Optimized Size and Distribution of Silver Nanoparticles on the Surface of Titanium Implant Regarding Cell Viability
Abstract
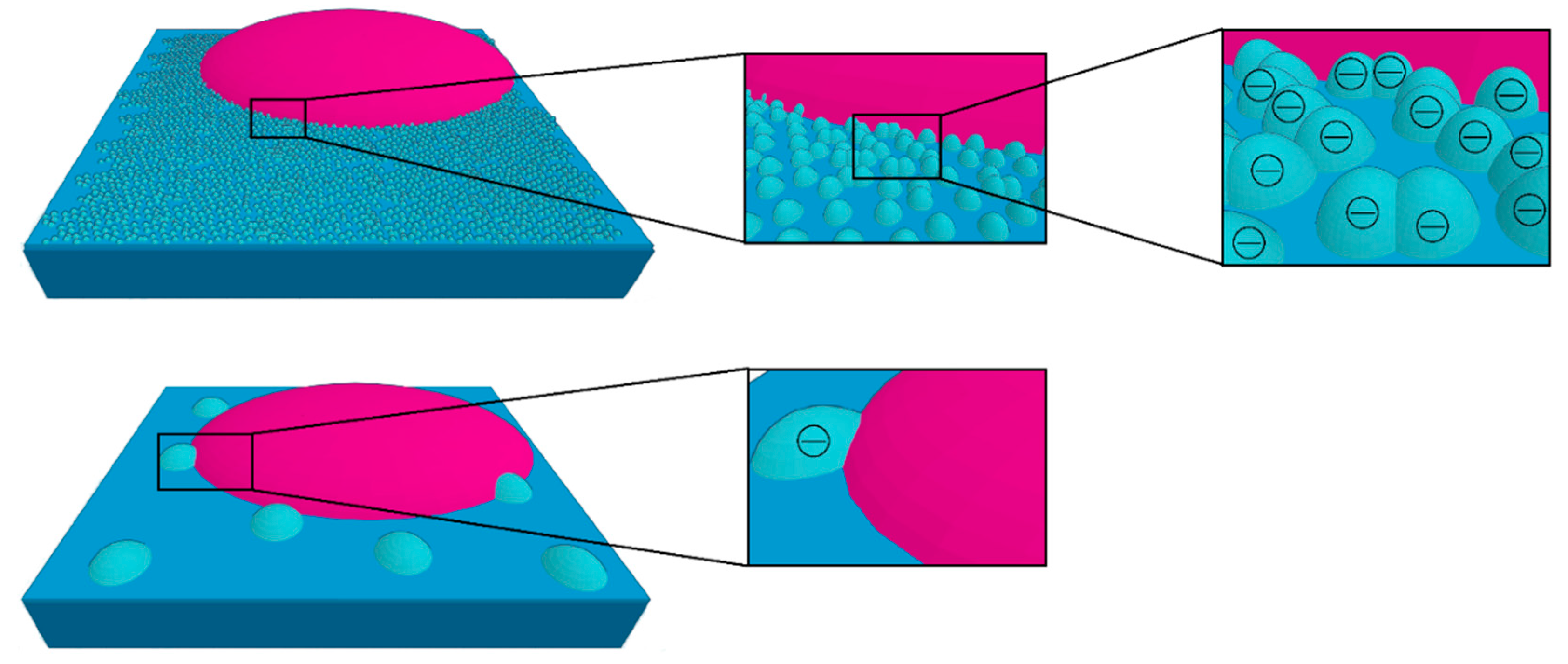
:1. Introduction
2. Sample Preparation
2.1. Stage I: First Annealing
2.2. Stage II: Silver Deposition
2.3. Stage III: Second Annealing
3. Experimental Methods
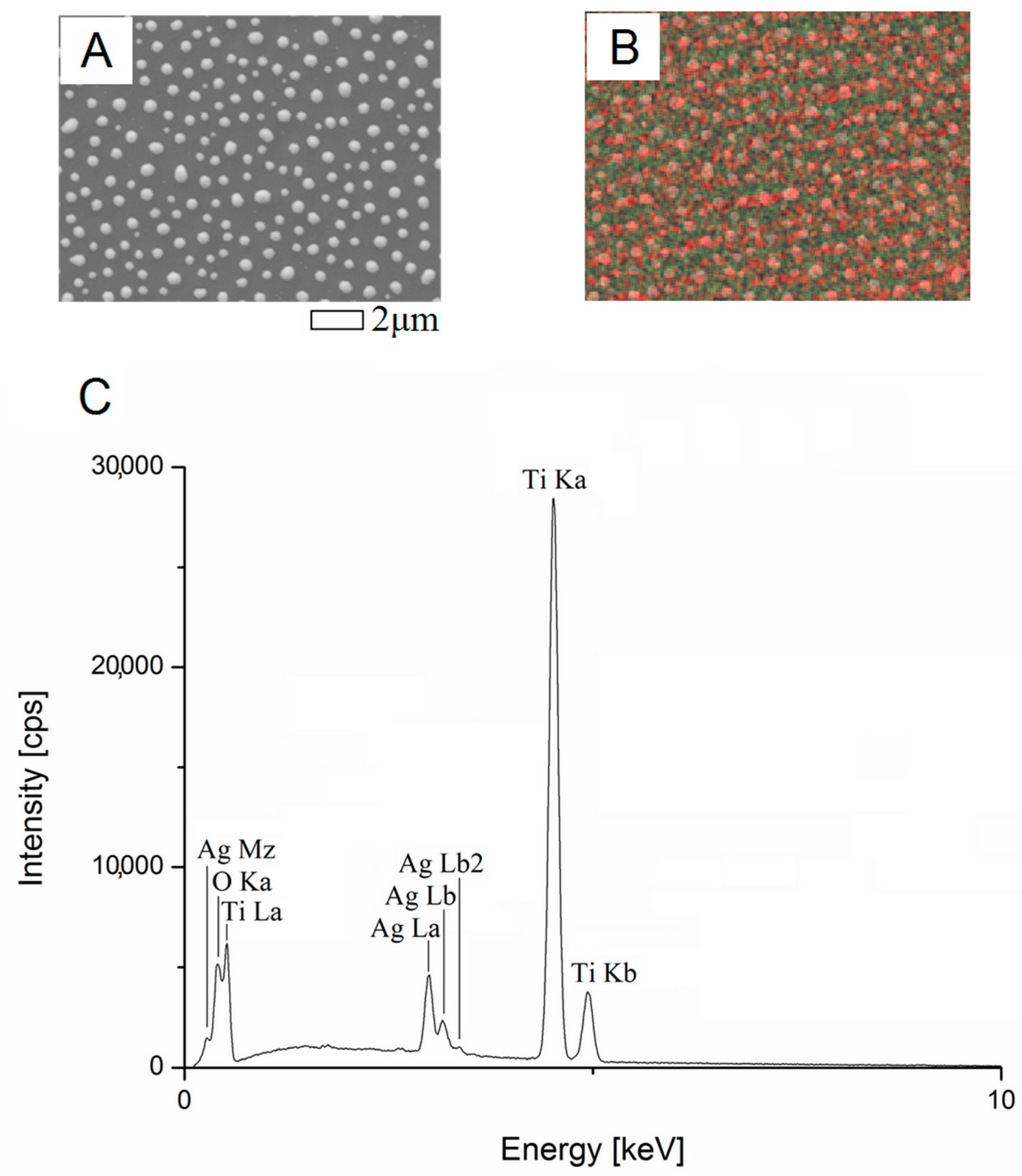
3.1. Secondary Neutral Mass Spectrometry
3.2. Scanning Electron Microscopy
3.3. Contact Angle Measurements
3.4. Cell Viability Assay
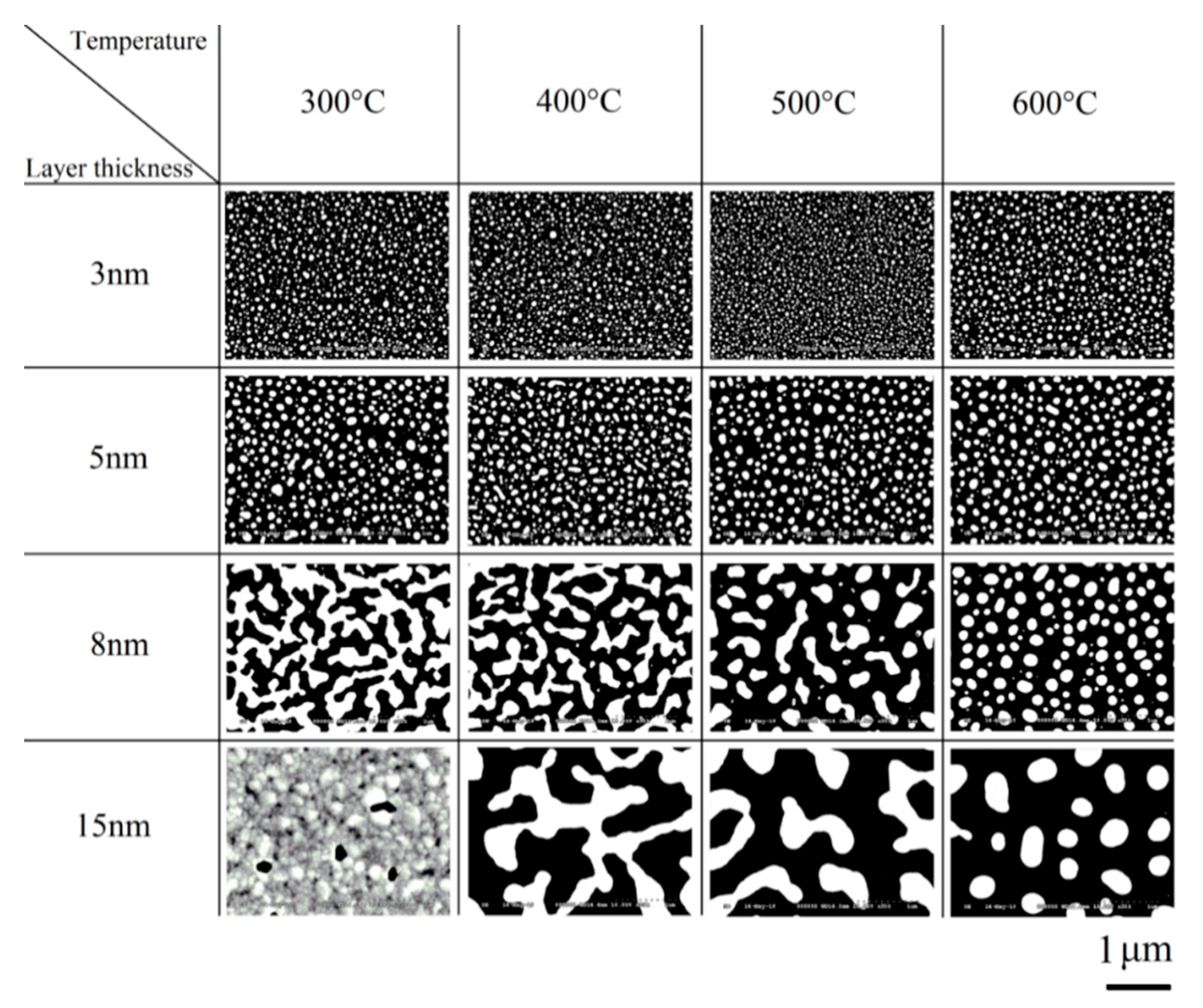
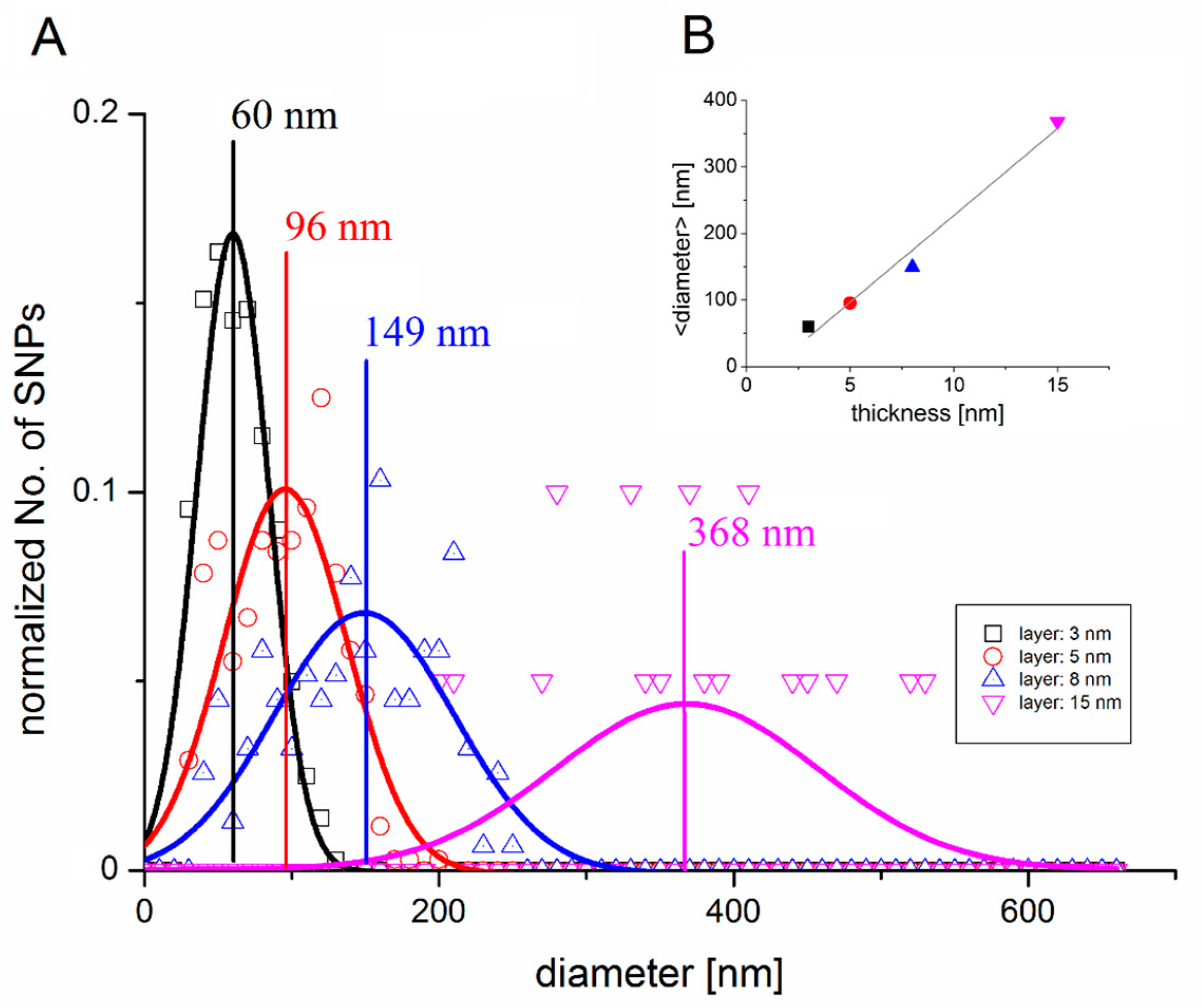
4. Results
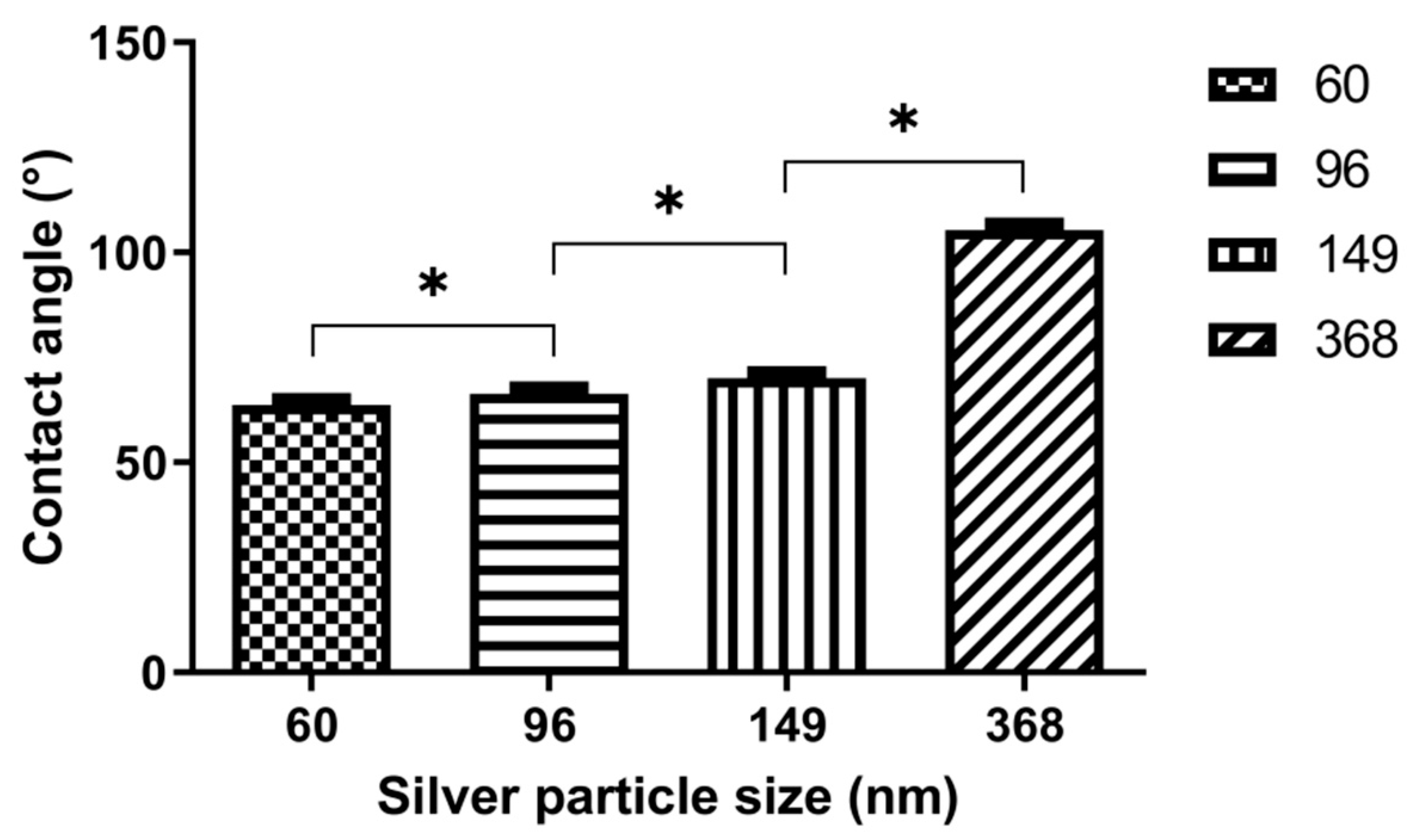
4.1. Contact Angle Measurements
4.2. Cell Growth
4.3. Cell Viability Assay
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Albrektsson, T.; Jacobsson, M. Bone-metal interface in osseointegration. J. Prosthet. Dent. 1987, 57, 597–607. [Google Scholar] [CrossRef]
- Albrektsson, T.; Branemark, P.I.; Hansson, A.; Lindstrom, J. Osseointegrated Titanium Implants: Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishnu, J.; Manivasagam, V.K.; Gopal, V.; Garcia, C.B.; Webster, T.J. Hydrothermal treatment of etched titanium: A potential surface nano-modification technique for enhanced biocompatibility. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102016. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, X.; Yan, W.; Chen, Z.; Wang, Y. Nanotubular topography enhances the bioactivity of titanium implants. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Tonda-Turo, C.; Mancuso, E.; Gentile, P. Multilayer nanoscale functionalization to treat disorders and enhance regeneration of bone tissue. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 22–38. [Google Scholar] [CrossRef]
- Özcan, I.; Uysal, H. Effects of silicon coating on bond strength of two different titaniumceramic to titanium. Dent. Mater. 2005, 21, 773–779. [Google Scholar] [CrossRef]
- Puckett, S.; Webster, T. Control of osteoblast alignment on nano patterned titanium. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 348. [Google Scholar] [CrossRef]
- Ma, T.; Ge, X.-Y.; Zhang, Y.; Lin, Y. Effect of Titanium Surface Modifications of Dental Implants on Rapid Osseointegration. Interface Oral Health Sci. 2016, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Chambrone, L.; Shibli, J.A.; Mercurio, C.E.; Cardoso, B.; Preshaw, P.E. Efficacy of standard (SLA) and modified sandblasted and acid-etched (SLActive) dental implants in promoting immediate and/or early loading protocols: A systematic review of prospective studies. Clin. Oral Implant. Res. 2014, 26, 359–370. [Google Scholar] [CrossRef]
- Hinkle, M.R.; Rimer, S.R.; Morgan, M.H.; Zeman, P. Loading of titanium implants with hydrophilic endosteal surface 3 weeks after insertion: Clinical and radiological outcome of a 12-month prospective clinical trial. J. Oral Maxillofac. Surg. 2014, 72, 1495–1502. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Erarlyosseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, F.E.; de Oliveira, G.J.P.L.; Aroni, M.A.T.; Marcantonio, R.A.C.; Marcantonio, E., Jr. Analysis of osseointegration of implants with hydrophilic surfaces in grafted areas: A Precilincal Study. Clin. Oral Implant. Res. 2018, 29, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Kangwansupamonkon, W.; Lauruengtana, V.; Surassmo, S.; Ruktanonchai, U. Antibacterial effect of apatite-coated titanium dioxide for textiles applications. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Tallósy, S.P.; Janovák, L.; Ménesi, J.; Nagy, E.; Juhász, Á.; Balázs, L.; Deme, I.; Buzás, N.; Dékány, I. Investigation of the antibacterial effects of silver-modified TiO2 and ZnO plasmonic photocatalysts embedded in polymer thin films. Environ. Sci. Pollut. Res. 2014, 21, 11155–11167. [Google Scholar] [CrossRef] [PubMed]
- Hajkova, P.; Spatenka, P.; Krumeich, J.; Exnar, P.; Kolouch, A.; Matoušek, J.; Kočí, P. Antibacterial effect of silver modified TiO2/PECVD films. Eur. Phys. J. 2009, 54, 189–193. [Google Scholar] [CrossRef]
- Li, H.; Cui, Q.; Feng, B.; Wang, J.; Lu, X.; Weng, J. Antibacterial activity of TiO2 nanotubes: Influence of crystal phase, morphology and Agdeposition. Appl. Surf. Sci. 2013, 284, 179–183. [Google Scholar] [CrossRef]
- Wang, G.; Jin, W.; Qasim, A.M.; Gao, A.; Peng, X.; Li, W.; Feng, H.; Chua, P.K. Antibacterial effect of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials 2017, 124, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Joya, Y.F.; Liu, Z.; Joya, K.S.; Wang, T. Preparation and antibacterial properties of laser-generated silver-anatase nanocomposite film against Escherichia coli and Staphylococcus aureus. Nanotechnology 2012, 23, 495708. [Google Scholar] [CrossRef]
- Song, H.Y.; Ko, K.K.; Oh, L.H.; Lee, B.T. Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur. Cells Mater. 2006, 11, 58. [Google Scholar]
- Raffi, M.; Hussain, F.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Hasan, M.M. Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. J. Mater. Sci. Technol. 2008, 24, 192–196. [Google Scholar]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lampé, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombrádi, Z.; Hajdu, P.; Hegedűs, V.; Rácz, R.; Varga, I.; et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef] [Green Version]
- de Matteis, V.; Malvindi, M.A.; Galeone, A.; Brunetti, V.; Pompa, P.P. Negligible particle-specific toxicity mechanism of silver nanoparticles: The role of Ag+ ion release in the cytosol. Nanomed. Nanotechnol. Biol. Med. 2015, 4, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bechert, T.; Steinrucke, P.; Wagener, M.; Seidel, P. An in vitro assessment of the antibacterial properties and cytotoxicity on nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef]
- Racz, R.; Biri, S.; Hajdu, P.; Csik, A.; Vad, K.; Kökényesi, S.; Csarnovics, I.; Hegedűs, C.; Radics, T.; Bakó, J.; et al. Application of an ECR ion source for ionic functionalization of implant materials on the nanoscale. In Proceedings of the 21st International Workshop on ECR Ion Sources (ECRIS2014); Institute of Applied Physics of the Russian Academy of Sciences: Nizhny Novgorod, Russia, 2014; ISBN 978-3-95450-158-8. [Google Scholar]
- Csarnovics, I.; Hajdu, P.; Biri, S.; Hegedűs, C.; Kökényesi, S.; Rácz, R.; Csik, A. Preliminary studies of creation of gold nanoparticles on titanium surface towards biomedical applications. Vacuum 2016, 126, 55–58. [Google Scholar] [CrossRef] [Green Version]
- Kökényesi, S.; Biri, S.; Hegedűs, C.; Csarnovics, I.; Csik, A. Functionalization of amorphous chalcogenide and titanium oxide layers by goldnanoparticles. Adv. Mater. Res. 2013, 747, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Racz, R.; Biri, S.; Csarnovics, I.; Kökényesi, S. Gold and calcium ion beams for materials research by the Atomki ECR Ion Source. Acta Phys. Deb. 2012, 46, 133–141. [Google Scholar]
- Beszeda, I.; Beke, D.L.; Gontier-Moya, E.G.; Kaganovskii, Y.S.; Ianetz, D. Calculation of Surface Self-Diffusion Coefficients from AES Data on Decay of Thin Metal Films. Defect Diffus. Forum 2005, 237, 727–732. [Google Scholar] [CrossRef]
- Mullins, W.W. Theory of Thermal Grooving. J. Appl. Phys. 1957, 28, 333. [Google Scholar] [CrossRef]
- Vad, K.; Csik, A.; Langer, G.A. Secondary neutral mass spectrometry—A powerful technique for quantitative elemental and depth profilinganalyses of nanostructures. Spectrosc. Eur. 2009, 21, 13–17. [Google Scholar]
- Oliveira, S.M.; Song, W.; Alves, N.M.; Mano, J.F. Chemical modification of bioinspired superhydrophobic polystyrene surfaces to control cell attachment/proliferation. Soft Matter 2011, 7, 8932–8941. [Google Scholar] [CrossRef]
- Khor, H.L.; Kuan, Y.; Kukula, H.; Tamada, K.; Knoll, W.; Moeller, M.; Hutmacher, D.W. Response of cells on surface-induced nanopatterns: Fibroblasts and mesenchymal progenitor cells. Biomacromolecules 2007, 8, 1530–1540. [Google Scholar] [CrossRef]
- Altankov, G.; Grinnell, F.; Groth, T.J. Studies on the biocompatibility of materials: Fibroblast reorganization of substratum-bound fibronectin on surfaces varying in wettability. J. Biomed. Mater. Res. 1996, 30, 385–391. [Google Scholar] [CrossRef]
- Webb, K.; Hlady, V.; Tresco, P.A. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J. Biomed. Mater. Res. 1998, 41, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Sun, Z. A mechanism for enhanced hydrophilicity of silver nanoparticles modified TiO2 thin films deposited by RF magnetron sputtering. Appl. Surf. Sci. 2009, 255, 6715–6720. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [Green Version]
- Milleret, V.; Lienemann, P.S.; Gasser, A.; Bauer, S.; Ehrbar, M.; Wennerberg, A. Rational design and in vitro characterization of novel dental implant and abutment surfaces for balancing clinical and biological needs. Clin. Implant. Dent. Relat. Res. 2019, 21, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Guo, H.; Yao, Z.; Mei, Y.; Tang, C.Y. Hydrophilic Silver Nanoparticles Induce Selective Nanochannels in Thin Film Nanocomposite Polyamide Membranes. Environ. Sci. Technol. 2019, 53, 5301–5308. [Google Scholar] [CrossRef]
- Cai, S.; Bhushan, B. Meniscus and viscous forces during separation of hydrophilic and hydrophobic smooth/rough surfaces with symmetric and asymmetric contact angles. Philos. Trans. R. Soc. A 2008, 366, 1627–1647. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajdu, P.; Lampé, I.; Rácz, R.; Biri, S.; Csík, A.; Tóth, F.; Szalóki, M.; Hegedűs, V.; Dombrádi, Z.; Varga, I.; et al. Optimized Size and Distribution of Silver Nanoparticles on the Surface of Titanium Implant Regarding Cell Viability. Appl. Sci. 2020, 10, 7063. https://doi.org/10.3390/app10207063
Hajdu P, Lampé I, Rácz R, Biri S, Csík A, Tóth F, Szalóki M, Hegedűs V, Dombrádi Z, Varga I, et al. Optimized Size and Distribution of Silver Nanoparticles on the Surface of Titanium Implant Regarding Cell Viability. Applied Sciences. 2020; 10(20):7063. https://doi.org/10.3390/app10207063
Chicago/Turabian StyleHajdu, Péter, István Lampé, Richárd Rácz, Sándor Biri, Attila Csík, Ferenc Tóth, Melinda Szalóki, Viktória Hegedűs, Zsuzsanna Dombrádi, István Varga, and et al. 2020. "Optimized Size and Distribution of Silver Nanoparticles on the Surface of Titanium Implant Regarding Cell Viability" Applied Sciences 10, no. 20: 7063. https://doi.org/10.3390/app10207063
APA StyleHajdu, P., Lampé, I., Rácz, R., Biri, S., Csík, A., Tóth, F., Szalóki, M., Hegedűs, V., Dombrádi, Z., Varga, I., Csarnovics, I., Kökényesi, S., Beke, D. L., & Hegedűs, C. (2020). Optimized Size and Distribution of Silver Nanoparticles on the Surface of Titanium Implant Regarding Cell Viability. Applied Sciences, 10(20), 7063. https://doi.org/10.3390/app10207063






