Effect of Cu, Cr, S Doped TiO2 for Transparent Plastic Bar Reinforced Concrete
Abstract
:Featured Application
Abstract
1. Introduction
2. Experimental Programs
2.1. Procedure of Transparent Plastic Bar Reinforced Concrete
2.2. Materials
2.3. Test Procedure
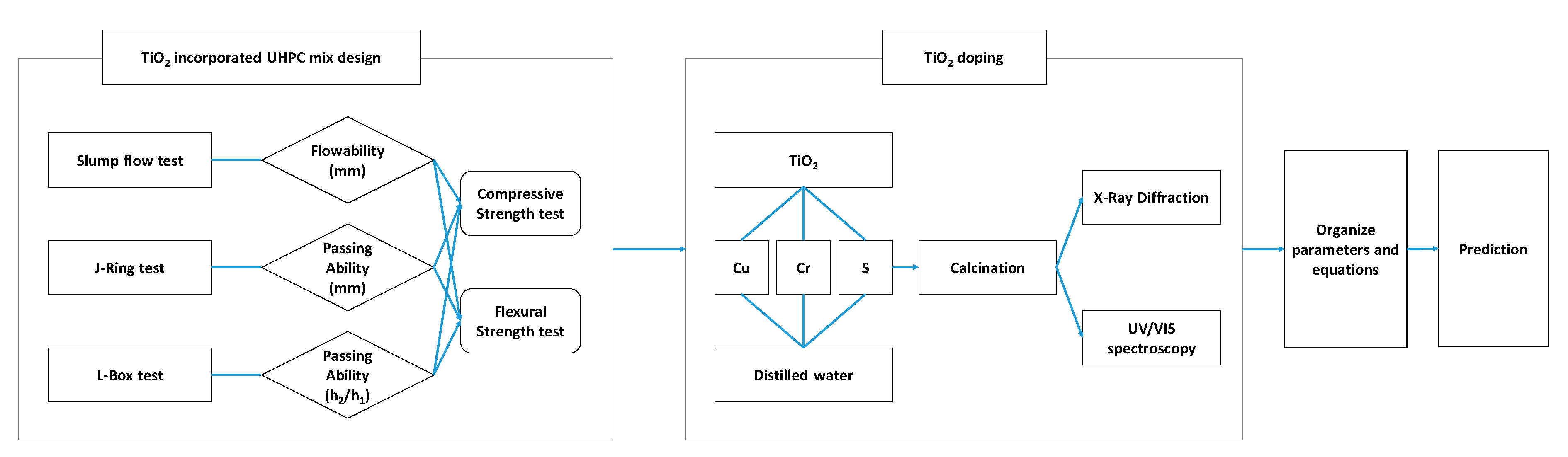
2.4. Mixing Design and Test Methods
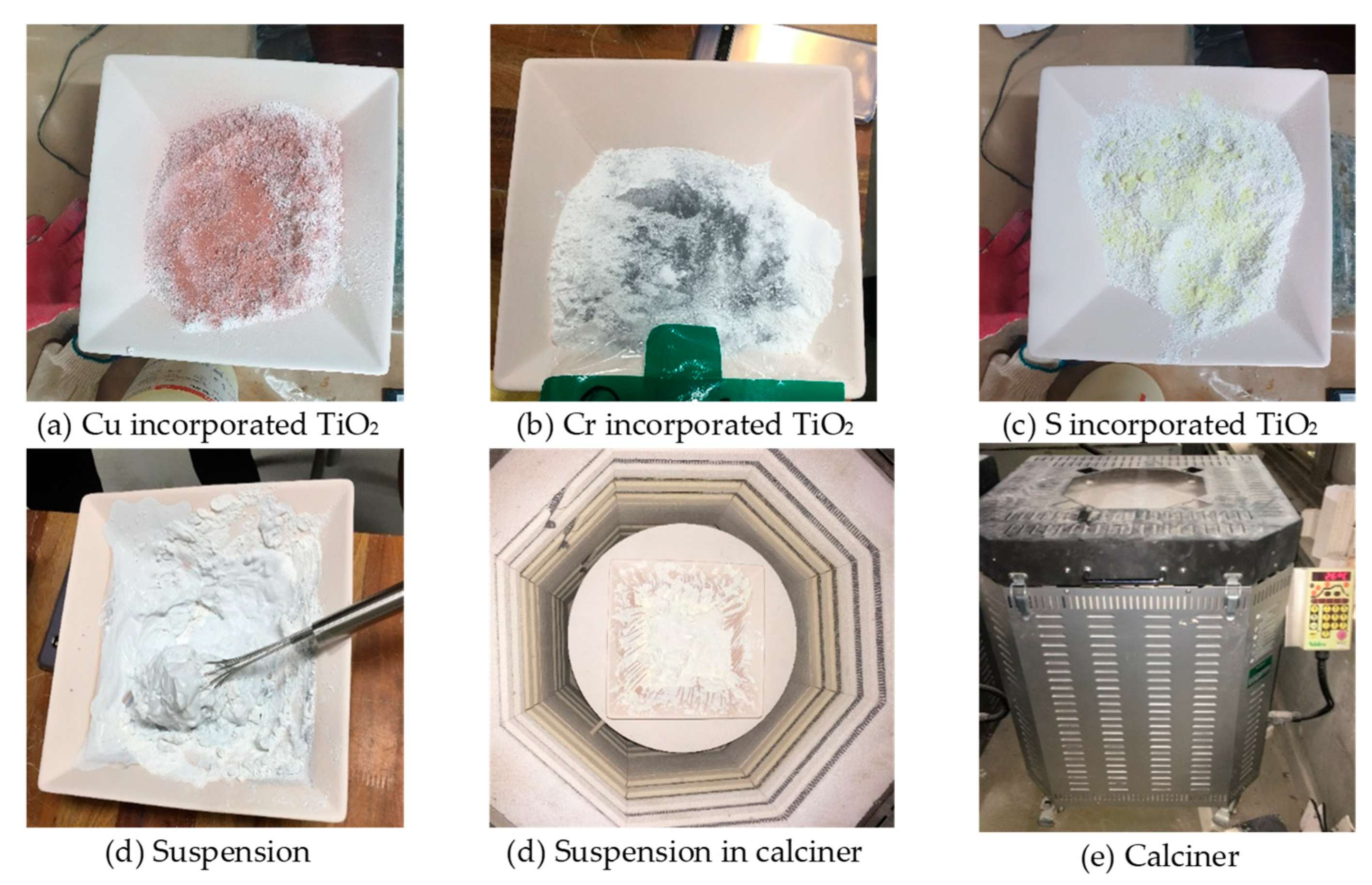
2.5. Doping TiO2 Photocatalyst
2.6. Photocatalytic NO Removal Performance Test
3. Experimental Results
3.1. Optimum Mix Design Results
3.2. Doping TiO2 Photocatalyst Results
3.3. Results of Photocatlaytic NO Removal Removal Test
4. Discussion
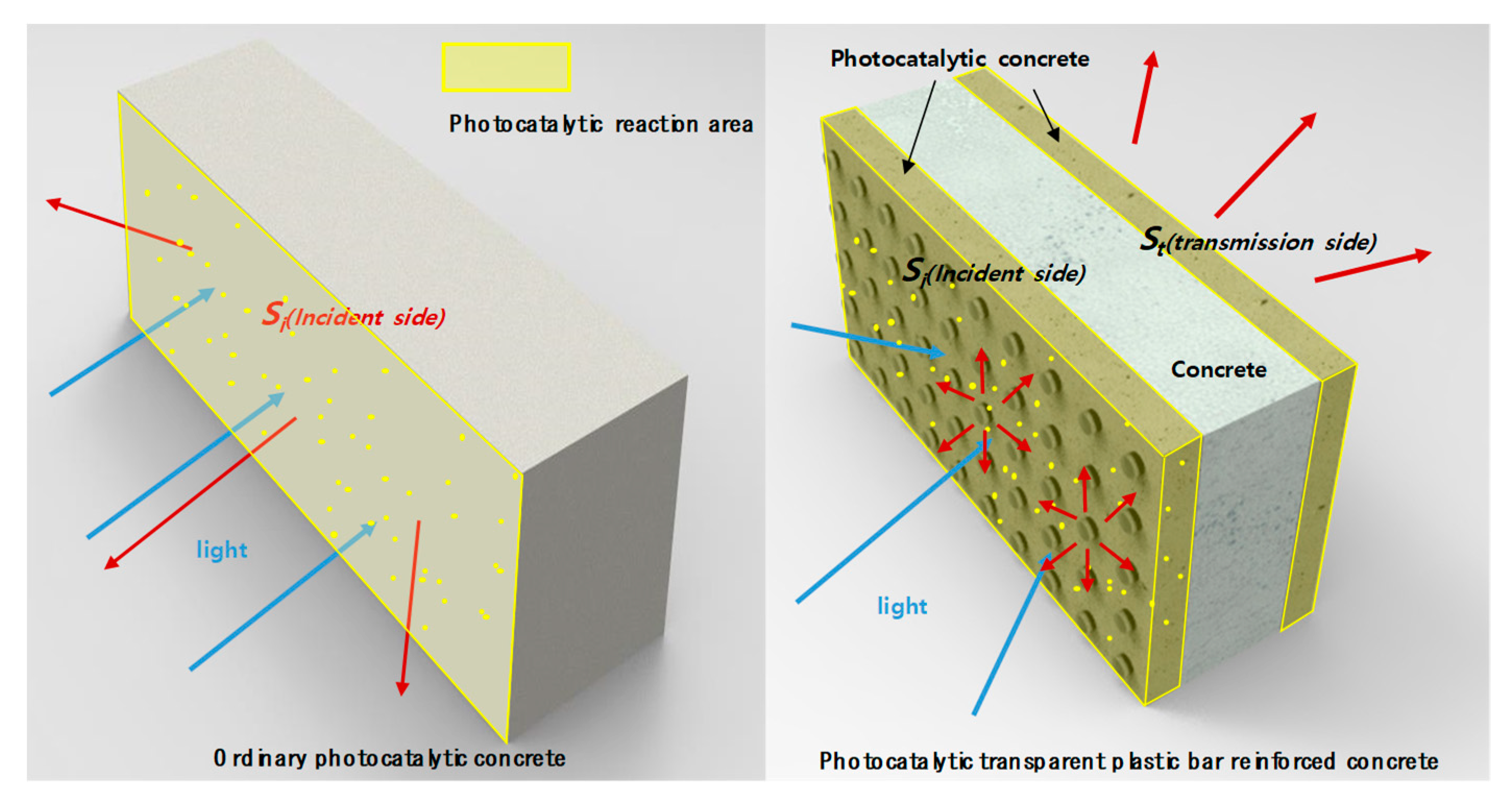
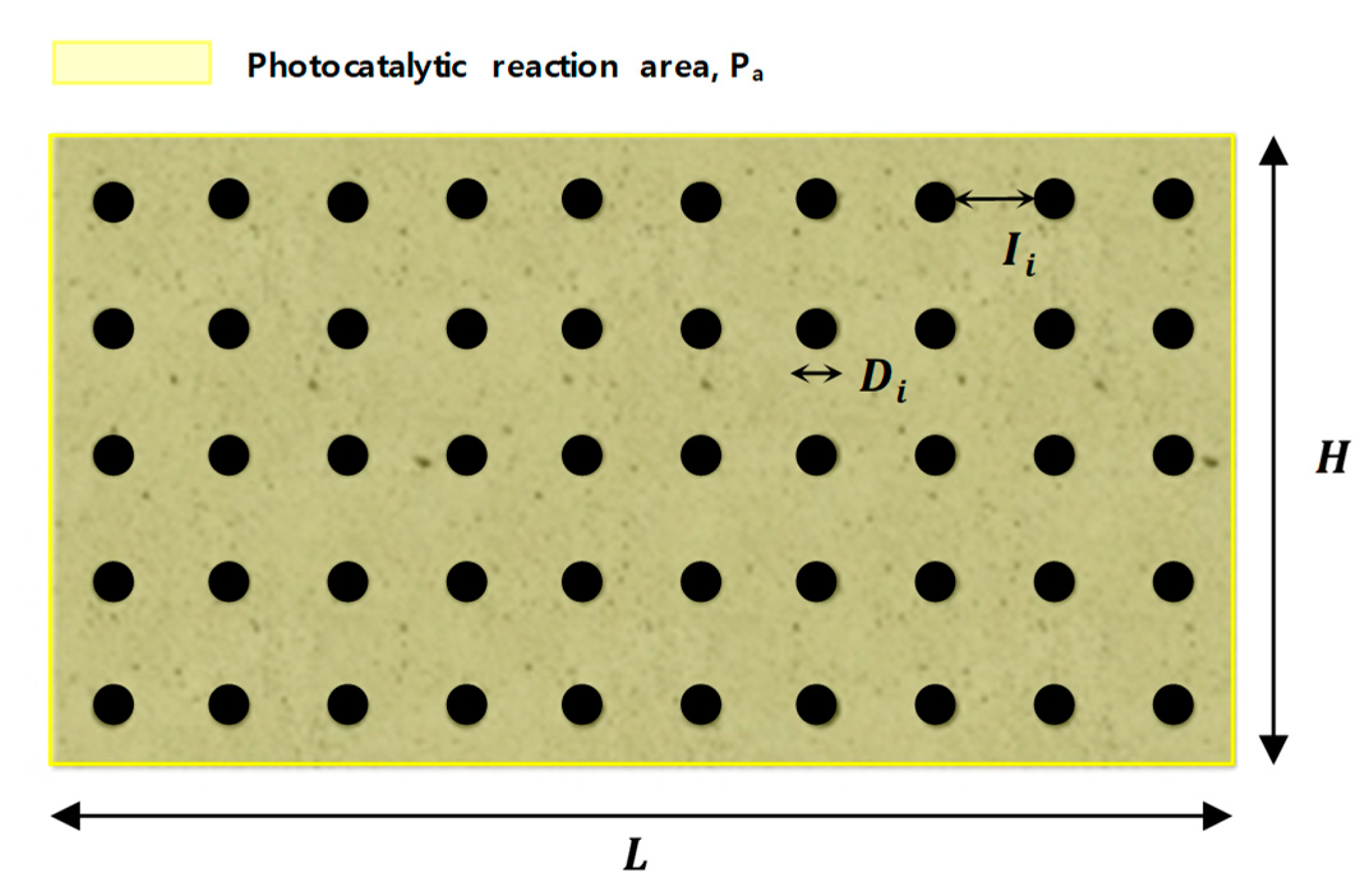
4.1. Photocatlaytic Reaction Mechanism of Transparent Plastic Bar Reinforced Concrete
4.2. Estimation of Efficiency of Visible Light Response Photocatalytic Transparent Bar Reinforced Concrete
5. Conclusions
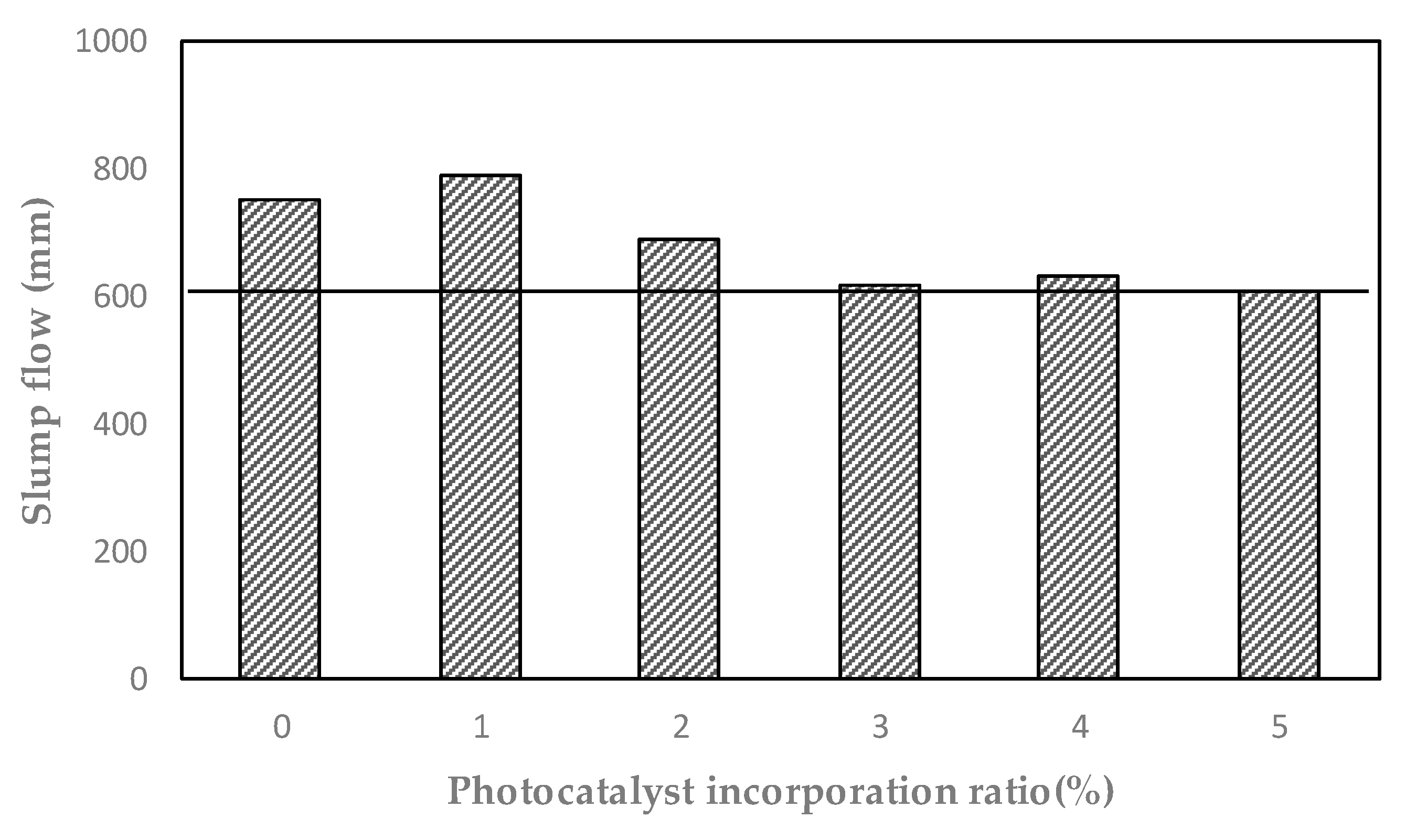
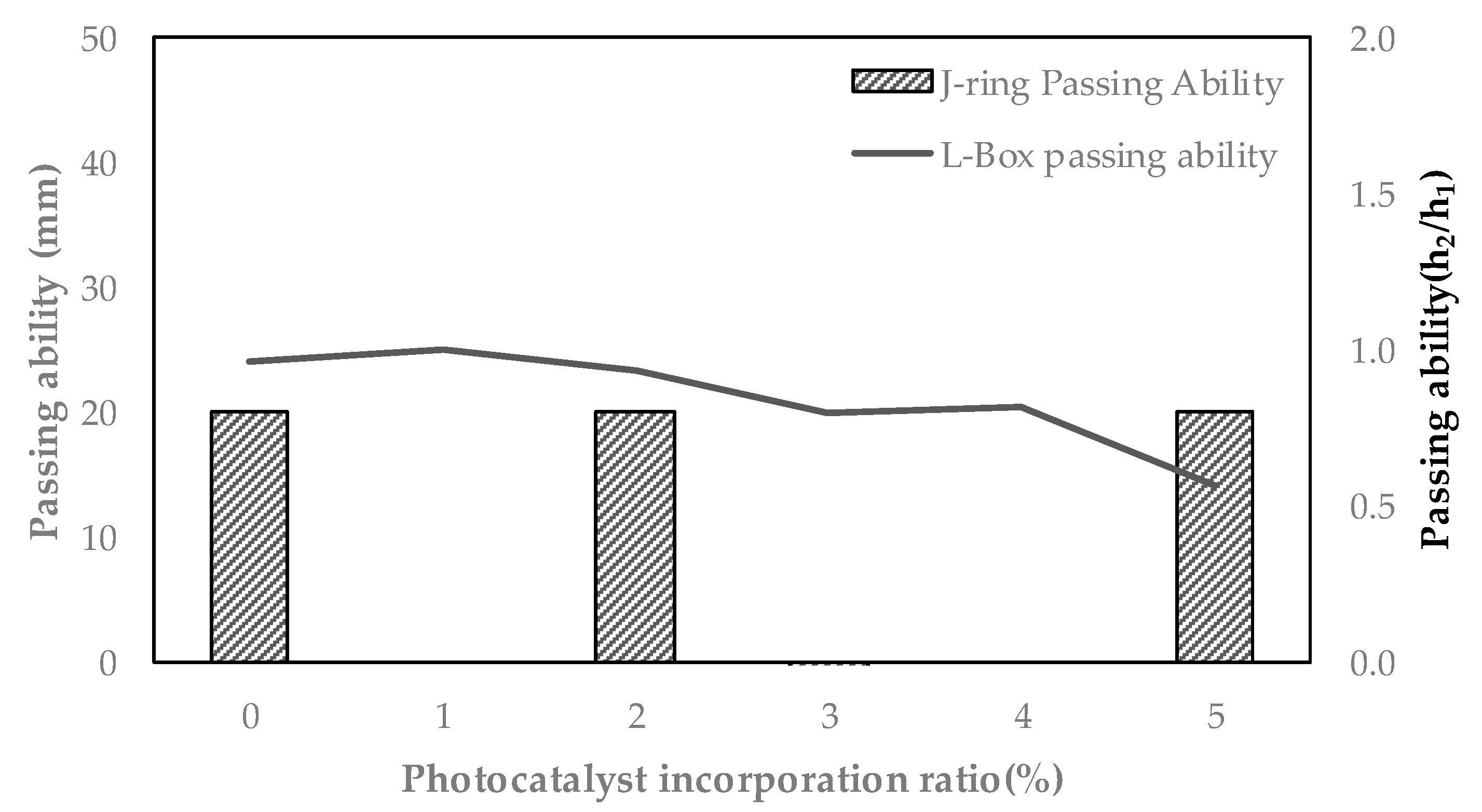
- As the photocatalyst mixing rate increased, the slump flow of UHPC gradually decreased. It is believed that an increase in the amount of mixed photocatalyst fineness induces a decrease in slump flow. As a result of the J-Ring test, all test specimens showed a pass rate of less than 25 mm, and the L-Box test had a pass rate of 0.8 or higher, so it was judged that good material dispersion during the pouring process was possible.
- Compressive strength is 100 MPa or more, and the flexural test has confirmed the test results of 7.0 to 8.4 MPa, and it is judged that there will be no major difficulties in manufacturing interior and exterior materials in the future.
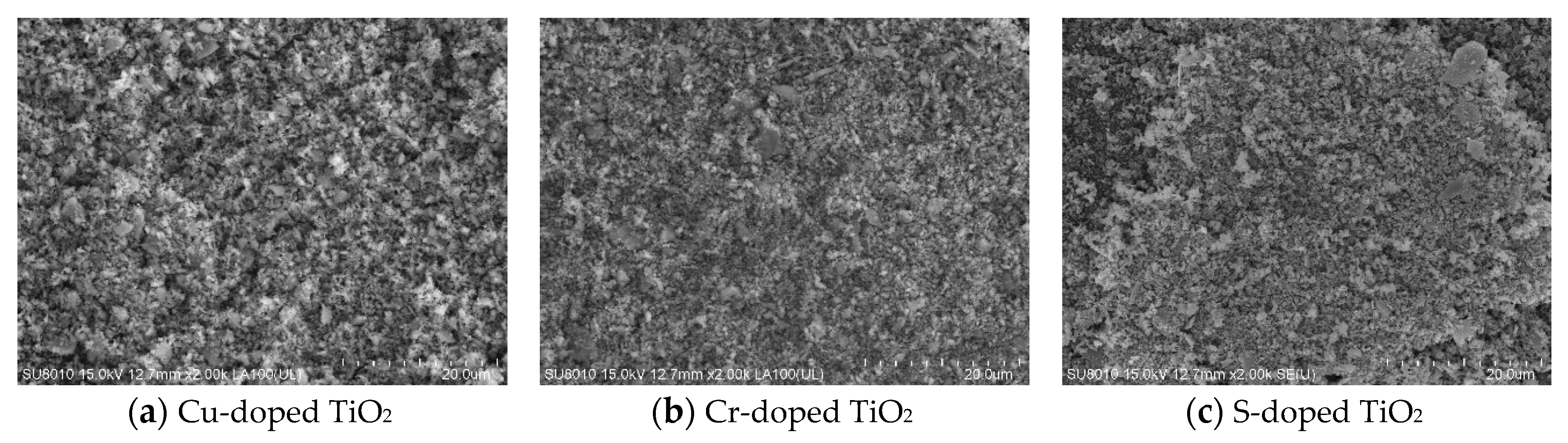
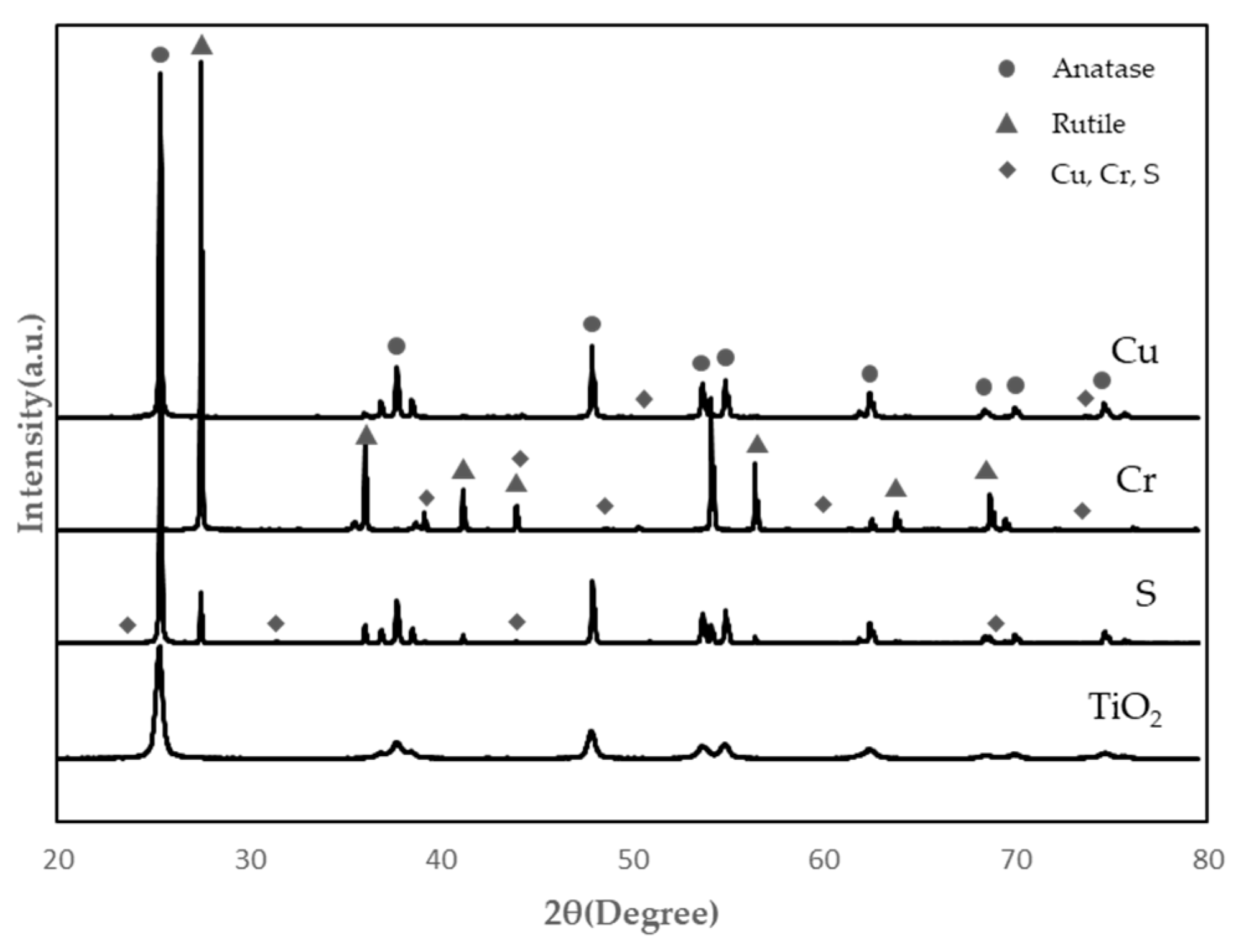
- SEM and EDS analysis of TiO2 in powder form after calcination at 1000 °C for 24 h showed that TiO2 doped with Cu had the best microstructure and EDS peak value. In addition, as a result of the XRD test, anatase peaks were confirmed for Cu, Cr, and S.
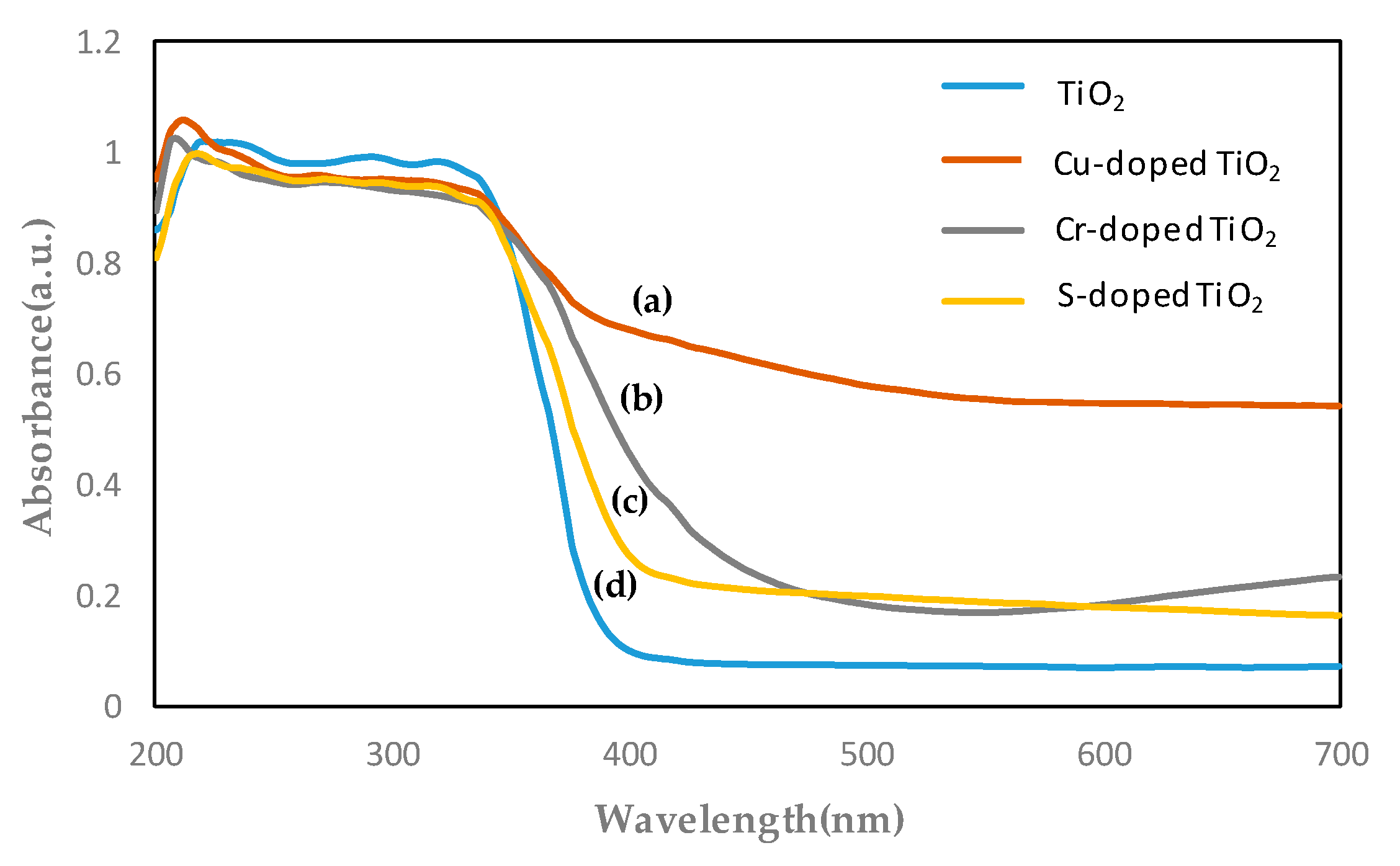
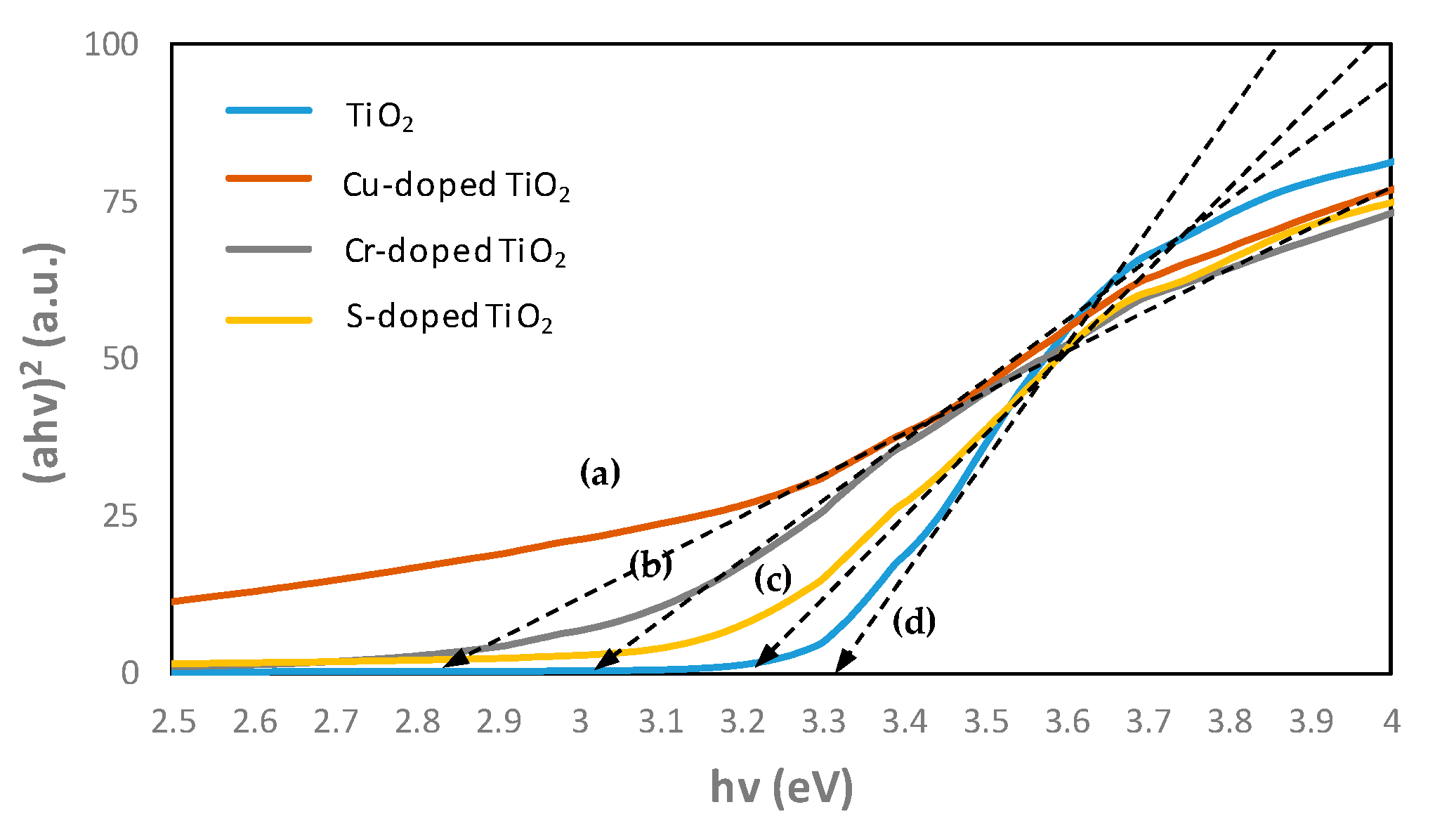
- As a result of confirming the TiO2 energy band gap in the UV-visible absorption spectrum test, TiO2 doped with Cu has a strong absorption of 0.8 up to 700 nm, and the inherent band gaps have also decreased from 3.3 to 2.8 eV. It was confirmed that the TiO2 test specimen doped with Cr and S had a sharp drop in absorption from 400 nm.
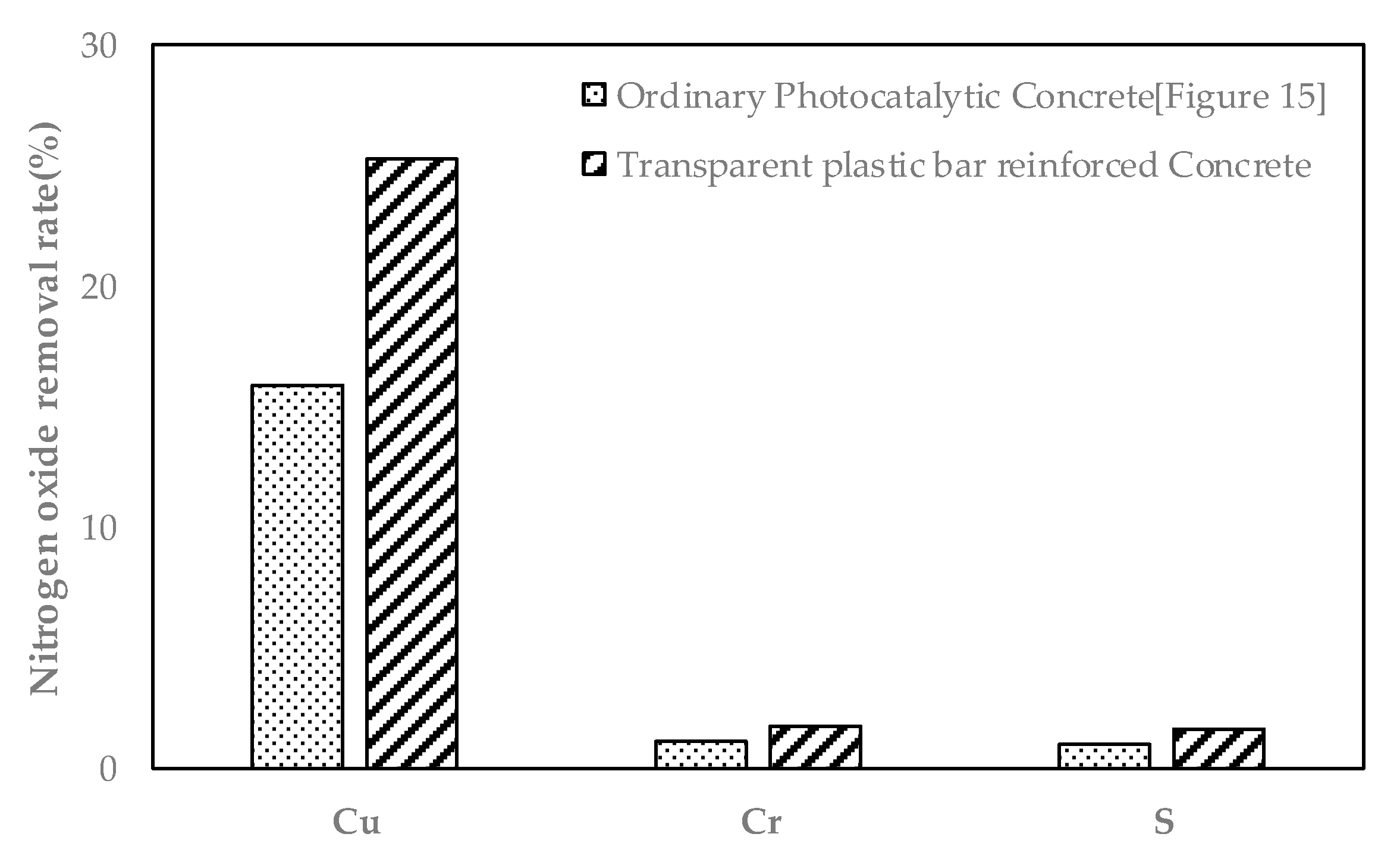
- The specimen mixed with TiO2 doped with Cu showed a removal rate of about 15.8% for 5 h in the NO removal test, showing the best results among the specimens. When applied to TPBRC using this method, visible light is transmitted through the inside of the test specimen. Considering the photocatalytic effect on the other side of the specimen, it is believed that about 25.3% of NO removal efficiency can be secured.
- Although the test results for the NOx removal test could not be derived due to the lack of the test method for the TPBRC test body, it confirmed the possibility that harmful substances in the indoor space can be improved due to photocatalytic reaction under visible light when considering the light transmittance and the exposed area on the concrete surface.
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Li, C.; Zhang, J. One-step preparation, characterization and visible-light photocatalytic activity of Cr-doped TiO2 with anatase and rutile bicrystalline phases. Chem. Eng. J. 2012, 191, 402–409. [Google Scholar] [CrossRef]
- Li, C.; Ma, Z.; Zhang, L.; Qian, R. Preparation of Ni/TiO2 nanoparticles and their catalytic performance on the thermal decomposition of ammonium perchlorate. Chin. J. Chem. 2009, 27, 1863–1867. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Yu, J.C. Thermally stable ordered mesoporous CeO2/TiO2 visible-light photocatalysts. Phys. Chem. Chem. Phys. 2009, 11, 3775–3782. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.; Kim, W.; Jeon, K. Experimental research about photocatalyst heat treatment doping level analysis. J. Archit. Inst. Korea Struct. Constr. 2010, 26, 67–74. [Google Scholar]
- Colón, G.; Maicu, M.; Hidalgo, M.C.; Navío, J.A. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B Environ. 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Nolan, M.; Pillai, S.C. Cu-Doped TiO2: Visible light assisted photocatalytic antimicrobial activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef] [Green Version]
- Praliaud, H.; Mikhailenko, S.; Chajar, Z.; Primet, M. Surface and bulk properties of Cu-ZSM-5 and Cu/Al2O3 solids during redox treatments. Correlation with the selective reduction of nitric oxide by hydrocarbons. Appl. Catal. B Environ. 1998, 16, 359–374. [Google Scholar] [CrossRef]
- Kim, W.; Jung, J.; Jeon, K. Development of visible ray photocatalyst for reinforcement of air cleaning function indoor and outdoors. J. Archit. Inst. Korea Struct. Constr. 2009, 25, 121–128. [Google Scholar]
- Ghicov, A.; Schmidt, B.; Kunze, J.; Schmuki, P. Photoresponse in the visible range from Cr doped TiO2 nanotubes. Chem. Phys. Lett. 2007, 433, 323–326. [Google Scholar] [CrossRef]
- Peng, Y.H.; Huang, G.F.; Huang, W.Q. Visible-light absorption and photocatalytic activity of Cr-doped TiO2 nanocrystal films. Adv. Powder Technol. 2012, 23, 8–12. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.; Lee, J.; Kim, W. An experiment research about a repository visible light reaction TiO2 development. Proc. Korea Concr. Inst. 2007, 5, 911–914. [Google Scholar]
- Ohno, T.; Akiyoshi, M.; Umebayashi, T.; Asai, K.; Mitsui, T.; Matsumura, M. Preparation of S-doped TiO2 photocatalysts and their photocatalytic activities under visible light. Appl. Catal. A Gen. 2004, 265, 115–121. [Google Scholar] [CrossRef]
- Nolan, M.; Elliott, S.D.; Mulley, J.S.; Bennett, R.A.; Basham, M.; Mulheran, P. Electronic structure of point defects in controlled self-doping of the TiO2 (110) surface: Combined photoemission spectroscopy and density functional theory study. Phys. Rev. B—Condens. Matter Mater. Phys. 2008, 77, 14–18. [Google Scholar] [CrossRef]
- Michalow, K.A.; Logvinovich, D.; Weidenkaff, A.; Amberg, M.; Fortunato, G.; Heel, A.; Graule, T.; Rekas, M. Synthesis, characterization and electronic structure of nitrogen-doped TiO2 nanopowder. Catal. Today 2009, 144, 7–12. [Google Scholar] [CrossRef]
- El Mragui, A.; Logvina, Y.; Pinto da Silva, L.; Zegaoui, O.; Esteves da Silva, J.C. Synthesis of fe-and co-doped TiO2 with improved photocatalytic activity under visible irradiation toward carbamazepine degradation. Materials 2019, 12, 3874. [Google Scholar] [CrossRef] [Green Version]
- Kim, B. Light Transmitting lightweight concrete with transparent plastic bar. Open Civ. Eng. J. 2017, 11, 615–626. [Google Scholar] [CrossRef]
- Kim, B.; Seo, S. Prediction model of flexural properties of LEFC using foaming agent. J. Korea Inst. Build. Constr. 2019, 19, 9–18. [Google Scholar] [CrossRef]
- Kim, B.; Han, Y. Flexural performance of transparent plastic bar reinforced concrete. Appl. Sci. 2018, 8, 325. [Google Scholar] [CrossRef]
- Kim, B.; Oh, S.; Seo, S. A Study on the development of light emotion friendly concrete block for efficient application of titan-oxide photocatalyst. J. Korea Inst. Struct. Maint. Insp. 2019, 23, 120–131. [Google Scholar] [CrossRef]
- Ryu, G.; Kim, S.; Ahn, G.; Koh, K. Evaluation of the direct tensile behavioral characteristics of UHPC using twisted steel fibers. Adv. Mater. Res. 2013, 602–604, 96–101. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. The effect of TiO2 nanoparticles on water permeability and thermal and mechanical properties of high strength self-compacting concrete. Mater. Sci. Eng. A 2010, 528, 756–763. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, S.; Park, J. Comparative flexural behavior of ultra-high-performance concrete reinforced with hybrid straight steel fibers. Constr. Build. Mater. 2017, 132, 219–229. [Google Scholar] [CrossRef]
- Kang, S.; Park, J.; Ryu, G.; Kim, S. Influence of the filler’s size on the mechanical properties of ultra high performance concrete(UHPC). J. Korean Soc. Civ. Eng. A 2008, 28, 573–580. [Google Scholar]
- ASTM C1611/C1611M-18. Standard Test Method for Slump Flow of Self-Consolidating Concrete; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar] [CrossRef]
- ASTM C1621/C1621M-17. Standard Test Method for Passing Ability of Self-Consolidating Concrete by J-Ring; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar] [CrossRef]
- BS EN 12350-10:2010. Testing Fresh Concrete-Part 10: Selfcompacting Concrete-L Box Test; Technical Standard, British Standards Institution: London, UK, 2010. [Google Scholar]
- KS F 2405. Standard Test Method for Compressive Strength of Concrete; Korea Standards Associations: Seoul, Korea, 2017. [Google Scholar]
- KS F 2408. Standard Test Method for Flexural Strength of Concrete; Korea Standards Associations: Seoul, Korea, 2016. [Google Scholar]
- ISO 17168-1. Fine Cerimics—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials under Indoor Light Environment—Part 1: Removal of Nitic Oxie; International Standards: Geneva, Switzerland, 2018. [Google Scholar]
- De Castro Monsores, K.G.; da Silva, A.O.; Oliveira, S.D.; Rodrigues, J.G.; Weber, R.P. Influence of ultraviolet radiation on polymethylmethacrylate (PMMA). J. Mater. Res. Technol. 2019, 8, 3713–3718. [Google Scholar] [CrossRef]
- Wang, B.; Lin, Q.; Shen, C.; Han, Y.; Tang, J.; Chen, H. Synthesis of MA POSS-PMMA as an intraocular lens material with high light transmittance and good cytocompatibility. RSC Adv. 2014, 4, 52959–52966. [Google Scholar] [CrossRef]
- Karapati, S.; Giannakopoulou, T.; Todorova, N.; Boukos, N.; Antiohos, S.; Papageorgiou, D.; Chaniotakis, E.; Dimotikali, D.; Trapalis, C. TiO2 functionalization for efficient NOx removal in photoactive cement. Appl. Surf. Sci. 2019, 319, 29–36. [Google Scholar] [CrossRef]






| Substance | Cu | Cr | S |
|---|---|---|---|
| Purity (%) | 99 | 99 | 98 |
| Form | powder | powder | powder |
| Mesh | 150 | 150 | - |
| Density (g/cm3) | 8.96 | 7.19 | 2.07 |
| Specimen | W/C (%) | Unit Weight (kg/m3) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | Cement | Silica Fume | Silica Sand | Filler | Super Plasticizer | Antifoamer | TiO2 | ||
| P-0 | 30 | 7.05 | 23.51 | 5.88 | 25.86 | 7.05 | 0.94 | 0.07 | 0 |
| P-1 | 23.28 | 0.235 | |||||||
| P-2 | 23.04 | 0.470 | |||||||
| P-3 | 22.81 | 0.705 | |||||||
| P-4 | 22.57 | 0.940 | |||||||
| P-5 | 22.34 | 1.176 | |||||||
| Difference between Slump Flow and J-Ring Flow | Blocking Assessment |
|---|---|
| 0 to 25 mm | no visible blocking |
| >25 to 50 mm | minimal to noticeable blocking |
| >50 mm | noticeable to extreme blocking |
| Specimen | Slump Flow (mm) | J-Ring Flow (mm) | J-Ring Passing Ability (mm) | L-Box Passing Ability (h2/h1) |
|---|---|---|---|---|
| P-0 | 750 | 730 | 20 | 0.96 |
| P-1 | 790 | 790 | 0 | 1.00 |
| P-2 | 690 | 670 | 20 | 0.93 |
| P-3 | 620 | 630 | 10 | 0.80 |
| P-4 | 630 | 630 | 0 | 0.82 |
| P-5 | 610 | 590 | 20 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, S.-H.; Kim, B.-I. Effect of Cu, Cr, S Doped TiO2 for Transparent Plastic Bar Reinforced Concrete. Appl. Sci. 2020, 10, 7334. https://doi.org/10.3390/app10207334
Seo S-H, Kim B-I. Effect of Cu, Cr, S Doped TiO2 for Transparent Plastic Bar Reinforced Concrete. Applied Sciences. 2020; 10(20):7334. https://doi.org/10.3390/app10207334
Chicago/Turabian StyleSeo, Seung-Hoon, and Byoung-Il Kim. 2020. "Effect of Cu, Cr, S Doped TiO2 for Transparent Plastic Bar Reinforced Concrete" Applied Sciences 10, no. 20: 7334. https://doi.org/10.3390/app10207334







