas, G.Z.; Symeonidou, M.P.; Matis, K.A. Technologies of winery wastewater treatment: A critical approach. Desalin. Water Treat. 2016, 57, 3372–3386. [Google Scholar] [CrossRef]
- Vlyssides, A.G.; Barampouti, E.M.; Mai, S. Wastewater characteristics from Greek wineries and distilleries. Water Sci. Technol. 2005, 51, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C.M.; Glasser, D.; Hildebrandt, D.; Petersen, J.; Rohwer, J. An annual and seasonal characterisation of winery effuent in South Africa. S. Afr. J. Enol. Vitic. 2011, 32, 1–8. [Google Scholar]
- Petruccioli, M.; Duarte, J.C.; Federici, F. High-rate aerobic treatment of winery wastewater using bioreactors with free and immobilized activated sludge. J. Biosci. Bioeng. 2000, 90, 381–386. [Google Scholar] [CrossRef]
- Amor, C.; Rodríguez-Chueca, J.; Fernandes, J.L.; Domínguez, J.R.; Lucas, M.S.; Peres, J.A. Winery wastewater treatment by sulphate radical based-advanced oxidation processes (SR-AOP): Thermally vs UV-assisted persulphate activation. Process Saf. Environ. Prot. 2019, 122, 94–101. [Google Scholar] [CrossRef]
- Holtman, G.A.; Haldenwang, R.; Welz, P.J. Biological sand filter system treating winery effluent for effective reduction in organic load and pH neutralisation. J. Water Process Eng. 2018, 25, 118–127. [Google Scholar] [CrossRef]
- de Beer, D.M.; Botes, M.; Cloete, T.E. The microbial community of a biofilm contact reactor for the treatment of winery wastewater. J. Appl. Microbiol. 2018, 124, 598–610. [Google Scholar] [CrossRef]
- Andreottola, G.; Foladori, P.; Ziglio, G. Biological treatment of winery wastewater: An overview. Water Sci. Technol. 2009, 60, 1117–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreottola, G.; Foladori, P.; Nardelli, P.; Denicolo, A. Treatment of winery wastewater in a full-scale fixed bed biofilm reactor. Water Sci. Technol. 2005, 51, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorpas, A.A.; Saranti, A. Multi-criteria analysis of sustainable environmental clean technologies for the treatment of winery’s wastewater. Int. J. Glob. Environ. Issues 2016, 15, 151–168. [Google Scholar] [CrossRef] [Green Version]
- Ioannou, L.A.; Puma, G.L.; Fatta-Kassinos, D. Treatment of winery wastewater by physicochemical, biological and advanced processes: A review. J. Hazard. Mater. 2015, 286, 343–368. [Google Scholar] [CrossRef]
- Mosse, K.P.M.; Patti, A.F.; Christen, E.W.; Cavagnaro, T.R. Review: Winery wastewater quality and treatment options in Australia. Aust. J. Grape Wine Res. 2011, 17, 111–122. [Google Scholar] [CrossRef]
- Rodríguez, E.; Márquez, G.; Carpintero, J.C.; Beltrán, F.J.; Álvarez, P. Sequential use of bentonites and solar photocatalysis to treat winery wastewater. J. Agric. Food Chem. 2008, 56, 11956–11961. [Google Scholar] [CrossRef]
- Brito, A.G.; Peixoto, J.; Oliveira, J.M.; Oliveira, J.A.; Costa, C.; Nogueira, R.; Rodrigues, A. Brewery and winery wastewater treatment: Some focal points of design and operation. Util. Prod. Treat. Waste Food Ind. 2007, 109–131. [Google Scholar] [CrossRef] [Green Version]
- Welz, P.J.; Holtman, G.; Haldenwang, R.; Le Roes-Hill, M. Characterisation of winery wastewater from continuous flow settling basins and waste stabilisation ponds over the course of 1 year: Implications for biological wastewater treatment and land application. Water Sci. Technol. 2016, 74, 2036–2050. [Google Scholar] [CrossRef] [Green Version]
- Pipolo, M.; Martins, R.C.; Quinta-Ferreira, R.M.; Costa, R. Integrating the Fenton’s Process with Biofiltration by Corbicula fluminea to Reduce Chemical Oxygen Demand of Winery Effluents. J. Environ. Qual. 2017, 46, 436–442. [Google Scholar] [CrossRef]
- Ferreira, R.; Gomes, J.; Martins, R.C.; Costa, R.; Quinta-Ferreira, R.M. Winery wastewater treatment by integrating Fenton’s process with biofiltration by Corbicula fluminea. J. Chem. Technol. Biotechnol. 2018, 93, 333–339. [Google Scholar] [CrossRef]
- Souza, B.S.; Moreira, F.C.; Dezotti, M.W.C.; Vilar, V.J.P.; Boaventura, R.A.R. Application of biological oxidation and solar driven advanced oxidation processes to remediation of winery wastewater. Catal. Today 2013, 209, 201–208. [Google Scholar] [CrossRef]
- Mosteo, R.; Sarasa, J.; Ormad, M.P.; Ovelleiro, J.L. Sequential solar photo-fenton-biological system for the treatment of winery wastewaters. J. Agric. Food Chem. 2008, 56, 7333–7338. [Google Scholar] [CrossRef] [PubMed]
- Benitez, F.J.; Acero, J.L.; Gonzalez, T.; Garcia, J. The Use of Ozone, Ozone Plus Uv Radiation, and Aerobic Microorganisms in the Purification of Some Agro-Industrial Wastewaters. J. Environ. Sci. Health Part A 2002, 37, 1307–1325. [Google Scholar] [CrossRef]
- Benitez, F.J.; Beltran-Heredia, J.; Real, F.J.; Acero, J.L. Wine vinasses treatments by ozone and an activated sludge system in continuous reactors. Bioprocess Eng. 2000, 23, 149–154. [Google Scholar] [CrossRef]
- Benitez, F.J.; Beltran-Heredia, J.; Real, F.J.; Acero, J.L. Enhancement of the ozonation of wine distillery wastewaters by an aerobic pretreatment. Bioprocess Eng. 1999, 21, 459–464. [Google Scholar] [CrossRef]
- Lucas, M.S.; Mouta, M.; Pirra, A.; Peres, J.A. Winery wastewater treatment by a combined process: Long term aerated storage and Fenton’s reagent. Water Sci. Technol. 2009, 60, 1089–1095. [Google Scholar] [CrossRef]
- Anastasiou, N.; Monou, M.; Mantzavinos, D.; Kassinos, D. Monitoring of the quality of winery influents/effluents and polishing of partially treated winery flows by homogeneous Fe(II) photo-oxidation. Desalination 2009, 248, 836–842. [Google Scholar] [CrossRef]
- Beltran de Heredia, J.; Torregrosa, J.; Dominguez, J.R.; Partido, E. Degradation of wine distillery wastewaters by the combination of aerobic biological treatment with chemical oxidation by Fenton’s reagent. Water Sci. Technol. 2005, 51, 167–174. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Michael, C.; Vakondios, N.; Drosou, K.; Xekoukoulotakis, N.P.; Diamadopoulos, E.; Fatta-Kassinos, D. Winery wastewater purification by reverse osmosis and oxidation of the concentrate by solar photo-Fenton. Sep. Purif. Technol. 2013, 118, 659–669. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Fatta-Kassinos, D. Solar photo-Fenton oxidation against the bioresistant fractions of winery wastewater. J. Environ. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Ioannou, L.; Michael, C.; Kyriakou, S.; Fatta-Kassinos, D. Solar Fenton: From pilot to industrial scale application for polishing winery wastewater pretreated by MBR. J. Chem. Technol. Biotechnol. 2014, 89, 1067–1076. [Google Scholar] [CrossRef]
- Braz, R.; Pirra, A.; Lucas, M.S.; Peres, J.A. Combination of long term aerated storage and chemical coagulation/flocculation to winery wastewater treatment. Desalination 2010, 263, 226–232. [Google Scholar] [CrossRef]
- Solís, R.R.; Rivas, F.J.; Ferreira, L.C.; Pirra, A.; Peres, J.A. Integrated aerobic biological–chemical treatment of winery wastewater diluted with urban wastewater. LED-based photocatalysis in the presence of monoperoxysulfate. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2018, 53, 124–131. [Google Scholar] [CrossRef]
- Amaral-Silva, N.; Martins, R.C.; Paiva, C.; Castro-Silva, S.; Quinta-Ferreira, R.M. A new winery wastewater treatment approach during vintage periods integrating ferric coagulation, Fenton reaction and activated sludge. J. Environ. Chem. Eng. 2016, 4, 2207–2215. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Remediation of a winery wastewater combining aerobic biological oxidation and electrochemical advanced oxidation processes. Water Res. 2015, 75, 95–108. [Google Scholar] [CrossRef]
- Sehar, S.; Nasser, H.A.A. Wastewater treatment of food industries through constructed wetland: A review. Int. J. Environ. Sci. Technol. 2019, 16, 6453–6472. [Google Scholar] [CrossRef]
- Flores, L.; García, J.; Pena, R.; Garfí, M. Constructed wetlands for winery wastewater treatment: A comparative Life Cycle Assessment. Sci. Total Environ. 2019, 659, 1567–1576. [Google Scholar] [CrossRef]
- Masi, F.; Bresciani, R.; Bracali, M. The Role of Natural and Constructed Wetlands in Nutrient Cycling and Retention on the Landscape. In The Role of Natural and Constructed Wetlands in Nutrient Cycling and Retention on the Landscape; Vymazal, J., Ed.; Springer: Cham, Switzerland, 2015; pp. 189–201. ISBN 978-3-319-08176-2. [Google Scholar]
- Masi, F.; Rochereau, J.; Troesch, S.; Ruiz, I.; Soto, M. Wineries wastewater treatment by constructed wetlands: A review. Water Sci. Technol. 2015, 71, 1113–1127. [Google Scholar] [CrossRef]
- Serrano, L.; de la Varga, D.; Ruiz, I.; Soto, M. Winery wastewater treatment in a hybrid constructed wetland. Ecol. Eng. 2011, 37, 744–753. [Google Scholar] [CrossRef]
- De la Varga, D.; Díaz, M.A.; Ruiz, I.; Soto, M. Avoiding clogging in constructed wetlands by using anaerobic digesters as pre-treatment. Ecol. Eng. 2013, 52, 262–269. [Google Scholar] [CrossRef]
- Kim, B.; Gautier, M.; Prost-Boucle, S.; Molle, P.; Michel, P.; Gourdon, R. Performance evaluation of partially saturated vertical-flow constructed wetland with trickling filter and chemical precipitation for domestic and winery wastewaters treatment. Ecol. Eng. 2014, 71, 41–47. [Google Scholar] [CrossRef] [Green Version]
- APHA Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2005.
- Tatoulis, T.I.; Tekerlekopoulou, A.G.; Akratos, C.S.; Pavlou, S.; Vayenas, D.V. Aerobic biological treatment of second cheese whey in suspended and attached growth reactors. J. Chem. Technol. Biotechnol. 2015, 90, 2040–2049. [Google Scholar] [CrossRef]
- Eusébio, A.; Petruccioli, M.; Lageiro, M.; Federici, F.; Duarte, J.C. Microbial characterisation of activated sludge in jet-loop bioreactors treating winery wastewaters. J. Ind. Microbiol. Biotechnol. 2004, 31, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Prades, G.; Sadowski, A.G. Activated sludge wastewater treatment plants optimisation to face pollution overloads during grape harvest periods. Water Sci. Technol. 2005, 51, 81–88. [Google Scholar] [CrossRef]
- Andreottola, G.; Foladori, P.; Ragazzi, M.; Villa, R. Treatment of winery wastewater in a sequencing batch biofilm reactor. Water Sci. Technol. 2002, 45, 347–354. [Google Scholar] [CrossRef]
- Michailides, M.; Tatoulis, T.; Sultana, M.-Y.; Tekerlekopoulou, A.; Konstantinou, I.; Akratos, C.S.; Pavlou, S.; Vayenas, D.V. Start-up of a free water surface constructed wetland for treating olive mill wastewater. Hem. Ind. 2015, 69, 577–583. [Google Scholar] [CrossRef]
- Tatoulis, T.I.; Zapantiotis, S.; Frontistis, Z.; Akratos, C.S.; Tekerlekopoulou, A.G.; Pavlou, S.; Mantzavinos, D.; Vayenas, D.V. A hybrid system comprising an aerobic biological process and electrochemical oxidation for the treatment of black table olive processing wastewaters. Int. Biodeterior. Biodegrad. 2016, 109, 104–112. [Google Scholar] [CrossRef]
- Torrijos, M.; Moletta, R. Winery wastewater depollution by sequencing batch reactor. Water Sci. Technol. 1997, 35, 249–257. [Google Scholar] [CrossRef]
- Petruccioli, M.; Cardoso Duarte, J.; Eusebio, A.; Federici, F. Aerobic treatment of winery wastewater using a jet-loop activated sludge reactor. Process Biochem. 2002, 37, 821–829. [Google Scholar] [CrossRef]
- Malandra, L.; Wolfaardt, G.; Zietsman, A.; Viljoen-Bloom, M. Microbiology of a biological contactor for winery wastewater treatment. Water Res. 2003, 37, 4125–4134. [Google Scholar] [CrossRef]
- Coetzee, G.; Malandra, L.; Wolfaardt, G.M.; Viljoen-Bloom, M. Dynamics of a microbial biofilm in a rotating biological contactor for the treatment of winery effluent. Water SA 2004, 30, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Montalvo, S.; Guerrero, L.; Borj, R.; Cortés, I.; Sánchez, E.; Colmenarejo, M.F. Effect of the influent COD concentration on the anaerobic digestion of winery wastewaters from grape-red and tropical fruit (Guava) wine production in fluidized bed reactors with Chilean natural zeolite for biomass immobilization. Chem. Biochem. Eng. Q. 2010, 24, 219–226. [Google Scholar]
- Ramond, J.B.; Welz, P.J.; Tuffin, M.; Burton, S.; Cowan, D.A. Assessment of temporal and spatial evolution of bacterial communities in a biological sand filter mesocosm treating winery wastewater. J. Appl. Microbiol. 2013, 115, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valderrama, C.; Ribera, G.; Bahí, N.; Rovira, M.; Giménez, T.; Nomen, R.; Lluch, S.; Yuste, M.; Martinez-Lladó, X. Winery wastewater treatment for water reuse purpose: Conventional activated sludge versus membrane bioreactor (MBR). A comparative case study. Desalination 2012, 306, 1–7. [Google Scholar] [CrossRef]
- Artiga, P.; González, F.; Mosquera-Corral, A.; Campos, J.L.; Garrido, J.M.; Ficara, E.; Méndez, R. Multiple analysis reprogrammable titration analyser for the kinetic characterization of nitrifying and autotrophic denitrifying biomass. Biochem. Eng. J. 2005, 26, 176–183. [Google Scholar] [CrossRef]
- Artiga, P.; Carballa, M.; Garrido, J.M.; Méndez, R. Treatment of winery wastewaters in a membrane submerged bioreactor. Water Sci. Technol. 2007, 56, 63–69. [Google Scholar] [CrossRef]
- Lobos, J.; Wisniewski, C.; Heran, M.; Grasmick, A. Continuous and sequencing membrane bioreactors applied to food industry effluent treatment. Water Sci. Technol. 2007, 56, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.S.; Peres, J.A. Removal of Emerging Contaminants by Fenton and UV-Driven Advanced Oxidation Processes. Water Air Soil Pollut. 2015, 226, 273. [Google Scholar] [CrossRef]
- Litaor, M.I.; Meir-Dinar, N.; Castro, B.; Azaizeh, H.; Rytwo, G.; Levi, N.; Levi, M.; MarChaim, U. Treatment of winery wastewater with aerated cells mobile system. Environ. Nanotechnol. Monit. Manag. 2015, 4, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Republic, H. 2011/354B of 8 March 2011 on the Establishment of Measures, Conditions and Procedures for the Reuse of Treated Wastewater and Other Provisions; Gazette of the Government (GR): Athens, Greece, 2011; pp. 5229–5243.
- Sultana, M.-Y.; Mourti, C.; Tatoulis, T.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Effect of hydraulic retention time, temperature, and organic load on a horizontal subsurface flow constructed wetland treating cheese whey wastewater. J. Chem. Technol. Biotechnol. 2016, 91, 726–732. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Akratos, C.S.; TSihrintzis, V.A. Vertical Flow Constructed Wetlands: Eco-Engineering Systems for Wastewater and Sludge Treatment, 1st ed.; Elsevier: Burlington, NJ, USA, 2014. [Google Scholar]
- Tatoulis, T.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V.; Stefanakis, A.I. A novel horizontal subsurface flow constructed wetland: Reducing area requirements and clogging risk. Chemosphere 2017, 186, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Akratos, C.S.; Tsihrintzis, V.A. Effect of temperature, HRT, vegetation and porous media on removal efficiency of pilot-scale horizontal subsurface flow constructed wetlands. Ecol. Eng. 2007, 29, 173–191. [Google Scholar] [CrossRef]
- Herouvim, E.; Akratos, C.S.; Tekerlekopoulou, A.; Vayenas, D.V. Treatment of olive mill wastewater in pilot-scale vertical flow constructed wetlands. Ecol. Eng. 2011, 37, 931–939. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Seeger, E.; Dorer, C.; Sinke, A.; Thullner, M. Performance of pilot-scale horizontal subsurface flow constructed wetlands treating groundwater contaminated with phenols and petroleum derivatives. Ecol. Eng. 2016, 95, 514–526. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Akratos, C.S.; Melidis, P.; Tsihrintzis, V.A. Surplus activated sludge dewatering in pilot-scale sludge drying reed beds. J. Hazard. Mater. 2009, 172, 1122–1130. [Google Scholar] [CrossRef]
- Bruch, I.; Fritsche, J.; Bänninger, D.; Alewell, U.; Sendelov, M.; Hürlimann, H.; Hasselbach, R.; Alewell, C. Improving the treatment efficiency of constructed wetlands with zeolite-containing filter sands. Bioresour. Technol. 2011, 102, 937–941. [Google Scholar] [CrossRef]
- Zhu, W.L.; Cui, L.H.; Ouyang, Y.; Long, C.F.; Tang, X.D. Kinetic Adsorption of Ammonium Nitrogen by Substrate Materials for Constructed Wetlands. Pedosphere 2011, 21, 454–463. [Google Scholar] [CrossRef]
- Saeed, T.; Sun, G. Enhanced denitrification and organics removal in hybrid wetland columns: Comparative experiments. Bioresour. Technol. 2011, 102, 967–974. [Google Scholar] [CrossRef]
- Yalcuk, A.; Ugurlu, A. Comparison of horizontal and vertical constructed wetland systems for landfill leachate treatment. Bioresour. Technol. 2009, 100, 2521–2526. [Google Scholar] [CrossRef]
- Vymazal, J.; Kröpfelová, L. Removal of organics in constructed wetlands with horizontal sub-surface flow: A review of the field experience. Sci. Total Environ. 2009, 407, 3911–3922. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.C. Plants as ecosystem engineers in subsurface-flow treatment wetlands. Water Sci. Technol. 2001, 44, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kaseva, M.E. Performance of a sub-surface flow constructed wetland in polishing pre-treated wastewater—A tropical case study. Water Res. 2004, 38, 681–687. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akratos, C.S.; Tatoulis, T.I.; Tekerlekopoulou, A.G. Biotreatment of Winery Wastewater Using a Hybrid System Combining Biological Trickling Filters and Constructed Wetlands. Appl. Sci. 2020, 10, 619. https://doi.org/10.3390/app10020619
Akratos CS, Tatoulis TI, Tekerlekopoulou AG. Biotreatment of Winery Wastewater Using a Hybrid System Combining Biological Trickling Filters and Constructed Wetlands. Applied Sciences. 2020; 10(2):619. https://doi.org/10.3390/app10020619
Chicago/Turabian StyleAkratos, Christos S., Triantafyllos I. Tatoulis, and Athanasia G. Tekerlekopoulou. 2020. "Biotreatment of Winery Wastewater Using a Hybrid System Combining Biological Trickling Filters and Constructed Wetlands" Applied Sciences 10, no. 2: 619. https://doi.org/10.3390/app10020619




