Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism, Media and Culture Conditions
2.2. Analyses
3. Results and Discussion
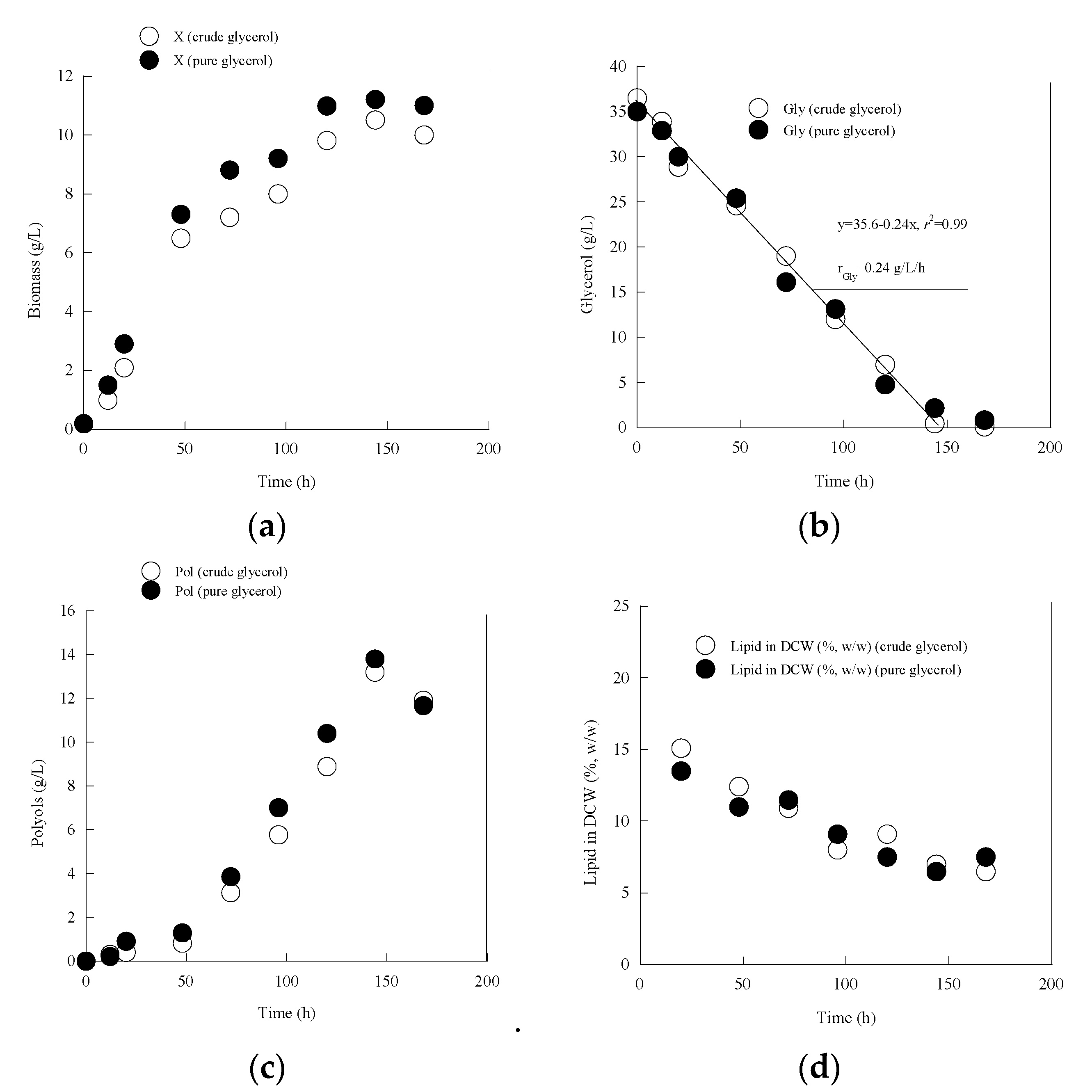
3.1. Metabolic Characterization of Yarrowia lipolytica LMBF Y-46: Effect of Different Glycerol Sources under Nitrogen Limitation
3.2. Metabolic Characterization of Yarrowia lipolytica LMBF Y-46: Effect of Glycerol Concentration in Media with Constant Initial Nitrogen in Shake-Flask Trials
3.3. Metabolic Characterization of Yarrowia lipolytica LMBF Y-46: Scale-Up in Laboratory-Scale Bioreactor
3.4. Modeling the Production of Polyols by Yarrowia lipolytica LMBF Y-46 Growing on Glycerol in Shake-Flask Experiments
3.5. Cellular Lipids of Yarrowia lipolytica LMBF Y-46
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Chatzifragkou, A.; Papanikolaou, S. Effect of impurities in biodiesel-derived waste glycerol on the performance and feasibility of biotechnological processes. Appl. Microbiol. Biotechnol. 2012, 95, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Robak, M.; Lazar, Z.; Tomaszewska, L.; Rymowicz, W. Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass Bioenergy 2013, 48, 148–166. [Google Scholar] [CrossRef]
- Russmayer, H.; Egermeier, M.; Kalemasi, D.; Sauer, M. Spotlight on biodiversity of microbial cell factories for glycerol conversion. Biotechnol. Adv. 2019, 37, 107395. [Google Scholar] [CrossRef]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 2018, 38, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdani, S.S.; Gonzalez, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef]
- Clomburg, J.M.; Gonzalez, R. Anaerobic fermentation of glycerol: A platform for renewable fuels and chemicals. Trend Biotechnol. 2013, 31, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Papanikolaou, S.; Fakas, S.; Fick, M.; Chevalot, I.; Galiotou-Panayotou, M.; Komaitis, M.; Marc, I.; Aggelis, G. Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl-esters) manufacturing process: Production of 1,3-propanediol, citric acid and single cell oil. Biomass Bioenergy 2008, 32, 60–71. [Google Scholar] [CrossRef]
- Kothri, M.; Mavrommati, M.; Elazzazy, A.M.; Baeshen, M.N.; Moussa, T.A.; Aggelis, G. Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol. Lett. 2020, 367, fnaa028. [Google Scholar] [CrossRef]
- Fickers, P.; Cheng, H.; Lin, C.S.K. Sugar alcohols and organic acids synthesis in Yarrowia lipolytica: Where are we? Microorganisms 2020, 8, 574. [Google Scholar] [CrossRef]
- Bankar, V.A.; Kumar, R.A.; Zinjarde, S.S. Environmental and industrial applications of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2009, 84, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Zinjarde, S.S.; Apte, M.; Mohite, P. Yarrowia lipolytica and pollutants: Interactions and applications. Biotechnol. Adv. 2014, 32, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Carly, F.; Fickers, P. Erythritol production by yeasts: A snapshot of current knowledge. Yeast 2018, 35, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Timumi, A.; Guillouet, S.E.; Molina-Jouve, C.; Fillaudeau, L.; Gorret, N. Impacts of environmental conditions on product formation and morphology of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2018, 102, 3831–3848. [Google Scholar] [CrossRef]
- Egermeier, M.; Sauer, M.; Marx, H. Golden gate-based metabolic engineering strategy for wild-type strains of Yarrowia lipolytica. FEMS Microbiol. Lett. 2019, 366, fnz022. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Filippousi, R.; Antoniou, D.; Varfi, E.; Xenopoulos, E.; Sarris, D.; Papanikolaou, S. Production of added-value microbial metabolites during growth of yeast strains on media composed of biodiesel-derived crude glycerol and glycerol/xylose blends. FEMS Microbiol. Lett. 2020, 367, fnaa063. [Google Scholar] [CrossRef]
- Τryfinopoulou, P.; Tsakalidou, E.; Nychas, G.J.E. Characterization of Pseudomonas spp. associated with spoilage of gilt-head sea-bream stored under various conditions. Appl. Environ. Microbiol. 2002, 68, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanikolaou, S.; Kampisopoulou, E.; Blanchard, F.; Rondags, E.; Gardeli, C.; Koutinas, A.A.; Chevalot, I.; Aggelis, G. Production of secondary metabolites through glycerol fermentation under carbon-excess conditions by the yeasts Yarrowia lipolytica and Rhodosporidium toruloides. Eur. J. Lipid Sci. Technol. 2017, 119, 1600507. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Citric acid production by Yarrowia lipolytica cultivated on olive-mill wastewater-based media. Bioresour. Technol. 2008, 99, 2419–2428. [Google Scholar] [CrossRef]
- Palaiogeorgou, A.M.; Papanikolaou, S.; de Castro, A.M.; Freire, D.M.G.; Kookos, I.K.; Koutinas, A.A. A newly isolated Enterobacter sp. strain produces 2,3-butanediol during its cultivation on low-cost carbohydrate-based substrates. FEMS Microbiol. Lett. 2019, 366, fny280. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, I.K.; Zeng, A.P.; et al. Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2017, 17, 262–281. [Google Scholar] [CrossRef] [PubMed]
- Chatzifragkou, A.; Makri, A.; Belka, A.; Bellou, S.; Mavrou, M.; Mastoridou, M.; Mystrioti, P.; Onjaro, G.; Aggelis, G.; Papanikolaou, S. Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 2011, 36, 1097–1108. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J. Appl. Microbiol. 2002, 92, 737–744. [Google Scholar] [CrossRef]
- Gardeli, C.; Athenaki, M.; Xenopoulos, E.; Mallouchos, A.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Lipid production and characterization by Mortierella (Umbelopsis) isabellina cultivated on lignocellulosic sugars. J. Appl. Microbiol. 2017, 123, 1461–1477. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Sarantou, S.; Komaitis, M.; Aggelis, G. Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple-limited media. J. Appl. Microbiol. 2004, 97, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Moustogianni, A.; Makri, A.; Aggelis, G. Lipids containing polyunsaturated fatty acids synthesized by Zygomycetes grown on glycerol. App. Biochem. Biotechnol. 2012, 166, 146–158. [Google Scholar] [CrossRef]
- Metsoviti, M.; Paraskevaidi, K.; Koutinas, A.; Zeng, A.P.; Papanikolaou, S. Production of 1,3-propanediol, 2,3-butanediol and ethanol by a newly isolated Klebsiella oxytoca strain growing on biodiesel-derived glycerol based media. Proc. Biochem. 2012, 47, 1872–1882. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Dietz, D.; Komaitis, M.; Zeng, A.P.; Papanikolaou, S. Effect of biodiesel-derived waste glycerol impurities on biomass and 1,3-propanediol production of Clostridium butyricum VPI 1718. Biotechnol. Bioeng. 2010, 107, 76–84. [Google Scholar] [CrossRef]
- Tomaszewska, L.; Rywińska, A.; Gładkowski, W. Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1333–1343. [Google Scholar] [CrossRef] [Green Version]
- Venkataramanan, K.P.; Boatman, J.J.; Kurniawan, Y.; Taconi, K.A.; Bothun, G.D.; Scholz, C. Impact of impurities in biodiesel-derived crude glycerol on the fermentation by Clostridium pasteurianum ATCC 6013. Appl. Microbiol. Biotechnol. 2012, 93, 1325–1335. [Google Scholar] [CrossRef]
- Mirończuk, A.M.; Furgala, I.; Rakicka, M.; Rymowicz, W. Enhanced production of erythritol by Yarrowia lipolytica on gycerol in repeated batch cultures. J. Ind. Microbiol. Biotechnol. 2014, 41, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrowolski, A.; Mituła, P.; Rymowicz, W.; Mirończuk, A.M. Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour. Technol. 2016, 207, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Uprety, B.K.; Dalli, S.S.; Rakshit, S.K. Bioconversion of crude glycerol to microbial lipid using a robust oleaginous yeast Rhodosporidium toruloides ATCC 10788 capable of growing in the presence of impurities. Energy Conv. Manag. 2017, 135, 117–128. [Google Scholar] [CrossRef]
- Chebbi, H.; Leiva-Candia, D.; Carmona-Cabello, M.; Jaouani, A.; Dorado, M.P. Biodiesel production from microbial oil provided by oleaginous yeasts from olive oil mill wastewater growing on industrial glycerol. Ind. Crop. Prod. 2019, 139, 111535. [Google Scholar] [CrossRef]
- Carly, F.; Vandermies, M.; Telek, S.; Steels, S.; Thomas, S.; Nicaud, J.M.; Fickers, P. Enhancing erythritol productivity in Yarrowia lipolytica using metabolic engineering. Metab. Eng. 2017, 42, 19–24. [Google Scholar] [CrossRef]
- Egermeier, M.; Russmayer, H.; Sauer, M.; Marx, H. Metabolic flexibility of Yarrowia lipolytica growing on glycerol. Front. Microbiol. 2017, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Filippoussi, R.; Antoniou, D.; Tryfinopoulou, P.; Nisiotou, A.A.; Nychas, G.J.; Koutinas, A.A.; Papanikolaou, S. Isolation, identification and screening of yeasts towards their ability to assimilate biodiesel-derived crude glycerol: Microbial production of polyols, endopolysaccharides and lipid. J. Appl. Microbiol. 2019, 127, 1080–1100. [Google Scholar] [CrossRef]
- André, A.; Chatzifragkou, A.; Diamantopoulou, P.; Sarris, D.; Philippoussis, A.; Galiotou-Panayotou, M.; Komaitis, M.; Papanikolaou, S. Biotechnological conversions of bio-diesel derived crude glycerol by Yarrowia lipolytica strains. Eng. Life Sci. 2009, 9, 468–478. [Google Scholar] [CrossRef]
- Makri, A.; Fakas, S.; Aggelis, G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010, 101, 2351–2358. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Beopoulos, A.; Koletti, A.; Thevenieau, F.; Koutinas, A.A.; Nicaud, J.M.; Aggelis, G. Importance of the methyl-citrate cycle on glycerol metabolism in the yeast Yarrowia lipolytica. J. Biotechnol. 2013, 168, 303–314. [Google Scholar] [CrossRef]
- Evans, C.T.; Ratledge, C. Phosphofructokinase and its regulation of the flux of carbon from glucose to lipid in the oleaginous yeast Rhodosporidium toruloides. J. Gen. Microbiol 1984, 130, 3251–3264. [Google Scholar]
- Park, W.S.; Murphy, P.A.; Glatz, B.A. Lipid metabolism and cell composition of the oleaginous yeast Apiotrichum curvatum grown at different carbon to nitrogen ratios. Can. J. Microbiol. 1990, 36, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, A.; Perdikouli, D.; Aggeli, D.; Aggelis, G. Laboratory evolution strategies for improving lipid accumulation in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2019, 103, 8585–8596. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywińska, A.; Zarowska, B.; Juszczyk, P. Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica. Chem. Pap. 2006, 60, 391–394. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywińska, A.; Gladkowski, W. Simultaneous production of citric acid and erythritol from crude glycerol by Yarrowia lipolytica Wratislavia K1. Chem. Pap. 2008, 62, 239–246. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywińska, A.; Marcinkiewicz, M. High-yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica. Biotechnol. Lett. 2009, 31, 377–380. [Google Scholar] [CrossRef]
- Rymowicz, W.; Fatykhova, A.R.; Kamzolova, S.V.; Rywińska, A.; Morgunov, I.G. Citric acid production from glycerol-containing waste of biodiesel industry by Yarrowia lipolytica in batch, repeated batch, and cell recycle regimes. Appl. Microbiol. Biotechnol. 2010, 87, 971–979. [Google Scholar] [CrossRef]
- Tomaszewska, L.; Rakicka, M.; Rymowicz, W.; Rywińska, A. A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res. 2014, 14, 966–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarris, D.; Sampani, Z.; Rapti, A.; Papanikolaou, S. Valorization of crude glycerol, residue deriving from biodiesel-production process, with the use of wild-type new isolated Yarrowia lipolytica strains: Production of metabolites with pharmaceutical and biotechnological interest. Curr. Pharm. Biotechnol. 2019, 20, 881–894. [Google Scholar] [CrossRef]
- Mirończuk, A.M.; Dobrowolski, A.; Rakicka, M.; Rywińska, A.; Rymowicz, W. Newly isolated mutant of Yarrowia lipolytica MK1 as a proper hostfor efficient erythritol biosynthesis from glycerol. Proc. Biochem. 2015, 50, 61–68. [Google Scholar] [CrossRef]
- Rakicka, M.; Kieron, A.; Hapeta, P.; Neuvéglise, C.; Lazar, Z. Sweet and sour potential of yeast from the Yarrowia clade. Biomass Bioenergy 2016, 92, 48–54. [Google Scholar] [CrossRef]
- Rakicka, M.; Biegalska, A.; Rymowicz, W.; Dobrowolski, A.; Mirończuk, A.M. Polyol production from waste materials by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 393–399. [Google Scholar] [CrossRef]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of added-value chemical compounds through bioconversions of olive-mill wastewaters blended with crude glycerol by a Yarrowia lipolytica strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yu, X.; Wang, Z.; Xia, J.; Yan, Y.; Hu, L.; Wang, X.; Xu, J.; He, A.; Zhao, P. Enhanced erythritol production by a Snf1-deficient Yarrowia lipolytica strain under nitrogen-enrichedfermentation condition. Food Bioprod. Proc. 2020, 119, 306–316. [Google Scholar] [CrossRef]
- Rywińska, A.; Rymowicz, W.; Zarowska, B.; Skrzypiński, A. Comparison of citric acid production from glycerol and glucose by different strains of Yarrowia lipolytica. World J. Microbiol. Biotechnol. 2010, 26, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Morgunov, I.; Aurich, A.; Perevoznikova, O.A.; Shishkanova, N.V.; Stottmeister, U.; Finogenova, T.V. Lipase secretion and citric acid production in Yarrowia lipolytica yeast grown on animal and vegetable fat. Food Technol. Biotechnol. 2005, 43, 113–122. [Google Scholar]
- Förster, Α.; Aurich, Α.; Mauersberger, S.; Barth, G. Citric acid production from sucrose using a recombinant strain of the yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 75, 1409–1417. [Google Scholar]
- Rywińska, A.; Rymowicz, W. Citric acid production from raw glycerol by Yarrowia lipolytica Wratislavia 1.31. In Microbial Conversions of Raw Glycerol; Aggelis, G., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2009; pp. 19–30. [Google Scholar]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. The citric acid production from raw glycerol by Yarrowia lipolytica yeast and its regulation. Appl. Microbiol. Biotechnol. 2013, 97, 7387–7397. [Google Scholar] [CrossRef]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Rymowicz, W. Chemostat study of citric acid production from glycerol by Yarrowia lipolytica. J. Biotechnol. 2011, 152, 54–57. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Fatykhova, A.R.; Dedyukhina, E.G.; Anastassiadis, S.G.; Golovchenko, N.P.; Morgunov, I.G. Citric acid production by yeast grown on glycerol-containing waste from biodiesel industry. Food Technol. Biotechnol. 2011, 49, 65–74. [Google Scholar]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Erten, H. Screening various Yarrowia lipolytica strains for citric acid production. Yeast 2019, 36, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Aseptic production of citric and isocitric acid from crude glycerol by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2019, 38, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.; Lopes, M.; Mota, M.; Belo, I. Oxygen transfer rate and pH as major operating parameters of citric acid production from glycerol by Yarrowia lipolytica W29 and CBS 2073. Chem. Pap. 2016, 70, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, P.; Lopes, M.; Mota, M.; Belo, I. Oxygen mass transfer impact on citric acid production by Yarrowia lipolytica from crude glycerol. Biochem. Eng. J. 2016, 110, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Rywińska, A.; Musiał, I.; Rymowicz, W.; Żarowska, B.; Boruczkowski, T. Effect of agitation and aeration on the citric acid production by Yarrowia lipolytica grown on glycerol. Prep. Biochem. Biotechnol. 2012, 42, 279–291. [Google Scholar] [CrossRef]
- Tan, M.-J.; Chen, X.; Wang, Y.-K.; Liu, G.-L.; Chi, Z.-M. Enhanced citric acid production by a yeast Yarrowia lipolytica over-expressing a pyruvate carboxylase gene. Bioprocess. Biosyst. Eng. 2016, 39, 1289–1296. [Google Scholar] [CrossRef]
- Moresi, C. Effect of glucose concentration on citric acid production by Yarrowia lipolytica—Kinetics of the trophophase, citrate lag phase and idiophase. J. Chem. Technol. Biotechnol. 1994, 60, 387–395. [Google Scholar] [CrossRef]
- Klasson, T.K.; Clausen, E.C.; Gaddy, J.L. Continuous fermentation for the production of citric acid from glucose. Appl. Biochem. Biotechnol. 1989, 21, 491–509. [Google Scholar] [CrossRef]
- Rane, K.; Sims, K. Production of citric acid by Candida lipolytica Y 1095: Effect of glucose concentration on yield and productivity. Enz. Microb. Technol. 1993, 15, 646–651. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Shishkanova, N.; Morgunov, I.G.; Finogenova, T.V. Oxygen requirements for growth and citric acid production of Yarrowia lipolytica. FEMS Yeast Res. 2003, 3, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Rywińska, A.; Rymowicz, W.; Marcinkiewicz, M. Valorization of raw glycerol for citric acid production by Yarrowia lipolytica yeast. Electron. J. Biotechnol. 2010, 13, 4. [Google Scholar] [CrossRef] [Green Version]
- Rywińska, A.; Rymowicz, W. High-yield production of citric acid by Yarrowia lipolytica on glycerol in repeated-batch bioreactors. J. Ind. Microbiol. Biotechnol. 2010, 37, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Lunina, J.N.; Morgunov, I.G. Biochemistry of citric acid production from rapeseed oil by Yarrowia lipolytica yeast. J. Am. Oil Chem. Soc. 2011, 88, 1965–1976. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chi, Z.; Liu, G.L.; Madzak, C.; Chi, Z.M. Both decrease in ACL1 gene expression and increase in ICL1 gene expression in marine-derived yeast Yarrowia lipolytica expressing INU1 gene enhance citric acid production from inulin. Mar. Biotechnol. 2013, 15, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Morgunov, I.G. Metabolic peculiarities of the citric acid overproduction from glucose in yeasts Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 433–440. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. Citric acid production by Yarrowia lipolytica yeast on different renewable raw materials. Fermentation 2018, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Diamantopoulou, P.; Stoforos, N.G.; Xenopoulos, E.; Sarris, D.; Psarianos, D.; Philippoussis, A.; Papanikolaou, S. Lipid production by Cryptococcus curvatus growing on commercial xylose and subsequent valorization of fermentation waste-waters for the production of edible and medicinal mushrooms. Biochem. Eng. J. 2020, 162, 107706. [Google Scholar] [CrossRef]
- Sarris, D.; Stoforos, N.G.; Mallouchos, A.; Kookos, I.K.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Production of added-value metabolites by Yarrowia lipolytica growing in olive mill wastewater-based media under aseptic and non-aseptic conditions. Eng. Life Sci. 2017, 17, 695–709. [Google Scholar] [CrossRef] [Green Version]
- Aggelis, G.; Sourdis, J. Prediction of lipid accumulation-degradation in oleaginous micro-organisms growing on vegetable oils. Antonie Leeuwenhoek 1997, 72, 159–165. [Google Scholar] [CrossRef]
- Galiotou-Panayotou, M.; Kalantzi, O.; Aggelis, G. Modelling of simultaneous production of polygalacturonase and exopolysaccharide by Aureobasidium pullulans ATHUM 2915. Antonie Leeuwenhoek 1998, 73, 155–162. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Modelling aspects of the biotechnological valorization of raw glycerol: Production of citric acid by Yarrowia lipolytica and 1,3-propanediol by Clostridium butyricum. J. Chem. Technol. Biotechnol. 2003, 78, 542–547. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Sources of microbial oils with emphasis to Mortierella (Umbelopsis) isabellina fungus. World J. Microbiol. Biotechnol. 2019, 35, 63. [Google Scholar] [CrossRef]
- Ochoa-Estopier, A.; Guillouet, S.E. D-stat culture for studying the metabolic shifts from oxidative metabolism to lipid accumulation and citric acid production in Yarrowia lipolytica. J. Biotechnol. 2014, 170, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Triantaphyllidou, I.E.; Mizerakis, P.; Aggelis, G. High lipid accumulation in Yarrowia lipolytica cultivated under double limitation of nitrogen and magnesium. J. Biotechnol. 2016, 234, 116–126. [Google Scholar] [CrossRef]
- Beopoulos, A.; Haddouche, R.; Kabran, P.; Dulermo, T.; Chardot, T.; Nicaud, J.M. Identification and characterization of DGA2, an acyltransferase of the DGAT1 acyl-CoA:diacylglycerol acyltransferase family in the oleaginous yeast Yarrowia lipolytica. New insights into the storage lipid metabolism of oleaginous yeasts. Appl. Microbiol. Biotechnol. 2012, 93, 1523–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, M.; Stephanopoulos, G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab. Eng. 2013, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Imam Abidi, S.H.; Liu, H.; Zhang, H.; Chakraborty, S.; Watson, N.; Ajikumar, P.K.; Stephanopoulos, G. Engineering lipid overproduction in the oleaginous yeast Yarrowia lipolytica. Metab. Eng. 2015, 29, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.J.; Holdsworth, J.E.; Reader, S.L. The effect of low oxygen uptake rate on the fatty acid profile of the oleaginous yeast Apiotrichum curvatum. Appl. Microbiol. Biotechnol. 1990, 33, 569–573. [Google Scholar] [CrossRef]






| Gly0 (g/L) | Time (h) | Glycons (g/L) | rGly (g/L/h) | X (g/L) | Ml (g/L) | Ery (g/L) | Ara (g/L) | Pol (g/L) | YPol/Gly (g/g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| ≈70 | a, b, c | 134 | 65.0 ± 4.5 | 0.54 | 16.1 ± 1.5 | 9.2 ± 1.4 | 7.3 ± 1.2 | 4.1 ± 0.7 | 20.6 ± 3.3 | 0.32 |
| d | 183 | 65.6 ± 3.9 | 22.4 ± 2.0 | 6.1 ± 0.8 | 3.1 ± 0.6 | 1.9 ± 0.4 | 11.1 ± 1.8 | 0.17 | ||
| ≈100 | a, b, c, d | 183 | 95.4 ± 7.1 | 0.52 | 15.0 ± 1.9 | 13.8 ± 2.2 | 17.1 ± 2.5 | 7.3 ± 1.2 | 38.2 ± 5.9 | 0.40 |
| ≈120 | a, b, c, d | 250 | 109.2 ± 9.0 | 0.45 | 13.5 ± 2.1 | 15.7 ± 2.4 | 20.6 ± 2.8 | 7.1 ± 1.1 | 43.4 ± 6.3 | 0.40 |
| ≈150 | a, b, c, d | 280 | 120.5 ± 12.8 | 0.37 | 11.1 ± 1.8 | 13.1 ± 2.0 | 24.1 ± 4.0 | 14.1 ± 2.1 | 51.3 ± 8.1 | 0.43 |
| Strain | Ery (g/L) | Ml (g/L) | Ara (g/L) | Pol (g/L) | YPol/Gly (g/g) | Cultivation Type | Reference |
|---|---|---|---|---|---|---|---|
| 1.22 & | 93.5 | 34.0 | - | 127.5 | 0.43 | Fed-batch reactor | Rymowicz et al. [46] |
| Wratislavia 1.31 * | 132.0 | 23.0 | - | 155.0 | 0.52 | Fed-batch reactor | Rymowicz et al. [46] |
| Wratislavia K1 * | 170.0 | 12.0 | - | 182.0 | 0.60 | Fed-batch reactor | Rymowicz et al. [46] |
| CCY-29–26-3 & | 23.0 | 2.6 | 2.3 | 27.9 | 0.40 | Batch flasks | Tomaszewska et al. [29] |
| CCY-29–26-4 & | 26.7 | 1.0 | 2.2 | 29.9 | 0.40 | Batch flasks | Tomaszewska et al. [29] |
| A-15 & | 71.0 | 8.0 | 1.8 | 80.8 | 0.50 | Batch reactor | Tomaszewska et al. [29] |
| A UV’1 * | 63.0 | 8.8 | 9.2 | 81.0 | 0.50 | Batch reactor | Tomaszewska et al. [29]) |
| Wratislavia K1 * | 80.0 | 2.6 | 0.3 | 82.9 | 0.51 | Batch reactor | Tomaszewska et al. [29] |
| Wratislavia K1 * | 135.5 | 3.9 | 0.1 | 139.5 | 0.58 | Repeated-batch reactor | Mirończuk et al. [31] |
| Wratislavia K1 * | 208.0 | 0.2 | - | 208.2 | 0.41 | Repeated-batch reactor | Mirończuk et al. [31] |
| Wratislavia 1.31 * | 26.2 | 16.8 | 3.7 | 46.7 | 0.36 | Batch reactor | Tomaszewska et al. [48] |
| Wratislavia AWG7 * | 25.7 | 17.1 | 2.7 | 45.5 | 0.30 | Batch reactor | Tomaszewska et al. [48] |
| Wratislavia K1 * | 40.7 | 15.1 | 2.9 | 58.7 | 0.40 | Batch reactor | Tomaszewska et al. [48] |
| MK1 * | 79.5 | 2.7 | 0.4 | 82.6 | 0.55 | Batch reactor | Mirończuk et al. [50] |
| MK1 * | 138.8 | 3.3 | 0.3 | 142.4 | 0.69 | Repeated-batch reactor | Mirończuk et al. [50] |
| MK1 * | 177.3 | 2.2 | - | 179.5 | 0.67 | Repeated-batch reactor | Mirończuk et al. [50] |
| CBS10146 ╬ | 44.6 | 5.2 | 10.5 | 60.3 | 0.53 | Batch reactor | Rakicka et al. [51] |
| CBS4855 ╬╬ | 33.4 | 7.6 | 6.8 | 47.8 | 0.43 | Batch reactor | Rakicka et al. [51] |
| CBS11013 ╬╬╬ | 35.4 | 0.6 | 8.9 | 44.9 | 0.41 | Batch reactor | Rakicka et al. [51] |
| FCY 214 * | 79.4 | n.i. | n.i. | 79.4 | 0.48 | Batch reactor | Carly et al. [35] |
| FCY 218 * | 80.6 | n.i. | n.i. | 80.6 | 0.53 | Batch reactor | Carly et al. [35] |
| HA 829 &$ | ≈4 | ≈28 | ≈6 | ≈38 | n.i. | Batch reactor | Egermeier et al. [36] |
| HA 1251 &$ | ≈4 | ≈32 | ≈5 | ≈41 | n.i. | Batch reactor | Egermeier et al. [36] |
| ACA YC 5029 &$ | 33.6 | 28.9 | - | 62.5 | 0.45 | Batch flasks | Papanikolaou et al. [18] |
| ACA YC 5030 &$ | 35.5 | 32.1 | - | 67.6 | 0.49 | Batch flasks | Papanikolaou et al. [18] |
| AIB & | 56.7 | 12.6 | 6.0 | 75.3 | 0.49 | Fed-batch reactor | Rakicka et al. [52] |
| AIB pADUTGUT1 * | 82.2 | 11.0 | 7.5 | 100.7 | 0.67 | Fed-batch reactor | Rakicka et al. [52] |
| ACA-DC 5029 &$ | 65.8 | 6.5 | 3.4 | 75.7 | 0.44 | Batch flasks | Sarris et al. [53] |
| ACA-DC 5029 &$ | 15.6 | 10.5 | 3.4 | 29.5 | 0.39 | Batch flasks | Sarris et al. [49] |
| M53-S * | 69.9 | 10.1 | - | 80.0 | 0.80 | Batch flasks | Liu et al. [54] |
| M53-S * | 72.5 | 10.0 | - | 82.5 | 0.82 | Batch reactor | Liu et al. [54] |
| LMBF Y-46 | 24.1 | 13.1 | 14.1 | 51.3 | 0.43 | Batch flasks | Present study |
| Gly0 (g/L) | Time (h) | Glycons (g/L) | X (g/L) | Ml (g/L) | Ery (g/L) | Ara (g/L) | CA (g/L) | Pol (g/L) | YCA/Gly (g/g) | YPol/Gly (g/g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≈100 Flasks | a, c | 183 | 95.4 ± 7.1 | 15.0 ± 1.9 | 13.8 ± 2.2 | 17.1 ± 2.5 | 7.3 ± 1.2 | - | 38.2 ± 5.9 | - | 0.40 |
| ≈100 | a, c | 41.5 | 86.8 ± 6.1 | 21.0 ± 3.1 | 3.0 ± 0.5 | - | - | 20.4 ± 3.3 | 3.0 ± 0.5 | 0.24 | 0.03 |
| Bioreactor | b | 65 | 103.5 ± 9.9 | 19.0 ± 3.4 | 0.5 ± 0.1 | - | - | 42.4 ± 5.5 | 0.5 ± 0.1 | 0.41 | 0.004 |
| Strain | Citric Acid (g/L) | Substrate | Yield (g/g) | Bioreactor Configuration | Reference |
|---|---|---|---|---|---|
| NRRL Y-7576 $ | 51.5 | Glucose | 0.71 | Fed-batch | Klasson et al. [69] |
| Y-1095 $ | 78.5 | Glucose | 0.79 | Fed-batch | Rane and Sims [70] |
| ATCC 20346 | 69.0 | Glucose | 0.52 | Fed-batch | Moresi [68] |
| N1 * | 120.0 | Ethanol | 0.85 | Fed-batch | Kamzolova et al. [71] |
| 187/1 $ | 135.1 | Rapeseed oil | 1.55 | Fed-batch | Kamzolova et al. [56] |
| Wratislavia AWG7 * | 88.1 | Crude glycerol | 0.46 | Batch | Rymowicz et al. [44] |
| Wratislavia K1 * | 75.7 | Crude glycerol | 0.40 | Batch | Rymowicz et al. [44] |
| H222-S4(p67ICL1)T5 * | 91.0 & | Sucrose | 0.53 | Fed-batch | Förster et al. [57] |
| H222-S4(p67ICL1)T5 * | 133.0 & | Sucrose | 0.78 | Fed-batch | Förster et al. [57] |
| A-101–1.22 * | 119.1 & | Crude glycerol | 0.64 | Fed-batch | Rymowicz et al. [47] |
| A-101–1.22 * | 115.6 & | Crude glycerol | 0.68 | Repeated batch | Rymowicz et al. [47] |
| A-101 $ | 69.3 | Glucose | 0.45 | Batch | Rywińska et al. [55] |
| A-101 $ | 66.5 | Pure glycerol | 0.44 | Batch | Rywińska et al. [55] |
| A-101 $ | 66.8 | Crude glycerol | 0.43 | Batch | Rywińska et al. [55] |
| Wratislavia 1.31 * | 76.4 | Glucose | 0.52 | Batch | Rywińska et al. [55] |
| Wratislavia 1.31 * | 63.9 | Pure glycerol | 0.40 | Batch | Rywińska et al. [55] |
| Wratislavia 1.31 * | 82.0 | Crude glycerol | 0.53 | Batch | Rywińska et al. [55] |
| Wratislavia 1.31 * | 126.0 & | Crude glycerol | 0.63 | Fed-batch | Rywińska et al. [72] |
| Wratislavia AWG7 * | 157.5 & | Crude glycerol | 0.58 | Fed-batch | Rywińska et al. [72] |
| Wratislavia AWG7 * | 160.5 & | Crude glycerol | 0.81 | Repeated batch | Rywińska and Rymowicz [73] |
| Wratislavia 1.31 * | 124.9 & | Crude glycerol | 0.59 | Repeated batch | Rywińska and Rymowicz [73] |
| N15$ | 98.0 | Pure glycerol | 0.70 | Fed-batch | Kamzolova et al. [61] |
| NG40/UV7 * | 175.0 | Rapeseed oil | 1.50 | Fed-batch | Kamzolova et al. [74] |
| Wratislavia AWG7 * | 63.3 | Pure glycerol | 0.67 | Continuous | Rywińska et al. [60] |
| Wratislavia 1.31 * | 92.8 | Pure glycerol | 0.63 | Batch | Rywińska et al. [66] |
| SWJ-1b * | 84.0 | Inulin | 0.89 | Batch | Liu et al. [75] |
| NG40/UV7 * | 115.0 | Pure glycerol | 0.64 | Fed-batch | Morgunov et al. [59] |
| NG40/UV7 * | 112.0 | Crude glycerol | 0.90 | Fed-batch | Morgunov et al. [59] |
| Wratislavia AWG7 * | 85.7 | Crude glycerol | 0.52 | Batch | Tomaszewska et al. [48] |
| Wratislavia K1 * | 65.0 | Crude glycerol | 0.43 | Batch | Tomaszewska et al. [48] |
| SWJ-1b * | 101.6 | Glucose | 0.89 | Fed-batch | Tan et al. [67] |
| CBS 6114 $ | ≈55 & | Pure glycerol | n.i. | Batch | Egermeier et al. [36] |
| H222 $ | ≈50 & | Pure glycerol | n.i. | Batch | Egermeier et al. [36] |
| DSM 1345 $ | ≈52 & | Pure glycerol | n.i. | Batch | Egermeier et al. [36] |
| VKM Y-2373 $ | 80–85 | Glucose | 0.70–0.75 | Batch | Kamzolova and Morgunov [76] |
| ACA YC 5029 $ | 39.0 | Crude glycerol | 0.42 | Batch | Papanikolaou et al. [18] |
| NG40/UV5 * | 140.0 | Rapeseed oil | 1.50 | Fed-batch | Morgunov et al. [77] |
| NG40/UV5 * | 108.8 | Glucose | 0.80 | Fed-batch | Morgunov et al. [77] |
| NG40/UV5 * | 87.0 | Crude glycerol | 0.64 | Fed-batch | Morgunov et al. [77] |
| K57 $ | 72.1 | Glucose | 0.77 | Batch | Carsanba et al. [62] |
| AJD pADUTGut 1/2 * | 75.9 && | Crude glycerol | 0.51 | Batch | Rzechonek et al. [63] |
| LMBF Y-46 $ | 42.4 & | Pure glycerol | 0.41 | Batch | Present study |
| LMBF Y-46 $ | 101.3 & | Pure glycerol | 0.46 | Fed-batch | Present study |
| Equation | Number of Data Points | Glycerol Concentration (g/L) | |||
|---|---|---|---|---|---|
| 100 | 120 | 150 | |||
| 11 | 12 | 13 | |||
| 1 | μmax (h−1) | 0.0296 | 0.0252 | 0.0350 | |
| Xmax (gX/L) | 20.63 | 16.94 | 10.62 | ||
| 2 | X at t = 0 (gX/L) | 2.26 | 2.22 | 2.23 | |
| SSE | 13.09 | 9.48 | 8.03 | ||
| R2 | 0.942 | 0.937 | 0.935 | ||
| 3 | qPolmax (gPol/(gXh)) | 0.0415 | 0.0420 | 0.0251 | |
| Polmax (gPol/L) | 40.14 | 48.86 | 162.87 | ||
| 4 | SSE | 127.02 | 59.43 | 68.33 | |
| R2 | 0.951 | 0.974 | 0.981 | ||
| 5 | YX/Gly (gX/gGlyc) | 0.2160 | 0.2749 | 0.1210 | |
| YPol/Gly (gPol/gGlyc) | 0.9997 | 0.6575 | 1.0586 | ||
| SSE | 445.2 | 187.1 | 1027.8 | ||
| R2 | 0.954 | 0.984 | 0.956 | ||
| Culture Type/Time | Fatty Acid Composition of Yeast Lipids (%, w/w) | |||||||||
| Shake-Flasks | ≤C12:0 * | C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C20:0 | ≥C22:0 $ | Other × |
| Gly0 ≈ 70 g/L, t ≈ 180 h | 2.5 ± 0.3 | 2.3 ± 0.4 | 21.1 ± 1.7 | 5.1 ± 0.6 | 15.6 ± 2.0 | 30.2 ± 3.5 | 11.5 ± 1.3 | 0.8 ± 0.1 | 2.2 ± 0.3 | 8.7 ± 0.9 |
| Gly0 ≈ 100 g/L, t ≈ 160 h | 0.8 ± 0.1 | 0.7 ± 0.1 | 22.2 ± 3.5 | 5.5 ± 0.4 | 18.5 ± 2.4 | 32.3 ± 4.0 | 12.2 ± 2.0 | 1.2 ± 0.2 | 2.5 ± 0.4 | 4.1 ± 0.3 |
| Gly0 ≈ 100 g/L, t ≈ 180 h | 1.3 ± 0.2 | 0.9 ± 0.2 | 17.5 ± 2.5 | 3.6 ± 0.5 | 25.3 ± 2.4 | 28.4 ± 4.1 | 8.8 ± 1.0 | 5.9 ± 1.1 | 3.0 ± 0.4 | 5.3 ± 0.6 |
| Gly0 ≈ 120 g/L, t ≈ 160 h | 1.8 ± 0.3 | 1.0 ± 0.1 | 19.9 ± 2.0 | 4.6 ± 0.7 | 18.9 ± 3.0 | 30.8 ± 5.0 | 9.0 ± 1.5 | 4.2 ± 0.9 | 2.0 ± 0.3 | 7.8 ± 1.4 |
| Gly0 ≈ 120 g/L, t ≈ 190 h | 0.2 ± 0.1 | 0.4 ± 0.1 | 21.7 ± 2.4 | 6.6 ± 0.8 | 15.9 ± 2.5 | 36.2 ± 5.2 | 12.8 ± 2.0 | 1.1 ± 0.1 | 2.9 ± 0.5 | 2.2 ± 0.4 |
| Gly0 ≈ 150 g/L, t ≈ 190 h | 3.9 ± 0.2 | 2.4 ± 0.2 | 19.5 ± 1.9 | 5.1 ± 0.4 | 18.0 ± 3.2 | 30.0 ± 5.0 | 10.7 ± 1.8 | 2.0 ± 0.2 | 2.0 ± 0.4 | 6.4 ± 0.9 |
| Culture Type/Time | Fatty Acid Composition of Yeast Lipids (%, w/w) | |||||||||
| Bioreactor | ≤C12:0 * | C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C20:0 | ≥C22:0 $ | Other × |
| t = 20.5 h | 1.9 ± 0.4 | 3.0 ± 0.4 | 21.1 ± 2.5 | 7.7 ± 0.8 | 17.0 ± 1.8 | 30.5 ± 3.9 | 17.7 ± 2.6 | - | - | 1.1 ± 0.3 |
| t = 41.5 h | - | 1.2 ± 0.2 | 18.4 ± 2.0 | 6.8 ± 0.9 | 12.0 ± 2.0 | 37.3 ± 4.0 | 16.2 ± 3.0 | - | 3.0 ± 0.4 | 5.1 ± 0.3 |
| t = 44.5 h | 1.5 ± 0.4 | 0.7 ± 0.3 | 16.5 ± 2.0 | 8.7 ± 1.0 | 7.7 ± 1.0 | 39.0 ± 5.1 | 15.4 ± 2.9 | 0.5 ± 0.1 | 2.6 ± 0.4 | 7.4 ± 1.2 |
| t = 66.0 h | 2.5 ± 0.2 | 1.2 ± 0.2 | 16.4 ± 2.1 | 7.3 ± 0.9 | 9.2 ± 1.4 | 36.2 ± 6.1 | 14.9 ± 3.0 | 0.5 ± 0.2 | 2.2 ± 0.4 | 9.9 ± 1.5 |
| t = 67.0 h | 1.7 ± 0.1 | 0.8 ± 0.1 | 16.3 ± 2.2 | 8.1 ± 1.2 | 8.1 ± 1.3 | 39.7 ± 5.8 | 16.7 ± 3.1 | 0.4 ± 0.1 | 2.8 ± 0.3 | 5.4 ± 0.9 |
| t = 89.0 h | 0.7 ± 0.1 | 0.6 ± 0.1 | 16.7 ± 2.3 | 9.0 ± 1.4 | 8.1 ± 1.6 | 40.3 ± 6.0 | 16.1 ± 3.2 | 0.4 ± 0.1 | 2.7 ± 0.3 | 5.4 ± 1.0 |
| t = 91.0 h | 2.2 ± 0.3 | 1.1 ± 0.2 | 17.0 ± 1.9 | 8.4 ± 1.7 | 8.4 ± 1.5 | 37.4 ± 5.1 | 16.2 ± 3.0 | 0.3 ± 0.1 | 1.5 ± 0.2 | 7.5 ± 1.3 |
| t = 97.0 h | 0.9 ± 0.1 | 0.7 ± 0.2 | 16.3 ± 2.5 | 8.5 ± 1.8 | 8.0 ± 1.6 | 40.2 ± 6.1 | 16.1 ± 2.7 | 0.5 ± 0.2 | 2.8 ± 0.3 | 6.0 ± 1.0 |
| t = 122.5 h | 0.9 ± 0.1 | 0.5 ± 0.2 | 16.1 ± 2.0 | 9.5 ± 1.8 | 7.0 ± 1.0 | 41.1 ± 4.9 | 16.9 ± 2.0 | 0.7 ± 0.2 | 2.5 ± 0.3 | 4.8 ± 1.2 |
| t = 164.0 h | 1.1 ± 0.2 | 0.8 ± 0.4 | 15.0 ± 1.9 | 9.5 ± 1.5 | 6.0 ± 1.0 | 43.0 ± 4.4 | 17.9 ± 3.1 | 0.8 ± 0.1 | 1.9 ± 0.3 | 4.0 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papanikolaou, S.; Diamantopoulou, P.; Blanchard, F.; Lambrinea, E.; Chevalot, I.; Stoforos, N.G.; Rondags, E. Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production. Appl. Sci. 2020, 10, 7373. https://doi.org/10.3390/app10207373
Papanikolaou S, Diamantopoulou P, Blanchard F, Lambrinea E, Chevalot I, Stoforos NG, Rondags E. Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production. Applied Sciences. 2020; 10(20):7373. https://doi.org/10.3390/app10207373
Chicago/Turabian StylePapanikolaou, Seraphim, Panagiota Diamantopoulou, Fabrice Blanchard, Eleni Lambrinea, Isabelle Chevalot, Nikolaos G. Stoforos, and Emmanuel Rondags. 2020. "Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production" Applied Sciences 10, no. 20: 7373. https://doi.org/10.3390/app10207373
APA StylePapanikolaou, S., Diamantopoulou, P., Blanchard, F., Lambrinea, E., Chevalot, I., Stoforos, N. G., & Rondags, E. (2020). Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production. Applied Sciences, 10(20), 7373. https://doi.org/10.3390/app10207373








