Lignocellulosic Biomass as a Substrate for Oleaginous Microorganisms: A Review
Abstract
1. Introduction
2. Oleaginous Microorganisms
2.1. Important Oleaginous Species
2.2. Growth Conditions That Promote Lipid Accumulation
2.3. Sugar Conversion into SCO and Regulatory Mechanisms in Sugar Assimilation
2.4. FA Composition of SCOs
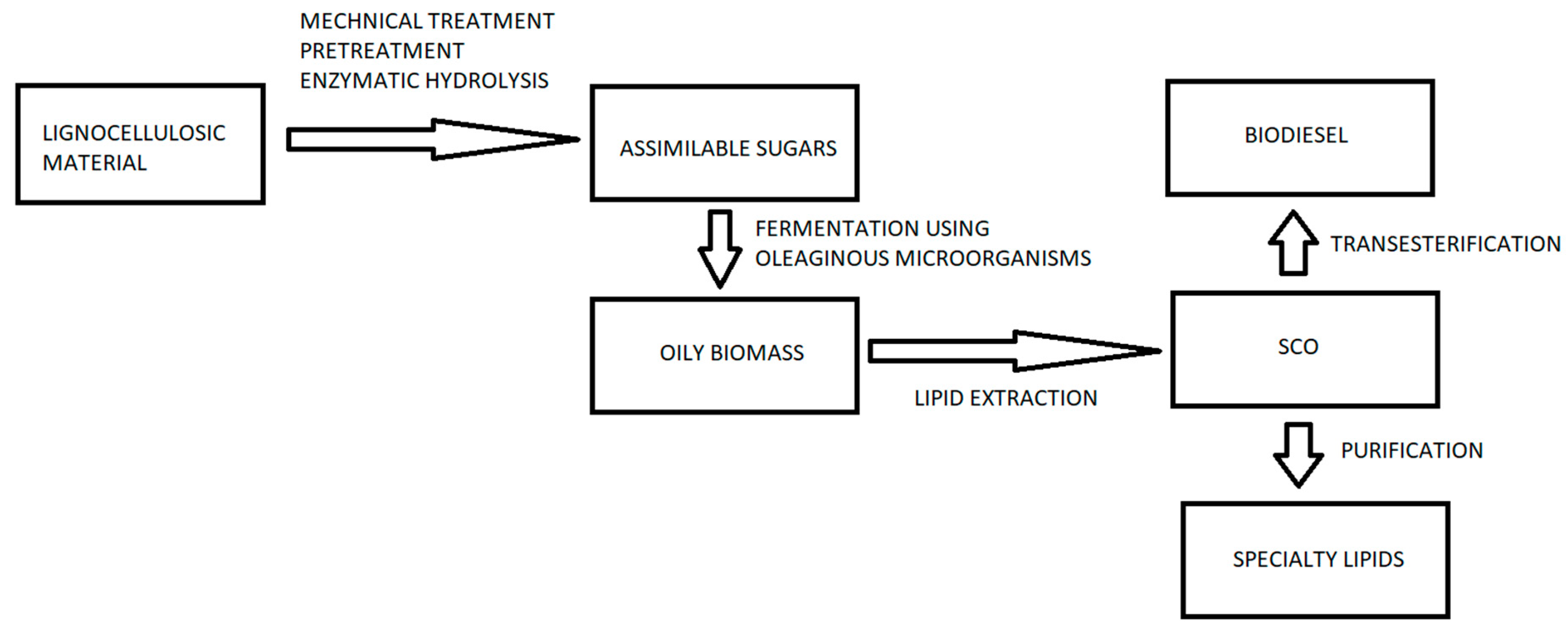
2.5. Importance of Lignocellulosic Biomass as Raw Material
2.6. Lignocellulose Structure and Chemical Composition
2.7. Recalcitrance of Lignocellulosic Biomass
3. Conversion of Lignocellulosic Biomass into Assimilable Sugars in Nature
4. Biomass Pretreatment to Decrease Recalcitrance
4.1. Principal Pretreatments
4.1.1. Acid Pretreatment
4.1.2. Alkaline Pretreatment
4.1.3. Sequential Acid–Alkaline Pretreatment
4.1.4. Steam Explosion Pretreatment
4.1.5. Organosolv Pretreatment
4.2. Optimization of Principal Pretreatments
5. Pretreatment By-Products Affecting Enzymatic Hydrolysis and Fermentation
6. Enzymatic Hydrolysis
7. SCO Production from Hydrolyzed Lignocellulosic Biomass
8. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Fakas, S.; Papanikolaou, S.; Batsos, A.; Galiotou-Panayotou, M.; Mallouchos, A.; Aggelis, G. Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenergy 2009, 33, 573–580. [Google Scholar] [CrossRef]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Triantaphyllidou, I.E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial oils as food additives: Recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef]
- Bellou, S.; Triantaphyllidou, I.E.; Mizerakis, P.; Aggelis, G. High lipid accumulation in Yarrowia lipolytica cultivated under double limitation of nitrogen and magnesium. J. Biotechnol. 2016, 234, 116–126. [Google Scholar] [CrossRef]
- Dourou, M.; Aggeli, D.; Papanikolaou, S.; Aggelis, G. Critical steps in carbon metabolism affecting lipid accumulation and their regulation in oleaginous microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 2509–2523. [Google Scholar] [CrossRef]
- Kothri, M.; Mavrommati, M.; Elazzazy, A.M.; Baeshen, M.N.; Moussa, T.A.A.; Aggelis, G. Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS. Microbiol. Lett. 2020, 367. [Google Scholar] [CrossRef]
- Sreeharsha, R.V.; Mohan, S.V. Obscure yet promising oleaginous yeasts for fuel and chemical production. Trends Biotechnol. 2020, 38, 873–887. [Google Scholar] [CrossRef]
- Adrio, J.L. Oleaginous yeasts: Promising platforms for the production of oleochemicals and biofuels. Biotechnol. Bioeng. 2017, 114, 1915–1920. [Google Scholar] [CrossRef]
- Singh, J.; Gu, S. Commercialization potential of microalgae for biofuels production. Renew. Sustain. Energy Rev. 2010, 14, 2596–2610. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Wu, Q. Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng. 2007, 98, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Reyes, P.; Mendonça, R.T.; Aguayo, M.G.; Rodríguez, J.; Vega, B.; Fardim, P. Extraction and characterization of hemicelluloses from Pinus radiata and its feasibility for bioethanol production. Rev. Arvore. 2013, 37, 175–180. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic biomass for bioethanol: An overview on pretreatment, hydrolysis and fermentation processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, J.; Bao, J. Acceleration of biodetoxification on dilute acid pretreated lignocellulose feedstock by aeration and the consequent ethanol fermentation evaluation. Biotechnol. Biofuels 2016, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Nicaud, J.M.; Ledesma-Amaro, R. The engineering potential of Rhodosporidium toruloides as a workhorse for biotechnological applications. Trends Biotechnol. 2018, 36, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Ohashi, T.; Wu, C.C.; Bataa, D.; Misaki, R.; Limtong, S.; Fujiyama, K. Delta-9 fatty acid desaturase overexpression enhanced lipid production and oleic acid content in Rhodosporidium toruloides for preferable yeast lipid production. J. Biosci. Bioeng. 2019, 127, 430–440. [Google Scholar] [CrossRef]
- Singh, P.; Kumari, S.; Guldhe, A.; Misra, R.; Rawat, I.; Bux, F. Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew. Sustain. Energy Rev. 2016, 55, 1109–1128. [Google Scholar] [CrossRef]
- Daskalaki, A.; Perdikouli, N.; Aggeli, D.; Aggelis, G. Laboratory evolution strategies for improving lipid accumulation in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2019, 103, 8585–8596. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, S.; Xu, L.; Zou, Y.; Song, W. Study on diversity of endophytic bacterial communities in seeds of hybrid maize and their parental lines. Arch. Microbiol. 2012, 194, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Tsolcha, O.N.; Tekerlekopoulou, A.G.; Akratos, C.S.; Aggelis, G.; Genitsaris, S.; Moustaka-Gouni, M.; Vayenas, D.V. Biotreatment of raisin and winery wastewaters and simultaneous biodiesel production using a Leptolyngbya-based microbial consortium. J. Clean. Prod. 2017, 148, 185–193. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yi, D.; Kim, Y.; Kim, H.; Seo, H.; Lee, J.; Kim, J.; Jeon, J.; Jang, K.; Kim, Y.; et al. Development of semi-synthetic microbial consortia of Streptomyces coelicolor for increased production of biodiesel (fatty acid methyl esters). Fuel 2015, 159, 189–196. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, P. The new face of the lipid droplet: Lipid droplet proteins. Proteomics 2019, 19, 1700223. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Bellou, S.; Aggelis, G. Biochemical activities in Chlorella sp. and Nannochloropsis salina during lipid and sugar synthesis in a lab-scale open pond simulating reactor. J. Biotechnol. 2013, 164, 318–329. [Google Scholar] [CrossRef]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef]
- De Olivera-Finco, A.M.; Goyzueta-Mamani, L.D.; de Carvalho, J.C.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Soccol, C.R. Technological trends and market perspectives for production of microbial oils rich in omega-3. Crit. Rev. Biotechnol. 2017, 37, 656–671. [Google Scholar] [CrossRef]
- Dourou, M.; Tsolcha, O.N.; Tekerlekopoulou, A.; Bokas, D.; Aggelis, G. Fish farm effluents are suitable growth media for Nannochloropsis gaditana, a polyunsaturated fatty acid producing microalga. Eng. Life Sci. 2018, 18, 851–860. [Google Scholar] [CrossRef]
- Malibari, R.; Sayegh, F.; Elazzazy, A.M.; Baeshen, M.N.; Dourou, M.; Aggelis, G. Reuse of shrimp farm wastewater as growth medium for marine microalgae isolated from Red Sea—Jeddah. J. Clean. Prod. 2018, 198, 160–169. [Google Scholar] [CrossRef]
- Deshmukh, S.; Kumar, R.; Bala, K. Microalgae biodiesel: A review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process. Technol. 2019, 191, 232–247. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef]
- Alvarez, H.M.; Steinbüchel, A. Triacylglycerols in prokaryotic microorganisms. Appl. Microbiol. Biotechnol. 2002, 60, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Kosa, M.; Ragauskas, A.J. Bioconversion of lignin model compounds with oleaginous Rhodococci. Appl. Microbiol. Biotechnol. 2012, 93, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Kosa, M.; Ragauskas, A.J. Lignin to lipid bioconversion by oleaginous Rhodococci. Green Chem. 2013, 15, 2070–2074. [Google Scholar] [CrossRef]
- Gouda, M.K.; Omar, S.H.; Aouad, L.M. Single cell oil production by Gordonia sp. DG using agro-industrial wastes. World J. Microbiol. Biotechnol. 2008, 24, 1703–1711. [Google Scholar] [CrossRef]
- Ruan, Z.; Zanotti, M.; Wang, X.; Ducey, C.; Liu, Y. Evaluation of lipid accumulation from lignocellulosic sugars by Mortierella isabellina for biodiesel production. Bioresour. Technol. 2012, 110, 198–205. [Google Scholar] [CrossRef]
- Sitepu, I.; Selby, T.; Lin, T.; Zhu, S.; Boundy-Mills, K. Carbon source utilization and inhibitor tolerance of 45 oleaginous yeast species. J. Ind. Microbiol. Biothecnol. 2014, 41, 1061–1070. [Google Scholar] [CrossRef]
- Slininger, P.J.; Dien, B.S.; Kurtzman, C.P.; Moser, B.R.; Bakota, E.L.; Thompson, S.R.; O’Bryan, P.J.; Cotta, M.A.; Balan, V.; Jin, M.; et al. Comparative lipid production by oleaginous yeasts in hydrolyzates of lignocellulosic biomass and process strategy for high titers. Biotechnol. Bioeng. 2016, 113, 1676–1690. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, H.; Zhou, W.; Wang, Y.; Yang, X.; Zhao, Z.K. Efficient conversion of acetate into lipids by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Arous, F.; Frikha, F.; Triantaphyllidou, I.E.; Aggelis, G.; Nasri, M.; Mechichi, T. Potential utilization of agro-industrial wastewaters for lipid production by the oleaginous yeast Debaryomyces etchellsii. J. Clean. Prod. 2016, 133, 899–909. [Google Scholar] [CrossRef]
- Gardeli, C.; Athenaki, M.; Xenopoulos, E.; Mallouchos, A.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Lipid production and characterization by Mortierella (Umbelopsis) isabellina cultivated on lignocellulosic sugars. J. Appl. Microbiol. 2017, 123, 1461–1477. [Google Scholar] [CrossRef] [PubMed]
- Dourou, M.; Mizerakis, P.; Papanikolaou, S.; Aggelis, G. Storage lipid and polysaccharide metabolism in Yarrowia lipolytica and Umbelopsis isabellina. Appl. Microbiol. Biotechnol. 2017, 101, 7213–7226. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.U.; Park, J.M. Biodiesel production by various oleaginous microorganisms from organic wastes. Bioresour. Technol. 2018, 256, 502–508. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Sources of microbial oils with emphasis to Mortierella (Umbelopsis) isabellina fungus. World J. Microbiol. Biotechnol. 2019, 35, 63. [Google Scholar] [CrossRef]
- Chaiyaso, T.; Manowattana, A.; Techapun, C.; Watanabe, M. Efficient bioconversion of enzymatic corncob hydrolysate into biomass and lipids by oleaginous yeast Rhodosporidium paludigenum KM281510. Prep. Biochem. Biotechnol. 2019, 49, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, V.; Siva Prakash, G.; Chang, S.W.; Ravindran, B.; Nguyen, D.D.; Vo, D.V.N.; La, D.D.; Bach, Q.V.; Wong, J.W.C.; Gupta, S.; et al. Enhanced microbial biodiesel production from lignocellulosic hydrolysates using yeast isolates. Fuel 2019, 256, 115932. [Google Scholar] [CrossRef]
- Llamas, M.; Dourou, M.; González-Fernández, C.; Aggelis, G.; Tomás-Pejó, E. Screening of oleaginous yeasts for lipid production using volatile fatty acids as substrate. Biomass Bioenergy 2020, 138, 105553. [Google Scholar] [CrossRef]
- Sagia, S.; Sharma, A.; Singh, S.; Chaturvedi, S.; Singh-Nain, P.K.; Nain, L. Single cell oil production by a novel yeast Trichosporon mycotoxinivorans for complete and ecofriendly valorization of paddy straw. Electron. J. Biotechnol. 2020, 44, 60–68. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Lipid production by oleaginous Mucorales cultivated on renewable carbon sources. Eur. J. Lipid Sci. Technol. 2007, 109, 1060–1070. [Google Scholar] [CrossRef]
- Makri, A.; Fakas, S.; Aggelis, G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010, 101, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Economou, C.N.; Makri, A.; Aggelis, G.; Pavlou, S.; Vayenas, D.V. Semi-solid state fermentation of sweet sorghum for the biotechnological production of single cell oil. Bioresour. Technol. 2010, 101, 1385–1388. [Google Scholar] [CrossRef]
- Economou, C.N.; Vasiliadou, I.A.; Aggelis, G.; Pavlou, S.; Vayenas, D.V. Modeling of oleaginous fungal biofilm developed on semi-solid media. Bioresour. Technol. 2011, 102, 9697–9704. [Google Scholar] [CrossRef] [PubMed]
- Chatzifragkou, A.; Makri, A.; Belka, A.; Bellou, S.; Mavrou, M.; Mastoridou, M.; Mystrioti, P.; Onjaro, G.; Aggelis, G.; Papanikolaou, S. Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 2011, 36, 1097–1108. [Google Scholar] [CrossRef]
- Tsigie, Y.A.; Wang, C.; Kasim, N.S.; Diem, Q.; Huynh, L.; Ho, Q.; Truong, C.; Ju, Y. Oil Production from Yarrowia lipolytica Po1g using rice bran hydrolysate. J. Biomed. Biotechnol. 2012, 378384. [Google Scholar] [CrossRef]
- Beopoulos, A.; Nicaud, J.M. Yeast: A new oil producer? Oléagineux Corps Gras Lipides 2012, 19, 22–28. [Google Scholar] [CrossRef]
- Kazamia, E.; Czesnick, H.; Van Nguyen, T.T.; Croft, M.T.; Sherwood, E.; Sasso, S.; Hodson, S.J.; Warren, M.J.; Smith, A.G. Mutualistic interactions between vitamin B 12 -dependent algae and heterotrophic bacteria. Environ. Microbiol. 2012, 14, 1466–1476. [Google Scholar] [CrossRef]
- Xie, B.; Bishop, S.; Stessman, D.; Wright, D.; Spalding, M.H.; Halverson, L.J. Chlamydomonas reinhardtii thermal tolerance enhancement mediated by a mutualistic interaction with vitamin B 12-producing bacteria. ISME J. 2013, 7, 1544–1555. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. Biotechnological potential of Synechocystis salina co-cultures with selected microalgae and cyanobacteria: Nutrients removal, biomass and lipid production. Bioresour. Technol. 2016, 200, 279–286. [Google Scholar] [CrossRef]
- Cho, H.U.; Kim, Y.; Park, J.M. Enhanced microalgal biomass and lipid production from a consortium of indigenous microalgae and bacteria present in municipal wastewater under gradually mixotrophic culture conditions. Bioresour. Technol. 2017, 228, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Tsai, C.; Chen, K.; Liao, W.; Huang, H. Enhancing high quality oil accumulation and carbon dioxide fixation by a mixed culture of Chlorella sp and Saccharomyces cerevisiae. J. Taiwan Inst. Chem. Eng. 2013, 44, 936–942. [Google Scholar] [CrossRef]
- Kitcha, S.; Cheirsilp, B. Enhanced lipid production by co-cultivation and co-encapsulation of oleaginous yeast Trichosporonoides spathulata with microalgae in alginate gel beads. Appl. Biochem. Biotechnol. 2014, 173, 522–534. [Google Scholar] [CrossRef]
- Ling, J.; Nip, S.; Cheok, W.L.; de Toledo, R.A.; Shim, H. Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour. Technol. 2014, 173, 132–139. [Google Scholar] [CrossRef]
- McNeil, B.A.; Stuart, D.T. Lipomyces starkeyi: An emerging cell factory for production of lipids, oleochemicals and biotechnology applications. World J. Microbiol. Biotechnol. 2018, 34, 147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sen, B.; Liu, X.; He, Y.; Xie, Y.; Wang, G. Enhanced saturated fatty acids accumulation in cultures of newly-isolated strains of Schizochytrium sp. and Thraustochytriidae sp. for large-scale biodiesel production. Sci. Total Environ. 2018, 631, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.; Hussain, M.S.; Gambill, L.; Blenner, M. Alternative substrate metabolism in Yarrowia lipolytica. Front. Microbiol. 2018, 9, 1077. [Google Scholar] [CrossRef]
- Spagnuolo, M.; Yaguchi, A.; Blenner, M. Oleaginous yeast for biofuel and oleochemical production. Curr. Opin. Biotechnol. 2019, 57, 73–81. [Google Scholar] [CrossRef]
- Economou, C.N.; Aggelis, G.; Pavlou, S.; Vayenas, D.V. Single cell oil production from rice hulls hydrolysate. Bioresour. Technol. 2011, 102, 9737–9742. [Google Scholar] [CrossRef]
- Kolouchová, I.; Maťátková, O.; Sigler, K.; Masák, J.; Řezanka, T. Lipid accumulation by oleaginous and non-oleaginous yeast strains in nitrogen and phosphate limitation. Folia Microbiol. 2016, 61, 431–438. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B.; Korstad, J. Utilization of lignocellulosic biomass by oleaginous yeast and bacteria for production of biodiesel and renewable diesel. Renew. Sustain. Energy Rev. 2017, 73, 654–671. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Z.; Chen, S.; Jin, M. Microbial lipid production from dilute acid and dilute alkali pretreated corn stover via Trichosporon dermatis. Bioresour. Technol. 2020, 295, 122253. [Google Scholar] [CrossRef] [PubMed]
- Economou, C.N.; Aggelis, G.; Pavlou, S.; Vayenas, D.V. Modeling of single-Cell oil production under nitrogen-limited and substrate inhibition conditions. Biotechnol. Bioeng. 2011, 108, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Xu, Z.; Du, C.; Qian, H.; Zhang, W.G. Effects of nitrogen on the lipid and carotenoid accumulation of oleaginous yeast Sporidiobolus pararoseus. Bioprocess Biosyst. Eng. 2016, 39, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Fakas, S.; Papanikolaou, S.; Komaitis, M.; Aggelis, G. Organic nitrogen of tomato waste hydrolysate enhances glucose uptake and lipid accumulation in Cunninghamella echinulata. J. Appl. Microbiol. 2008, 105, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Fakas, S.; Čertik, M.; Papanikolaou, S.; Aggelis, G.; Komaitis, M.; Galiotou-Panayotou, M. γ-Linolenic acid production by Cunninghamella echinulata growing on complex organic nitrogen sources. Bioresour. Technol. 2008, 99, 5986–5990. [Google Scholar] [CrossRef] [PubMed]
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.L.; Molina-Jouve, C.; Nicaud, J.M. Yarrowia lipolytica as a model for bio-oil production. Prog. Lipid Res. 2009, 48, 375–387. [Google Scholar] [CrossRef]
- Huang, X.; Luo, H.; Mu, T.; Shen, Y.; Yuan, M.; Liu, J. Enhancement of lipid accumulation by oleaginous yeast through phosphorus limitation under high content of ammonia. Bioresour. Technol. 2018, 262, 9–14. [Google Scholar] [CrossRef]
- Calvey, C.H.; Su, Y.K.; Willis, L.B.; McGee, M.; Jeffries, T.W. Nitrogen limitation, oxygen limitation, and lipid accumulation in Lipomyces starkeyi. Bioresour. Technol. 2016, 200, 780–788. [Google Scholar] [CrossRef]
- Wang, X.; Fosse, H.K.; Li, K.; Chauton, M.S.; Vadstein, O.; Reitan, K.I. Influence of nitrogen limitation on lipid accumulation and EPA and DHA content in four marine microalgae for possible use in aquafeed. Front. Mar. Sci. 2019, 6, 95. [Google Scholar] [CrossRef]
- Yodsuwan, N.; Sawayama, S.; Sirisansaneeyakul, S. Effect of nitrogen concentration on growth, lipid production and fatty acid pro fi les of the marine diatom Phaeodactylum tricornutum. Agric. Nat. Resour. 2017, 51, 190–197. [Google Scholar] [CrossRef]
- Zhao, L.; Li, K.; Wang, Q.; Song, X.; Su, H.; Xie, B.; Zhang, X.; Huang, F.; Chen, X.; Zhou, B.; et al. Nitrogen starvation impacts the photosynthetic performance of Porphyridium cruentum as revealed by chlorophyll a fluorescence. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Negi, S.; Barry, A.N.; Friedland, N.; Sudasinghe, N.; Subramanian, S.; Pieris, S.; Holguin, F.O.; Dungan, B.; Schaub, T.; Sayre, R. Impact of nitrogen limitation on biomass, photosynthesis, and lipid accumulation in Chlorella sorokiniana. Appl. Phycol. 2016, 28, 803–812. [Google Scholar] [CrossRef]
- Srinuanpan, S.; Cheirsilp, B.; Prasertsan, P.; Kato, Y.; Asan, Y. Strategies to increase the potential use of oleaginous microalgae as biodiesel feedstocks: Nutrient starvations and cost-effective harvesting process. Renew. Energy 2018, 122, 507–516. [Google Scholar] [CrossRef]
- Aratboni, H.A.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.B. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell. Fact. 2019, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Gao, L.; Zhao, W.; Chen, W.; Li, M. Effects of nitrogen forms and supply mode on lipid production of microalga Scenedesmus obliquus. Energies 2020, 13, 697. [Google Scholar] [CrossRef]
- Bellou, S.; Moustogianni, A.; Makri, A.; Aggelis, G. Lipids containing polyunsaturated fatty acids synthesized by Zygomycetes grown on glycerol. Appl. Biochem. Biotechnol. 2012, 166, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; Lasa, H.D. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Cho, H.U.; Kim, Y.; Choi, Y.; Xu, X.; Shin, D.Y.; Park, J.M. Effects of pH control and concentration on microbial oil production from Chlorella vulgaris cultivated in the effluent of a low-cost organic waste fermentation system producing volatile fatty acids. Bioresour. Technol. 2015, 184, 245–250. [Google Scholar] [CrossRef]
- Chiu, S.; Kao, C.; Tsai, M.; Ong, S.; Chen, C.; Lin, C. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Weldy, C.S.; Huesemann, M. Lipid production by Dunaliella salina in batch culture: Effects of nitrogen limitation and light intensity. Am. J. Undergrad. Res. 2014, 7, 115–122. [Google Scholar]
- Pandit, P.R.; Fulekar, M.H.; Karuna, M.S.L. Effect of salinity stress on growth, lipid productivity, fatty acid composition, and biodiesel properties in Acutodesmus obliquus and Chlorella vulgaris. Environ. Sci. Pollut. Res. 2017, 24, 13437–13451. [Google Scholar] [CrossRef] [PubMed]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Borghi, M. Del. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Mattsson, L.; Lindehoff, E.; Olofsson, M.; Legrand, C. Boosting algal lipids: Diurnal shifts in temperature exceed the effects of nitrogen limitation. Eng. Rep. 2019, 1, e12067. [Google Scholar] [CrossRef]
- Shi, K.; Gao, Z.; Shi, T.Q.; Song, P.; Ren, L.J.; Huang, H.; Ji, X.J. Reactive oxygen species-mediated cellular stress response and lipid accumulation in oleaginous microorganisms: The state of the art and future perspectives. Front. Microbiol. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Zhang, S.; He, Y.; Sen, B.; Wang, G. Reactive oxygen species and their applications toward enhanced lipid accumulation in oleaginous microorganisms. Bioresour. Technol. 2020, 307, 123234. [Google Scholar] [CrossRef]
- Ratledge, C. Biochemistry, stoichiometry, substrates and economics. In Single Cell Oil; Moreton, R.S., Ed.; Longman Scientific and Technical: Harlow, UK, 1988; pp. 33–70. [Google Scholar]
- Papanikolaou, S.; Aggelis, G. Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica. Lipid. Technol. 2009, 21, 83–87. [Google Scholar] [CrossRef]
- Kim, J.H.; Block, D.E.; Mills, D.A. Simultaneous consumption of pentose and hexose sugars: An optimal microbial phenotype for efficient fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2010, 88, 1077–1085. [Google Scholar] [CrossRef]
- Yamada, R.; Yamauchi, A.; Kashihara, T.; Ogino, H. Evaluation of lipid production from xylose and glucose/xylose mixed sugar in various oleaginous yeasts and improvement of lipid production by UV mutagenesis. Biochem. Eng. J. 2017, 128, 76–82. [Google Scholar] [CrossRef]
- Dai, C.C.; Tao, J.; Xie, F.; Dai, Y.J.; Zhao, M. Biodiesel generation from oleaginous yeast Rhodotorula glutinis with xylose assimilating capacity. Afr. J. Biotechnol. 2007, 6, 2130–2134. [Google Scholar]
- Joshua, C.J.; Dahl, R.; Benke, P.I.; Keasling, J.D. Absence of diauxie during simultaneous utilization of glucose and xylose by Sulfolobus acidocaldarius. J. Bacteriol. 2011, 193, 1293–1301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poontawee, R.; Yongmanitchai, W.; Limtong, S. Efficient oleaginous yeasts for lipid production from lignocellulosic sugars and effects of lignocellulose degradation compounds on growth and lipid production. Process Biochem. 2017, 53, 44–60. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, X.; Hua, Y.; Feng, B.; Zhao, Z. Medium optimization for lipid production through co-fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi. Eur. J. Lipid Sci. Technol. 2008, 110, 405–412. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.F.; Xiong, L.; Yang, X.Y.; Chen, X.D.; Ma, L.L.; Chen, Y. Microbial oil production from corncob acid hydrolysate by oleaginous yeast Trichosporon coremiiforme. Biomass Bioenergy 2013, 49, 273–278. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Diamantopoulou, P.; Chatzifragkou, A.; Philippoussis, A.; Aggelis, G. Suitability of low-cost sugars as substrates for lipid production by the fungus Thamnidium elegans. Energy Fuels 2010, 24, 4078–4086. [Google Scholar] [CrossRef]
- Dey, P.; Maiti, M.K. Molecular characterization of a novel isolate of Candida tropicalis for enhanced lipid production. J. Appl. Microbiol. 2013, 114, 1357–1368. [Google Scholar] [CrossRef]
- Rodriguez, A.; Ersig, N.; Geiselman, G.M.; Seibel, K.; Simmons, B.A.; Magnuson, J.K.; Eudes, A.; Gladden, J.M. Conversion of depolymerized sugars and aromatics from engineered feedstocks by two oleaginous red yeasts. Bioresour. Technol. 2019, 286, 121365. [Google Scholar] [CrossRef]
- Hu, C.; Wu, S.; Wang, Q.; Jin, G.; Shen, H.; Zhao, Z.K. Simultaneous utilization of glucose and xylose for lipid production by Trichosporon cutaneum. Biotechnol. Biofuels 2011, 4, 25. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Xiong, X.; Chen, S. Co-utilization of glucose, xylose and cellobiose by the oleaginous yeast Cryptococcus curvatus. Biomass Bioenergy 2014, 71, 340–349. [Google Scholar] [CrossRef]
- Fei, Q.; Fu, R.; Shang, L.; Brigham, C.J.; Chang, H.N. Lipid production by microalgae Chlorella protothecoides with volatile fatty acids (VFAs) as carbon sources in heterotrophic cultivation and its economic assessment. Bioproc. Biosystems. Eng. 2015, 38, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Poontawee, R.; Yongmanitchai, W.; Limtong, S. Lipid production from a mixture of sugarcane top hydrolysate and biodiesel- derived crude glycerol by the oleaginous red yeast, Rhodosporidiobolus fluvialis. Process. Biochem. 2018, 66, 150–161. [Google Scholar] [CrossRef]
- Yang, X.; Jin, G.; Gong, Z.; Shen, H.; Song, Y.; Bai, F.; Zhao, Z.K. Simultaneous utilization of glucose and mannose from spent yeast cell mass for lipid production by Lipomyces starkeyi. Bioresour. Technol. 2014, 158, 383–387. [Google Scholar] [CrossRef]
- Patel, A.; Pruthi, V.; Singh, R.P.; Pruthi, P.A. Synergistic effect of fermentable and non-fermentable carbon sources enhances TAG accumulation in oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour. Technol. 2015, 188, 136–144. [Google Scholar] [CrossRef]
- Tanimura, A.; Takashima, M.; Sugita, T.; Endoh, R.; Ohkuma, M.; Kishino, S.; Ogawa, J.; Shima, J. Lipid production through simultaneous utilization of glucose, xylose, and l-arabinose by Pseudozyma hubeiensis: A comparative screening study. AMB Express 2016, 6, 58. [Google Scholar] [CrossRef]
- Patel, A.; Sindhu, D.K.; Arora, N.; Singh, R.P.; Pruthi, V.; Pruthi, P.A. Biodiesel production from non-edible lignocellulosic biomass of Cassia fistula L. fruit pulp using oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour. Technol. 2015, 197, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tsigie, Y.A.; Wang, C.Y.; Truong, C.T.; Ju, Y.H. Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate. Bioresour. Technol. 2011, 102, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Valdés, G.; Mendonça, R.T.; Parra, C.; Aggelis, G. Patterns of lignocellulosic sugar assimilation and lipid production by newly isolated yeast strains from Chilean Valdivian forest. Appl. Biochem. Biotechnol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Sarantou, S.; Komaitis, M.; Aggelis, G. Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple-limited media. J. Appl. Microbiol. 2004, 97, 867–875. [Google Scholar] [CrossRef]
- Arous, F.; Mechichi, T.; Nasri, M.; Aggelis, G. Fatty acid biosynthesis during the life cycle of Debaryomyces etchellsii. Microbiology 2016, 162, 1080–1090. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Chen, H.; Chen, Y.Q.; Chen, W.; Song, Y.; Ratledge, C. Enhanced lipid accumulation in the yeast Yarrowia lipolytica by over-expression of ATP: Citrate lyase from Mus musculus. J. Biotechnol. 2014, 192, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Dulermo, T.; Lazar, Z.; Dulermo, R.; Rakicka, M.; Haddouche, R.; Nicaud, J.M. Analysis of ATP-citrate lyase and malic enzyme mutants of Yarrowia lipolytica points out the importance of mannitol metabolism in fatty acid synthesis. Biochim. Biophys. Acta 2015, 1851, 1107–1117. [Google Scholar] [CrossRef]
- Blatti, J.L.; Michaud, J.; Burkart, M.D. Engineering fatty acid biosynthesis in microalgae for sustainable biodiesel. Curr. Opin. Chem. Biol. 2013, 17, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Cronan, J.E.; Thomas, J. Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways. Methods Enzymol. 2009, 459, 395–433. [Google Scholar]
- Spaans, S.K.; Weusthuis, R.A.; van der Oost, J.; Kengen, S.W.M. NADPH-generating systems in bacteria and archaea. Front. Microbiol. 2015, 6, 742. [Google Scholar] [CrossRef] [PubMed]
- Wasylenko, T.M.; Ahn, W.S.; Stephanopoulos, G. The oxidative pentose phosphate pathway is the primary source of NADPH for lipid overproduction from glucose in Yarrowia lipolytica. Metab. Eng. 2015, 30, 27–39. [Google Scholar] [CrossRef]
- Ratledge, C. The role of malic enzyme as the provider of NADPH in oleaginous microorganisms: A reappraisal and unsolved problems. Biotechnol. Let. 2014, 36, 1557–1568. [Google Scholar] [CrossRef]
- Bommareddy, R.R.; Sabra, W.; Maheshwari, G.; Zeng, A.P. Metabolic network analysis and experimental study of lipid production in Rhodosporidium toruloides grown on single and mixed substrates. Microb. Cell. Factories 2015, 14, 36. [Google Scholar] [CrossRef]
- Park, Y.; Han, G.S.; Mileykovskaya, E.; Garrett, T.A.; Carman, G.M. Altered lipid synthesis by lack of yeast Pah1 phosphatidate phosphatase reduces chronological life span. J. Biol. Chem. 2015, 290, 25382–25394. [Google Scholar] [CrossRef]
- Fakas, S. Lipid biosynthesis in yeasts: A comparison of the lipid biosynthetic pathway between the model nonoleaginous yeast Saccharomyces cerevisiae and the model oleaginous yeast Yarrowia lipolytica. Eng. Life Sci. 2017, 17, 292–302. [Google Scholar] [CrossRef]
- Hardman, D.; McFalls, D.; Fakas, S. Characterization of phosphatidic acid phosphatase activity in the oleaginous yeast Yarrowia lipolytica and its role in lipid biosynthesis. Yeast 2017, 34, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Dulermo, T.; Nicaud, J.M. Involvement of the G3P shuttle and β-oxidation pathway in the control of TAG synthesis and lipid accumulation in Yarrowia lipolytica. Metab. Eng. 2011, 13, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Zanotti, M.; Zhong, Y.; Liao, W.; Ducey, C.; Liu, Y. Co-hydrolysis of lignocellulosic biomass for microbial lipid accumulation. Biotechnol. Bioeng. 2013, 110, 1039–1049. [Google Scholar] [CrossRef]
- Qian, X.; Gorte, O.; Chen, L.; Zhang, W.; Dong, W.; Ma, J.; Jiang, M.; Xin, F.; Ochsenreither, K. Co-production of single cell oil and gluconic acid using oleaginous Cryptococcus podzolicus DSM 27192. Biotechnol. Biofuels 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Citric acid production by Yarrowia lipolytica cultivated on olive-mill wastewater-based media. Bioresour. Technol. 2008, 99, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Chatzifragkou, A.; Fakas, S.; Galiotou-panayotou, M.; Komaitis, M.; Nicaud, J.; Aggelis, G. Biosynthesis of lipids and organic acids by Yarrowia lipolytica strains cultivated on glucose. Eur. J. Lipid. Sci. Technol. 2009, 111, 1221–1232. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Kampisopoulou, E.; Blanchard, F.; Rondags, E.; Gardeli, C.; Koutinas, A.A.; Chevalot, I.; Aggelis, G. Production of secondary metabolites through glycerol fermentation under carbon-excess conditions by the yeasts Yarrowia lipolytica and Rhodosporidium toruloides. Eur. J. Lipid. Sci. Technol. 2017, 119, 1600507. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, I.K.; Zeng, A.P.; et al. Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2017, 17, 262–281. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, H.; Wang, G.; Chi, Z.; Chi, Z. Disruption of the MIG1 gene enhances lipid biosynthesis in the oleaginous yeast Yarrowia lipolytica ACA-DC 50109. BBA—Mol. Cell Biol. Lipids 2013, 1831, 675–682. [Google Scholar] [CrossRef]
- Lazar, Z.; Dulermo, T.; Neuvéglise, C.; Crutz-Le Coq, A.M.; Nicaud, J.M. Hexokinase—A limiting factor in lipid production from fructose in Yarrowia lipolytica. Metab. Eng. 2014, 2, 89–99. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Chen, H.; Chen, W.; Ratledge, C.; Song, Y.; Chen, W. Regulatory properties of malic enzyme in the oleaginous yeast, Yarrowia lipolytica, and its non-involvement in lipid accumulation. Biotechnol. Lett. 2013, 35, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, L.R.; Pardo, J.P.; Lomelí, M.M.; Bocardo, O.I.L.; Juárez Oropeza, M.A.; Guerra Sánchez, G. Lipid droplets accumulation and other biochemical changes induced in the fungal pathogen Ustilago maydis under nitrogen-starvation. Arch. Microbiol. 2017, 199, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Skerker, J.M.; Rutter, C.D.; Maurer, M.J.; Arkin, A.P.; Rao, C.V. Engineering Rhodosporidium toruloides for increased lipid production. Biotechnol. Bioeng. 2016, 113, 1056–1066. [Google Scholar] [CrossRef]
- Shi, S.; Chen, Y.; Siewers, V.; Nielsen, J. Improving production of malonyl coenzyme a-derived metabolites by abolishing Snf1-dependent regulation of Acc1. MBio 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Q.; Zhang, H.; Bao, J. Inhibitor degradation and lipid accumulation potentials of oleaginous yeast Trichosporon cutaneum using lignocellulose feedstock. Bioresour. Technol. 2016, 218, 892–901. [Google Scholar] [CrossRef]
- Li, Z.; Sun, H.; Mo, X.; Li, X.; Xu, B.; Tian, P. Overexpression of malic enzyme (ME) of Mucor circinelloides improved lipid accumulation in engineered Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 4927–4936. [Google Scholar] [CrossRef]
- Xue, J.; Niu, Y.; Huang, T.; Yang, W.; Liu, J.; Li, H. Genetic improvement of the microalga Phaeodactylum tricornutum for boosting neutral lipid accumulation. Metab. Eng. 2015, 27, 1–9. [Google Scholar] [CrossRef]
- Runguphan, W.; Keasling, J.D. Metabolic engineering of Saccharomyces cerevisiae for production of fatty acid-derived biofuels and chemicals. Metab. Eng. 2014, 21, 103–113. [Google Scholar] [CrossRef]
- Chuang, L.; Chen, D.; Nicaud, J.; Madzak, C.; Chen, Y.; Huang, Y. Co-expression of heterologous desaturase genes in Yarrowia lipolytica. N. Biotechnol. 2010, 27, 277–282. [Google Scholar] [CrossRef]
- Xie, D.; Jackson, E.N.; Zhu, Q. Sustainable source of omega-3 eicosapentaenoic acid from metabolically engineered Yarrowia lipolytica: From fundamental research to commercial production. Appl. Microbiol. Biotechnol. 2015, 99, 1599–1610. [Google Scholar] [CrossRef]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Efficient concomitant production of lipids and carotenoids by oleaginous red yeast Rhodotorula glutinis cultured in palm oil mill effluent and application of lipids for biodiesel production. Biotechnol. Bioprocess Eng. 2011, 16, 23–33. [Google Scholar] [CrossRef]
- Xu, J.; Du, W.; Zhao, X.; Zhang, G.; Liu, D. Microbial oil production from various carbon sources and its use for biodiesel preparation. Biofuel. Bioprod. Bior. 2013, 7, 65–77. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Qadeer, S.; Khalid, A.; Mahmood, S.; Anjum, M.; Ahmad, Z. Utilizing oleaginous bacteria and fungi for cleaner energy production. J. Clean. Prod. 2017, 168, 917–928. [Google Scholar] [CrossRef]
- Shruthi, P.; Rajeshwari, T.; Mrunalini, B.R.; Girish, V.; Girisha, S.T. Evaluation of oleaginous bacteria for potential biofuel. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 47–57. [Google Scholar]
- Lin, C.Y.; Lin, Y.W. Fuel characteristics of biodiesel produced from a high-acid oil from soybean soapstock by supercritical-methanol transesterification. Energies 2012, 5, 2370–2380. [Google Scholar] [CrossRef]
- Ekpeni, L.E.N.; Benyounis, K.Y.; Nkem-ekpeni, F.F.; Stokes, J.; Olabi, A.G. Underlying factors to consider in improving energy yield from biomass source through yeast use on high-pressure homogenizer (hph). Energy 2015, 81, 74–83. [Google Scholar] [CrossRef]
- Vaz, S., Jr. Biomass and the Green Chemistry, 1st ed.; Springer: Brasília, Brazil, 2018; p. 264. [Google Scholar]
- Fatma, S.; Hameed, A.; Noman, M.; Ahmed, T.; Sohail, I.; Shahid, M.; Tariq, M.; Tabassum, R. Lignocellulosic biomass: A sustainable bioenergy source for future. Protein Pept. Lett. 2018, 25. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolysate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Carrillo, I.; Teixeira, R.; Ago, M.; Rojas, O. Comparative study of cellulosic components isolated from different Eucalyptus species. Cellulose 2018, 25, 1011–1029. [Google Scholar] [CrossRef]
- Borand, M.N.; Karaosmanoğlu, F. Effects of organosolv pretreatment conditions for lignocellulosic biomass in biorefinery applications: A review. J. Renew. Sustain. Energy 2018, 10, 033104. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic liquid based of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef] [PubMed]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions, 1st ed.; Verlag Kessel: Remagen, Germany, 2003; p. 613. [Google Scholar]
- Lim, W.; Lee, J. Influence of pretreatment condition on the fermentable sugar production and enzymatic hydrolysis of dilute acid-pretreated mixed softwood. Bioresour. Technol. 2013, 140, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jiang, D.; Zhang, H.; Lee, D.; Zhang, Z.; Zhang, Q.; Jing, Y.; Zhang, Y.; Xia, C. Effects of different pretreatment methods on the structural characteristics, enzymatic saccharification and photo-fermentative bio-hydrogen production performance of corn straw. Bioresour. Technol. 2020, 304, 122999. [Google Scholar] [CrossRef]
- Lima, D.R.; Silveira, M.H.L.; Del Rio, L.; Ramos, L.P. Pretreatment processes for cellulosic ethanol production: Processes integration and modeling for the utilization of lignocellulosics such as sugarcane straw. In Green Fuels Technology, 1st ed.; Soccol, C.R., Brar, S.K., Faulds, C., Ramos, L.P., Eds.; Springer: New York, NY, USA, 2016; Volume 1, pp. 107–131. [Google Scholar]
- Gellerstedt, G. The worldwide wood resource. In Pulp and Paper Chemistry and Technology Wood Chemistry and Wood Biotechnology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; Walter de Gruyter: Berlin, Germany, 2009; p. 321. [Google Scholar]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.D.; Porto de Souza, L.; Vandenberghe, L.P.; Neto, D.C.; Sydney, A.C.; Letti, L.A.; Karp, S.G.; Torres, L.A.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance–conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Arantes, V.; Goodell, B. Current understanding of brown-rot fungal biodegradation mechanisms: A review. In Deterioration and Protection of Sustainable Biomaterials; Schultz, T.P., Goodell, B., Nicholas, D., Eds.; American Chemical Society: Washington, DC, USA, 2014; pp. 3–21. [Google Scholar]
- Cragg, S.M.; Beckham, G.T.; Bruce, N.C.; Bugg, T.D.H.; Distel, D.L.; Dupree, P.; Etxabe, A.G.; Goodell, B.S.; Jellison, J.; Mcgeehan, J.E.; et al. Lignocellulose degradation mechanisms across the Tree of Life. Curr. Opin. Chem. Biol. 2015, 29, 108–119. [Google Scholar] [CrossRef]
- Potumarthi, R.; Raju, R.; Nayak, P.; Jetty, A. Simultaneous pretreatment and sacchariffication of rice husk by Phanerochete chrysosporium for improved production of reducing sugars. Bioresour. Technol. 2013, 128, 113–117. [Google Scholar] [CrossRef]
- Rodríguez-Couto, S. Industrial and environmental applications of white-rot fungi. Mycosphere 2017, 8, 456–466. [Google Scholar] [CrossRef]
- Martínez-patiño, J.C.; Lu-chau, T.A.; Gullón, B.; Ruiz, E.; Romero, I.; Castro, E.; Lema, J.M. Application of a combined fungal and diluted acid pretreatment on olive tree biomass. Ind. Crop. Prod. 2018, 121, 10–17. [Google Scholar] [CrossRef]
- Daniel, G. Fungal and bacterial biodegradation: White rots, brown rots, soft rots, and bacteria. ACS Symp. Ser. 2014, 1158, 23–58. [Google Scholar] [CrossRef]
- Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Insights into lignin degradation and its potential industrial applications. Adv. Appl. Microbiol. 2013, 82, 1–28. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Fariña, J.I.; Castrillo, M.L.; Rodríguez, M.D.; Nuñez, C.E.; Villalba, L.L.; Zapata, P.D. Biopulping of wood chips with Phlebia brevispora BAFC 633 reduces lignin content and improves pulp quality. Int. Biodeterior. Biodegr. 2014, 90, 29–35. [Google Scholar] [CrossRef]
- Barakat, A.; Mayer-laigle, C.; Solhy, A.; Arancon, R.A.D.; Vries, H.; Luque, R. Mechanical pretreatments of lignocellulosic biomass: Towards facile and environmentally sound technologies for biofuels production. RSC Adv. 2014, 4, 48109–48127. [Google Scholar] [CrossRef]
- Mayer-Laigle, C.; Rajaonarivony, R.K.; Blanc, N.; Rouau, X. Comminution of dry lignocellulosic biomass: Part II. Technologies, improvement of milling performances, and security issues. Bioengineering 2018, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Monlau, F.; Solhy, A.; Carrere, H. Mechanical dissociation and fragmentation of lignocellulosic biomass: Effect of initial moisture, biochemical and structural proprieties on energy requirement. Appl. Energy 2015, 142, 240–246. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzyme Res. 2011, 2011, 1–17. [Google Scholar] [CrossRef]
- Singh, J.; Suhag, M.; Dhaka, A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Romero-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid pretreatment of lignocellulosic biomass for energy vectors production: A review focused on operational conditions and techno-economic assessment for bioethanol production. Renew. Sustain. Energy Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Rigual, V.; Santos, T.M.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Evaluation of hardwood and softwood fractionation using autohydrolysis and ionic liquid microwave pretreatment. Biomass Bioenergy 2018, 117, 190–197. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Hsu, T.; Guo, G.; Chen, W.; Hwang, W. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 2010, 101, 4907–4913. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Cara, C.; Moya, M.; Ruiz, E.; Castro, E.; Romero, I. Fermentable sugar production from rapeseed straw by dilute phosphoric acid pretreatment. Ind. Crops Prod. 2013, 50, 525–531. [Google Scholar] [CrossRef]
- Wang, G.S.; Lee, J.; Zhu, J.Y.; Jeffries, T.W. Dilute Acid Pretreatment of corncob for efficient sugar production. Appl. Biochem. Biotechnol. 2011, 163, 658–668. [Google Scholar] [CrossRef]
- Amnuaycheewa, P.; Hengaroonprasan, R.; Rattanaporn, K.; Kirdponpattara, S.; Cheenkachorn, K.; Sriariyanund, M. Enhancing enzymatic hydrolysis and biogas production from rice straw by pretreatment with organic acids. Ind. Crop. Prod. 2016, 87, 247–254. [Google Scholar] [CrossRef]
- Noparat, P.; Prasertsan, P.; O.-thong, S.; Pan, X. Dilute Acid Pretreatment of oil palm trunk biomass at high temperature for enzymatic hydrolysis. Energy Proc. 2015, 79, 924–929. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, C.; Lin, Q.; Wang, X.; Cheng, B.; Li, H.; Ren, J. Microwave-assisted oxalic acid pretreatment for the enhancing of enzyme hydrolysis in the production of xylose and arabinose from bagasse. Molecules 2018, 23, 862. [Google Scholar] [CrossRef]
- Dziekonska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Pielech-Przybylska, K.; Balcerek, M. Nitric acid pretreatment of Jerusalem artichoke stalks for enzymatic saccharification and biorthanol production. Energies 2018, 11, 2153. [Google Scholar] [CrossRef]
- Sahu, S.; Pramanik, K. Evaluation and optimization of organic acid pretreatment of cotton gin waste for enzymatic hydrolysis and bioethanol production. Appl. Biochem. Biotechnol. 2018, 186, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wu, S.; Liu, L. Enzymatic saccharification of dilute acid pretreated eucalyptus chips for fermentable sugar production. Bioresour. Technol. 2012, 110, 302–307. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, Y.; Cort, J.R.; Ragauskas, A.J.; Yang, B. Revealing the molecular structural transformation of hardwood and softwood in dilute acid flow through pretreatment. ACS Sustain. Chem. Eng. 2016, 4, 6618–6628. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Dorgan, K.M.; Chen, S. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Beevi, S.; Kumar, A.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Du, Y.; Shang, X.; Zheng, Y.; Zhou, J. Enhancing fermentable sugar yield from cassava residue using a two-step dilute ultra-low acid pretreatment process. Ind. Crop. Prod. 2018, 124, 555–562. [Google Scholar] [CrossRef]
- Janga, K.K.; Hägg, M.-B.; Moe, S.T. Influence of acid concentration, temperature, and time on the concentrated sulfuric acid hydrolysis of pinewood and aspenwood: A statistical approach. BioResources 2012, 7, 391–411. [Google Scholar] [CrossRef]
- Bravo, C.; Garcés, D.; Faba, L.; Sastre, H.; Ordóñez, S. Selective arabinose extraction from Pinus sp. sawdust by two-step soft acid hydrolysis. Ind. Crop. Prod. 2017, 104, 229–236. [Google Scholar] [CrossRef]
- He, Y.; Zhang, J.; Bao, J. Dry dilute acid pretreatment by co-currently feeding of corn stover feedstock and dilute acid solution without impregnation. Bioresour. Technol. 2014, 158, 360–364. [Google Scholar] [CrossRef]
- Gupta, R.; Lee, Y.Y. Pretreatment of corn stover and hybrid poplar by sodium hydroxide and hydrogen peroxide. Biotechnol. Prog. 2010, 26, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, S.; Wang, H. Use of different alkaline pretreatments and enzyme models to improve low-cost cellulosic biomass conversion. Biomass Bioenergy 2012, 39, 182–191. [Google Scholar] [CrossRef]
- Sakuragi, K.; Igarashi, K.; Samejima, M. Application of ammonia pretreatment to enable enzymatic hydrolysis of hardwood biomass. Polym. Degrad. Stab. 2018, 148, 19–25. [Google Scholar] [CrossRef]
- An, S.; Li, W.; Liu, Q.; Xia, Y.; Zhang, T.; Huang, F.; Lin, Q.; Chen, L. Combined dilute hydrochloric acid and alkaline wet oxidation pretreatment to improve sugar recovery of corn stover. Bioresour. Technol. 2019, 271, 283–288. [Google Scholar] [CrossRef]
- Zu, S.; Li, W.; Zhang, M.; Li, Z.; Wang, Z.; Jameel, H.; Chang, H. Pretreatment of corn stover for sugar production using dilute hydrochloric acid followed by lime. Bioresour. Technol. 2014, 152, 364–370. [Google Scholar] [CrossRef]
- Yang, M.; Lan, M.; Gao, X.; Dou, Y.; Zhang, X. Sequential dilute acid/alkali pretreatment of corncobs for ethanol production. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 1–10. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.Y.; Jang, H.M.; Lee, M.W.; Park, J.M. Sequential dilute acid and alkali pretreatment of corn stover: Sugar recovery efficiency and structural characterization. Bioresour. Technol. 2015, 182, 296–301. [Google Scholar] [CrossRef]
- Cybulska, I.; Brudecki, G.P.; Zembrzuska, J.; Schmit, J.E.; Lopez, C.G.B.; Thomsen, M.H. Organosolv delignification of agricultural residues (date palm fronds, Phoenix dactylifera L.) of the United Arab Emirates. Appl. Energy 2016, 185, 1040–1050. [Google Scholar] [CrossRef]
- Koo, B.; Park, N.; Jeong, H.; Choi, J.; Yeo, H.; Choi, I. Characterization of by-products from organosolv pretreatments of yellow poplar wood (Liriodendron tulipifera) in the presence of acid and alkali catalysts. J. Ind. Eng. Chem. 2011, 17, 18–24. [Google Scholar] [CrossRef]
- Shahbazi, A.; Zhang, B. Dilute and concentrated acid hydrolysis of lignocellulosic biomass. In Bioalcohol Production: Biochemical Conversion of Lignocellulosic Biomass; Waldron, K.W., Ed.; Woodhead: Sawston, Cambridge, UK, 2010; p. 496. [Google Scholar]
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech. 2015, 5, 597–609. [Google Scholar] [CrossRef]
- Qi, W.; He, C.; Wang, Q.; Liu, S.; Yu, Q.; Wang, W.; Leksawasdi, N.; Wang, C.; Yuan, Z. Carbon-based solid acid pretreatment in corncob saccharification: Specific xylose production and efficient enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2018, 6, 3640–3648. [Google Scholar] [CrossRef]
- Xu, J.; Sun, R.C. Recent advances in alkaline pretreatment of lignocellulosic biomass. In Biomass Fractionation Technologies for A Lignocellulosic Feedstock Based Biorefiner; Mussatto, S., Ed.; Elsevier: Atlanta, GA, USA, 2016; pp. 311–324. [Google Scholar]
- Nitsos, C.; Rova, U. Organosolv Fractionation of softwood biomass for biofuel and biorefinery applications. Energies 2018, 11, 50. [Google Scholar] [CrossRef]
- Nargotra, P.; Sharma, V.; Gupta, M.; Kour, S.; Bajaj, B.K. Application of ionic liquid and alkali pretreatment for enhancing saccharification of sunflower stalk biomass for potential biofuel-ethanol production. Bioresour. Technol. 2018, 267, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Nieves, I.U.; Mullinnix, M.T.; Sagues, W.J.; Hoffman, R.W.; Fernández-Sandoval, M.T.; Tian, Z.; Rockwood, D.L.; Tamang, B.; Ingram, L.O. Optimization of dilute-phosphoric-acid steam pretreatment of Eucalyptus benthamii for biofuel production. Appl. Energy 2014, 125, 76–83. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Garay, L.A.; Enriquez, L.; Fry, R.; Butler, J.H.; Lopez, J.M.; Kanti, A.; Faulina, S.A.; Nugroho, A.J.; Simmons, B.A.; et al. 1-Ethyl-3-methylimidazolium tolerance and intracellular lipid accumulation of 38 oleaginous yeast species. Appl. Microbiol. Biotechnol. 2017, 101, 8621–8631. [Google Scholar] [CrossRef]
- Abdul, P.M.; Jahim, J.; Harun, S.; Markom, M.; Aminah, N.; Hassan, O.; Balan, V.; Dale, B.E.; Mohd-Nor, M.T. Effects of changes in chemical and structural characteristic of ammonia fibre expansion (AFEX) pretreated oil palm empty fruit bunch fiber on enzymatic saccharification and fermentability for biohydrogen. Bioresour. Technol. 2016, 211, 200–208. [Google Scholar] [CrossRef]
- Kim, S.M.; Dien, B.S.; Tumbleson, M.E.; Rausch, K.D.; Singh, V. Improvement of sugar yields from corn stover using sequential hot water pretreatment and disk milling. Bioresour. Technol. 2016, 216, 706–713. [Google Scholar] [CrossRef]
- Wells, J.M.; Drielak, E.; Surendra, K.C.; Khanal, S. Hot water pretreatment of lignocellulosic biomass: Modeling the effects of temperature, enzyme and biomass loadings on sugar yield. Bioresour. Technol. 2020, 300, 122593. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, M.M.; Radiah, D. Microwave-assisted pretreatment of lignocellulosic biomass: A review. J. Eng. Sci. Technol. 2015, 10, 97–109. [Google Scholar]
- Huang, C.; Zong, M.; Wu, H.; Liu, Q. Microbial oil production from rice straw hydrolysate by Trichosporon fermentans. Bioresour. Technol. 2009, 100, 4535–4538. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, F.; Du, W.; Liu, C.; Liu, D. Effects of some inhibitors on the growth and lipid accumulation of oleaginous yeast Rhodosporidium toruloides and preparation of biodiesel by enzymatic transesterification of the lipid. Bioproc. Biosyst. Eng. 2012, 35, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, N.; Bhatnagar, R.; Viswanathan, L. Inhibition of glycolysis by furfural in Saccharomyces cerevisiae. Eur. J. Appl. Microbiol. 1981, 11, 226–228. [Google Scholar] [CrossRef]
- Modig, T.; Liden, G.; Taherzadeh, M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.M.; Bertilsson, M.; Hahn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Carbon fluxes of xylose-consuming Saccharomyces cerevisiae strains are affected differently by NADH and NADPH usage in HMF reduction. Appl. Microbiol. Biotechnol. 2009, 84, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Quintero, A.; Rinco, L.E.; Cardona, C.A. Production of bioethanol from agroindustrial residues as feedstocks. In Biofuels Alternative Feedstocks and Conversion Processes; Pandey, A., Larroche, C., Ricke, S., Dussap, C.G., Gnansounou, E., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 251–285. [Google Scholar]
- Tu, W.; Hallett, J.P. Recent advances in the pretreatment of lignocellulosic biomass. Curr. Opin. Green Sustain. Chem. 2019, 20, 11–17. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Zhang, X.; Hu, F.; Ryu, D.D.Y.; Bao, J. Screening of oleaginous yeast strains tolerant to lignocellulose degradation compounds. Appl. Biochem. Biotechnol. 2009, 159, 591–604. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, X.; Zhao, J.; Wu, S.; Zhao, Z.K. Effects of biomass hydrolysis by-products on oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 2009, 100, 4843–4847. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, J.; Blomqvist, J.; Pickova, J.; Bonturi, N.; Sandgren, M.; Passoth, V. Lipid production from hemicellulose with Lipomyces starkeyi in a pH regulated fed-batch cultivation. Yeast 2016, 33, 451–462. [Google Scholar] [CrossRef]
- Pereira, I.; Madeira, A.; Prista, C.; Loureiro-Dias, M.C.; Leandro, M.J. Characterization of new polyol/H+ symporters in Debaryomyces hansenii. PLoS ONE 2014, 9. [Google Scholar] [CrossRef][Green Version]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuel. 2013, 6, 16. [Google Scholar] [CrossRef]
- Park, Y.C.; Oh, E.J.; Jo, J.H.; Jin, Y.S.; Seo, J.H. Recent advances in biological production of sugar alcohols. Curr. Opin. Biotechnol. 2016, 37, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Y.; Liao, Q.; Zhu, X.; Xu, T.F. Hydrolysates of lignocellulosic materials for biohydrogen production. BMB Rep. 2013, 46, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Liu, J.N.; Lu, L.J.; Peng, K.M.; Yang, G.X.; Liu, J. Culture strategies for lipid production using acetic acid as sole carbon source by Rhodosporidium toruloides. Bioresour. Technol. 2016, 206, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ho, S.H.; Hasunuma, T.; Chang, J.S.; Ren, N.Q.; Kondo, A. Recent advances in yeast cell-surface display technologies for waste biorefineries. Bioresour. Technol. 2016, 215, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Zhu, J.Y.; Ragauskas, A.; Deng, Y. Enhanced enzymatic hydrolysis of spruce by alkaline pretreatment at low temperature. Biotechnol. Bioeng. 2008, 99, 1320–1328. [Google Scholar] [CrossRef]
- Keshav, P.K.; Shaik, N.; Koti, S.; Linga, V.R. Bioconversion of alkali delignified cotton stalk using two-stage dilute acid hydrolysis and fermentation of detoxified hydrolysate into ethanol. Ind. Crop. Prod. 2016, 91, 323–331. [Google Scholar] [CrossRef]
- Pival, S.L.; Birner-Gruenberger, R.; Krump, C.; Nidetzky, B. D-Xylulose kinase from Saccharomyces cerevisiae: Isolation and characterization of the highly unstable enzyme, recombinantly produced in Escherichia coli. Protein Expr. Purif. 2011, 79, 223–230. [Google Scholar] [CrossRef][Green Version]
- Hirasawa, H.; Shioya, K.; Mori, K.; Tashiro, K.; Aburatani, S.; Shida, Y.; Kuhara, S.; Ogasawara, W. Cellulase productivity of Trichoderma reesei mutants developed in Japan varies with varying pH conditions. J. Biosci. Bioeng. 2019, 128, 264–273. [Google Scholar] [CrossRef]
- Claes, A.; Deparis, Q.; Foulquié-Moreno, M.R.; Thevelein, J.M. Simultaneous secretion of seven lignocellulolytic enzymes by an industrial second-generation yeast strain enables efficient ethanol production from multiple polymeric substrates. Metab. Eng. 2020, 59, 131–141. [Google Scholar] [CrossRef]
- Won, K.Y.; Kim, Y.S.; Oh, K.K. Comparison of bioethanol production of simultaneous saccharification and fermentation and separation hydrolysis and fermentation from cellulose-rich barley straw. Korean J. Chem. Eng. 2012, 29, 1341–1346. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, H.; Wang, Q.; Yang, X.; Xie, H.; Zhao, Z.K. Efficient conversion of biomass into lipids by using the simultaneous saccharification and enhanced lipid production process. Biotechnol. Biofuels 2013, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Yu, Z.; Bao, J. Simultaneous saccharification and microbial lipid fermentation of corn stover by oleaginous yeast Trichosporon cutaneum. Bioresour. Technol. 2012, 118, 13–18. [Google Scholar] [CrossRef]
- Peng, X.; Chen, H. Single cell oil production in solid-state fermentation by Microsphaeropsis sp. from steam-exploded wheat straw mixed with wheat bran. Bioresour. Technol. 2008, 99, 3885–3889. [Google Scholar] [CrossRef] [PubMed]
- Le, R.K.; Das, P.; Mahan, K.M.; Anderson, S.A.; Wells, T., Jr.; Yuan, J.S.; Ragauskas, A.J. Utilization of simultaneous saccharification and fermentation residues as feedstock for lipid accumulation in Rhodococcus opacus. AMB Express 2017, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Pejó, E.; Oliva, J.M.; González, A.; Ballesteros, I.; Ballesteros, M. Bioethanol production from wheat straw by the thermotolerant yeast Kluyveromyces marxianus CECT 10875 in a simultaneous saccharification and fermentation fed-batch process. Fuel 2009, 88, 2142–2147. [Google Scholar] [CrossRef]
- Zhang, J.; Lynd, L.R. Ethanol production from paper sludge by simultaneous saccharification and co-fermentation using recombinant xylose-fermenting microorganisms. Biotechnol. Bioenergy 2010, 107, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, J.; Singh, S.; Nain, L. Bioprospecting thermotolerant ethanologenic yeasts for simultaneous saccharification and fermentation from diverse environments. J. Biosci. Bioeng. 2017, 123, 342–346. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. Heterotrophic cultivation of Auxenochlorella protothecoides using forest biomass as a feedstock for sustainable biodiesel production. Biotechnol. Biofuels 2018, 11, 169. [Google Scholar] [CrossRef]
- Xiong, L.; Huang, C.; Yang, X.Y.; Lin, X.Q.; Chen, X.F.; Wang, C.; Wang, B.; Zeng, X.A.; Chen, X.D. Beneficial effect of corncob acid hydrolysate on the lipid production by oleaginous yeast Trichosporon dermatis. Prep. Biochem. Biotechnol. 2015, 45, 421–429. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chang, K.S.; Lee, C.F.; Hsu, C.L.; Huang, C.W.; Jang, H.D. Microbial lipid production by oleaginous yeast Cryptococcus sp. in the batch cultures using corncob hydrolysate as carbon source. Biomass Bioenergy 2015, 72, 95–103. [Google Scholar] [CrossRef]
- Chen, X.F.; Huang, C.; Xiong, L.; Ma, L.L.; Chen, X.D. Microbial oil production from Corncob acid hydrolysate by Trichosporon cutaneum. Biotechnol. Lett. 2012, 34, 1025–1028. [Google Scholar] [CrossRef]
- Gao, Q.; Cui, Z.; Zhang, J.; Bao, J. Lipid fermentation of corncob residues dhydrolysate by oleaginous yeast Trichosporon cutaneum. Bioresour. Technol. 2014, 152, 552–556. [Google Scholar] [CrossRef]
- Gong, Z.; Zhou, W.; Shen, H.; Yang, Z.; Wang, G.; Zuo, Z.; Hou, Y.; Zhao, Z.K. Co-fermentation of acetate and sugars facilitating microbial lipid production on acetate-rich biomass hydrolysates. Bioresour. Technol. 2016, 207, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, X.F.; Xiong, L.; Chen, X.D.; Ma, L.L. Oil production by the yeast Trichosporon dermatis cultured in enzymatic hydrolysates of corncobs. Bioresour. Technol. 2012, 110, 711–714. [Google Scholar] [CrossRef]
- Fei, Q.; O’Brien, M.; Nelson, R.; Chen, X.; Lowell, A.; Dowe, N. Enhanced lipid production by Rhodosporidium toruloides using different fed-batch feeding strategies with lignocellulosic hydrolysate as the sole carbon source. Biotechnol. Biofuels. 2016, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Liu, H.; Zhang, J. Enhanced lipid production with undetoxified corncob hydrolysate by Rhodotorula glutinis using a high cell density culture strategy. Bioresour. Technol. 2015, 180, 32–39. [Google Scholar] [CrossRef]
- Wells, T.; Wei, Z.; Ragauskas, A. Bioconversion of lignocellulosic pretreatment effluent via oleaginous Rhodococcus opacus DSM 1069. Biomass Bioenergy 2015, 72, 200–205. [Google Scholar] [CrossRef]
- Xu, J.; Du, W.; Zhao, X.; Liu, D. Renewable microbial lipid production from oleaginous yeast: Some surfactants greatly improved lipid production of Rhodosporidium toruloides. World J. Microbiol. Biotechnol. 2016, 32, 107. [Google Scholar] [CrossRef] [PubMed]
- Taoka, Y.; Nagano, N.; Okita, Y.; Izumida, H.; Sugimoto, S.; Hayashi, M. Effect of Tween 80 on the growth, lipid accumulation and fatty acid composition of Thraustochytrium aureum ATCC 34304. J. Biosci. Bioeng. 2011, 111, 420–424. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, L.K.; Ghosh, S. Bioconversion of lignocellulosic fraction of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to ethanol by Pichia stipitis. Bioresour. Technol. 2009, 100, 3293–3297. [Google Scholar] [CrossRef]
- Li, Y.; Qi, B.; Wan, Y. Separation of monosaccharides from pretreatment inhibitors by nanofiltration in lignocellulosic hydrolysate: Fouling mitigation by activated carbon adsorption. Biomass Bioenergy 2020, 136, 105527. [Google Scholar] [CrossRef]
- Kundu, C.; Lee, J. Bioethanol production from detoxified hydrolysate and the characterization of oxalic acid pretreated eucalyptus (Eucalyptus globulus) biomass. Ind. Crop. Prod. 2016, 83, 322–328. [Google Scholar] [CrossRef]
- Kim, Y.; Kreke, T.; Hendrickson, R.; Parenti, J.; Ladisch, M.R. Fractionation of cellulase and fermentation inhibitors from steam pretreated mixed hardwood. Bioresour. Technol. 2013, 135, 30–38. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, P.; Yadav, A.; Datta, S. Degradation of fermentation inhibitors from lignocellulosic hydrolysate liquor using immobilized bacterium, Bordetella sp. BTIITR. Chem. Eng. J. 2019, 361, 1152–1160. [Google Scholar] [CrossRef]
- Tai, M.; Stephanopoulos, G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab. Eng. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Quarterman, J.C.; Slininger, P.J.; Hector, R.E.; Dien, B.S. Engineering Candida phangngensis-an oleaginous yeast from the Yarrowia clade-for enhanced detoxification of lignocellulose-derived inhibitors and lipid overproduction. FEMS Yeast Res. 2018, 18, foy102. [Google Scholar] [CrossRef] [PubMed]
- Quarterman, J.; Slininger, P.J.; Kurtzman, C.P.; Thompson, S.R.; Dien, B.S. A survey of yeast from the Yarrowia clade for lipid production in dilute acid pretreated lignocellulosic biomass hydrolysate. Appl. Microbiol. Biotechnol. 2017, 101, 3319–3334. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Arora, N.; Sartaj, K.; Pruthi, V.; Pruthi, P.A. Sustainable biodiesel production from oleaginous yeasts utilizing hydrolysates of various non-edible lignocellulosic biomasses. Renew. Sustain. Energy Rev. 2016, 62, 836–855. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, H. Metabolic engineering of oleaginous yeasts for production of fuels and chemicals. Front. Microbiol. 2017, 8, 2185. [Google Scholar] [CrossRef]
- Chong, G.G.; Huang, X.J.; Di, J.H.; Xu, D.Z.; He, Y.C.; Pei, Y.N.; Tang, Y.; Ma, C.L. Biodegradation of alkali lignin by a newly isolated Rhodococcus pyridinivorans CCZU-B16. Bioproc. Biosyst. Eng. 2018, 41, 501–510. [Google Scholar] [CrossRef]
- Kumar, L.R.; Yellapu, S.K.; Tyagi, R.D.; Zhang, X. A review on variation in crude glycerol composition, bio-valorization of crude and purified glycerol as carbon source for lipid production. Bioresour. Technol. 2019, 293, 122155. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.; Allen, M.J.; Chuck, C.J. Coproducts of algae and yeast-derived single cell oils: A critical review of their role in improving biorefinery sustainability. Bioresour. Technol. 2020, 303, 122862. [Google Scholar] [CrossRef] [PubMed]


| After Hydrolysis | After EH | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reagent | Solid Fraction (% w/w, 100 g) | Liquid Fraction (% w/w, 100 g) | By-Products (g/L) | Digestibility (% w/w) | |||||||||||||||||||
| Pretreatment | Substrate | % v/v | S:L Ratio | T (°C) | t (min) | Total | Glc | H | L | Total | Glc | H | L | Furfural | HMF | PC | Formic | Acetic | Levulinic | Glc | H | Reference | |
| DA | Sugarcane bagasse | HCl | 2.5 | 1:15 | 121 | 45 | 21 | 4.0 | 16 | 0.12 | 0.61 | [118] | |||||||||||
| DA | Defatted rice Bran | H2SO4 | 3 | 1:8 | 90 | 360 | 50 | 43 | 7 | 0.03 | 0.3 | [56] | |||||||||||
| DA | Rice straw | H2SO4 | 1 | 1:10 | 180 | 1 | 59 | 1.9 | 21 | 5 | 14 | 1.6 | 0.2 | 1.6 | 70 | [191] | |||||||
| DA | Rapeseed straw | H3PO4 | 3 | 1:12 | 200 | 15 | 48 | 48 | 0 | 47 | 4 | 10 | 8.0 | 2.6 | 0.6 | 5.5 | 83 | [192] | |||||
| DA | Corncob | H2SO4 | 2.2 | 170 | 30 | 56 | 34 | 4.4 | 14 | 88 | [193] | ||||||||||||
| DA | Rice straw | C2H2O4 | 5.0 | 136 | 31 | 60 | 24 | 22 | 5 | 43 | [194] | ||||||||||||
| DA | Oil palm trunk | H2SO4 | 3 | 1:5 | 180 | 40 | 47 | 52 | ND | 37 | 79 | [195] | |||||||||||
| DA | Sugar bagasse | C2H2O4 | 3.6 | 1:20 | 120 | 15 | 3.3 | 5 | 93 | 80 | 92 | 96 | [196] | ||||||||||
| DA | Jerusalem artichoke | H2SO4 | 5 | 1:10 | 121 | 60 | 60 | 42 | 1.9 | 27 | 7 | 83 | 0.3 | 0.2 | 1.1 | 0.4 | 3.3 | 58 | [197] | ||||
| DA | Jerusalem artichoke | HNO3 | 5 | 1:10 | 121 | 60 | 66 | 77 | 1.3 | 21 | 15 | 97 | 0.04 | 0.01 | 0.5 | 1.2 | 3.2 | 89 | [197] | ||||
| DA | Cotton gin waste | C4H4O4 | 1.6 | 1:10 | 130 | 45 | 77 | 39 | 2.3 | 17 | 1.1 | 84 | 88 | 0.8 | 0.22 | 0.1 | 1.02 | 68 | [198] | ||||
| DA | Eucalyptus sp. | H2SO4 | 0.75 | 1:20 | 160 | 10 | 17 | 30 | 13 | 90 | 1.4 | 0.13 | 2.0 | 76 | [199] | ||||||||
| DA | Poplar | H2SO4 | 0.05 | 240 | 10 | 0 | 0 | 50 | 100 | [200] | |||||||||||||
| DA | Pine | H2SO4 | 0.05 | 240 | 10 | 0 | 65 | 70 | 95 | 30 | 70 | [200] | |||||||||||
| DA | Mixed softwood | C2H2 (CO)2O | 1:4 | 180 | 30 | 47 | 16 | 19 | 0.9 | 2.4 | 5.3 | 2.3 | 57 | [167] | |||||||||
| DA | Wheat straw | H2SO4 | 2 | 1:10 | 121 | 60 | 14 | 82 | 0.44 | 0.05 | 4 | [201] | |||||||||||
| DA | Corn stover | H2SO4 | 1 | 1:10 | 160 | 10 | 1.3 | 2.6 | 2.9 | 2.3 | [73] | ||||||||||||
| DA | Wild rice grass | H2SO4 | 2 | 1:10 | 121 | 60 | 85 | 38 | 6 | 36 | 45 | [202] | |||||||||||
| DA | Cassava residue | H2SO4 | 0.01 | 1:20 | 180 | 20 | 55 | 17 | 18 | 7.6 | 39 | 0.05 | 0.02 | 0.76 | 0.44 | 70 | [203] | ||||||
| TSDA | Cassava residue | H2SO4 | 0.05 | 1:20 | 190 | 10 | 60 | 6 | 21 | 2.5 | 58 | 0.12 | 0.02 | 0.13 | 0.18 | 73 | [203] | ||||||
| TSDA | Populus tremula | H2SO4 | 65 | 1:15 | 35 | 180 | 36 | 19 | 0.5 | 0.04 | 2.7 | 1.9 | 1.6 | [204] | |||||||||
| TSDA | Pinus sylvestris | H2SO4 | 73 | 1:15 | 53 | 60 | 44 | 17 | 0.2 | 0.05 | 6.8 | 3.5 | 1.9 | [204] | |||||||||
| TSDA | Pinus sp. | HCl | 3 | 1:10 | 65/80 | 108/240 | 38 | 14 | 42 | 38 | 54 | [205] | |||||||||||
| DDA | Corn stover | H2SO4 | 2.5 | 2:1 | 185 | 1440 | 39 | 21 | 85 | [206] | |||||||||||||
| Alk | Corn stover | NaOH | 2 | 1:10 | 121 | 20 | 6.4 | 5.3 | [73] | ||||||||||||||
| Alk | Corn stover | NaOH | 5 | 1:10 | 60 | 1440 | 74 | 28 | 7.6 | 3.3 | 19 | 65 | 81 | 99 | [207] | ||||||||
| Alk | Poplar | NaOH | 5 | 1:10 | 120 | 1440 | 88 | 42 | 8.1 | 23 | 5 | 46 | 21 | 80 | [207] | ||||||||
| Alk | Japanese silvergrass | Ca(OH)2 | 10 | 1:10 | 25 | 8 W | 72 | 98 | 37 | 77 | 28 | 2.4 | 63 | 23 | 60 | 50 | [208] | ||||||
| Alk | Japanese silvergrass | NH3 | 3 | 1:10 | 25 | 8 W | 71 | 93 | 53 | 90 | 29 | 6.1 | 47 | 9.8 | 45 | 10 | [208] | ||||||
| Alk | Napiergrass | Ca(OH)2 | 10 | 1:10 | 25 | 8 W | 68 | 92 | 32 | 86 | 32 | 8 | 68 | 2.8 | 65 | 45 | [208] | ||||||
| Alk | Napiergrass | NH3 | 3 | 1:10 | 25 | 8 W | 65 | 91 | 55 | 90 | 35 | 9 | 45 | 10 | 70 | 30 | [208] | ||||||
| Alk | Rice straw | Ca(OH)2 | 10 | 1:10 | 25 | 8 W | 70 | 96 | 30 | 49 | 31 | 4.2 | 71 | 51 | 45 | 40 | [208] | ||||||
| Alk | Rice straw | NH3 | 3 | 1:10 | 25 | 8 W | 66 | 92 | 55 | 54 | 34 | 8 | 45 | 46 | 50 | 10 | [208] | ||||||
| Alk | Hardwoods | NH4 | 140 | 60 | 95 | 100 | 98 | [209] | |||||||||||||||
| A/A | corn stover | HCl/NH3 | 1/13 | 1:10 | 120/130 | 40/40 | 42 | 85 | - | 14 | 1 | 83 | 0.9 | 72 | [210] | ||||||||
| A/A | Corn stover | HCl/Ca(OH)2 | 1/0.1 | 1:10 | 120/60 | 40/12h | 55 | 92 | 6 | 75 | 8 | 92 | 25 | 78 | 97 | [211] | |||||||
| A/A | Corncobs | H2SO4/NaOH | 1 | 1:10 | 120/60 | 60/120 | 62 | 75 | 8 | 3.2 | 4 | 89 | 88 | 93 | [212] | ||||||||
| A/A | Corn stover | H2SO4/NaOH | 0.5/2 | 1:10 | 180/80 | 1/60 | 62 | 83 | 4 | 5.4 | 14 | 71 | 89 | 0.9 | ND | 2.8 | 98 | 76 | [213] | ||||
| SE | Sugarcane bagasse | H2O | 1:10 | 121 | 120 | 61 | [48] | ||||||||||||||||
| SE | rice husk | H2O | 1:10 | 121 | 120 | 63 | [48] | ||||||||||||||||
| SE | Eucalyptus globulus | H2O | - | 8:1 | 200 | 30 | 69 | 37 | ND | 62 | 1.3 | 2.2 | 0.6 | 2.4 | 73 | [189] | |||||||
| SE | Pinus radiata | H2O | - | 10:1 | 200 | 90 | 67 | 50 | 0.7 | 47 | 0.9 | 2.0 | 0.4 | 0.8 | 10 | [189] | |||||||
| OS | Palm fronds | Ethanol/H2SO4 | 60/1.5 | 1:10 | 200 | 60 | 85 | 90 | 30 | 57 | 5 | 70 | 43 | 0.7 | 4 | 95 | 78 | [214] | |||||
| OS | Prairie cordgrass | Ethyl acetat-ethanol-H2O-H2SO4 | 37-25-38-0.4 | 1:10 | 140 | 20 | 51 | 4.0 | [214] | ||||||||||||||
| OS | Switchgrass | 1:10 | 140 | 20 | 59 | 6.0 | [214] | ||||||||||||||||
| OS | Corn stover | 1:10 | 140 | 20 | 68 | 2.5 | [214] | ||||||||||||||||
| OS | Yellow poplar | Ethanol/H2SO4 | 50/1 | 1:10 | 140 | 10 | 61 | 46 | 13 | 7.7 | 39 | 0.04 | 0.06 | 2.4 | [215] | ||||||||
| OS | Yellow poplar | Ethanol/NaOH | 50/1 | 1:10 | 140 | 10 | 73 | 59 | 16 | 0.3 | 27 | <0.01 | 0 | 4.6 | [215] | ||||||||
| Pretreatment | Advantage | Disadvantages | Action Mechanism | Work Condition | References |
|---|---|---|---|---|---|
| Ionic liquids (IL) | Applied with alkaline pretreatment Environmentally friendly Decreases crystallinity and increases porosity Up to 90% assimilable sugars are obtained after enzymatic hydrolysis | Expensive liquids Residual IL interfere with enzymatic hydrolysis | Cleavage of the β-O-4 bonds in lignin followed by dipole-ion formation. Cellulose is destabilized, and hemicelluloses are dissolved. IL are composed by organic cations and small inorganic or organic anions, linked by a strong ionic bond. Commonly IL used are imidazonium salts, AMIMCl (1-Allyl-3-methylimidazonium chloride) and BMIMCl (1-butyl-3-methylimidazolium chloride) | 80–160 °C 3–50% solid 30 min-8 h 60–80% w/w | [13,165,185,189,221,223] |
| AFEX | Partial disruption of the fibers leaving short cellulose chains and disrupt lignin Solid 99% recovered Low inhibitor concentration Removes acetyl groups by deacetylation Herbaceous biomass and agricultural residues are high susceptible Lignin removed > 85% Up to 95% assimilable sugars are obtained after enzymatic hydrolysis | Hardwood low susceptible Not suitable for softwoods The dosage of liquid ammonia is 1–2 kg of ammonia/kg of dry biomass, which increases the costs Residual lignin generates unspecific bonds in the enzymatic hydrolysis | Alkaline reagent is impregnated into the biomass, pressure is applied, and it quickly depressurizes Anhydrous liquid or gaseous ammonia | 60–200 °C 10–50% solid 5–60 min >100% w/w | [13,71,165,185,188,217,224] |
| Ammonia recycled percolation (ARP) and Soaking in aqueous ammonia (SAA) | Significant degree of delignification in hardwood and herbaceous woods Solids 99% recovered Used to preserve most of the glucan and xylan | High cost due to the solvent | Solubilization of hemicelluloses and lignin | 140–210 °C 10–90 min 5–15% w/w | [184,188,217] |
| CO2 explosion | Efficient to removing lignin in hard and softwoods and to dissolving cellulose and hemicelluloses No inhibitory compounds have been reported Low-cost pretreatment Acceptable environmental impact More cost-effective treatment than AFEX and less toxic than steam explosion | Lower yield than steam and AFEX explosion | Supercritical fluid, reacting with the moisture in the substrate to form carbonic acid that contributes to the degradation of the biomass | 31–250 °C 20–60 min 5–15% w/w | [71,185,186,217] |
| Hot water | Reduces the size of particles Effective for solubilizing hemicelluloses as oligomers not require catalysts, chemical products or corrosion-resistant materials Lignin removed > 73% Up to 95% assimilable sugars are obtained after enzymatic hydrolysis Inhibitor compounds in low concentrations compared to acid treatments. | Inhibitors can be produced requires a high demand for water and energy | Consists in cooking the lignocellulosic biomass in water at high temperature and pressure | 121–240 °C 10–20% solid 4–60 min | [12,71,185,225,226] |
| Oxidative (Wet oxidation | Used with alkaline solution (NaOH) reducing inhibiting products Combination with stem exploitation the conversion of cellulose and hemicelluloses is increased Removes hemicelluloses and lignin (between 50–70%). Xylan from hardwoods and herbaceous are affected Lignin removed > 60% Up to 95% assimilable sugars are obtained after enzymatic hydrolysis | Mannans from softwoods are low affected Cellulose is not affected Possibility of non-selective oxidation causing loss of hemicelluloses and cellulose components Large amount of acids and chemical compounds are generated High temperatures and pressure and oxidizing agents are costly | When the biomass is suspended in water, the oxide agents produce electrophilic substitution chain reactions that divide the aromatic lignin nuclei or the bonds between the alkyl and aryl groups Mainly degrades lignin by attacking aromatic ring. Oxide agents such as oxygen (O2), hydrogen peroxide (H2O2) or peracetic acid, ozone (O3) | 25–195 °C 10–20 min 1–2% w/v | [71,185,210] |
| Microwave pretreatment | Applied to acids and alkaline and steam exploitation Low cost Short reaction times Change ultra-structure of cellulose by degrading lignin and hemicelluloses Homogeneous heating of the reaction mixture Improve the recovery yields of glucose, xylose, and total sugar by 13–27%, 17–25%, and 20–21%, respectively | Increase the formation of inhibitors | Consists in radiating energy that accelerates the molecules, which begin to friction each other and quickly increasing the medium temperature generating physical, chemical, or biological reactions | 150–180 °C 3 min 1–2% w/v | [143,189,227] |
| Furan Compounds | Phenolic Compounds | Weak Acids | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Furfural | 5-HMF | Vanillin | Syringaldehyde | 4-HB | Formic | Acetic | Levulinic | ||||||||||||||||||
| g/L | % Inhibition (w/w) | g/L | % Inhibition (w/w) | g/L | % Inhibition (w/w) | g/L | % Inhibition (w/w) | g/L | %Inhibition (w/w) | g/L | % Inhibition (w/w) | g/L | % Inhibition (w/w) | g/L | % Inhibition (w/w) | ||||||||||
| Microorganism | S | X | L | S | X | L | S | X | L | S | X | L | S | X | L | S | X | L | S | X | L | S | X | L | Ref |
| Cryptococcus curvatus | 1 | - | 62 | 3 | - | 7.9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [201] |
| Trichosporon dematis | 4 | - | 59 | 2 | - | 22 | 1.2 | 71 | 80 | 1 | 88 | 98 | 1.5 | 100 | 100 | 4 | 100 | - | >9 | 30 | 57 | 10 | 27 | 54 | [73] |
| Trichosporon cutaneum | 1 | 40 | 30 | 2 | - | - | 2 | 26 | 21 | - | - | - | 1.5 | 42 | 38 | 5 | 38 | 7 | 5 | 25 | 59 | 10 | - | - | [235] |
| Rhodosporidium toruloides | - | - | - | - | - | - | 2 | 100 | - | 2.2 | 16 | - | 1.2 | 100 | - | 4 | 40 | - | - | - | - | - | - | - | [236] |
| Rhodosporidium toruloides | >1 | 50 | - | - | - | - | >2 | 50 | - | - | - | - | - | - | - | 4 | 50 | - | 15 | 50 | - | - | - | - | [229] |
| Lipomyces starkeyi | 1.4 | 100 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3.9 | 100 | - | - | - | - | [237] |
| Trichosporon cutaneum | 0.5 | 65 | 91 | 5 | 35 | 25 | 2 | 40 | 40 | 2.5 | 20 | 20 | 2 | 50 | 50 | 6 | 18 | 26 | 25 | 50 | 34 | 10 | 50 | 50 | [146] |
| Trichosporon mycotoxinivorans | 1 | 47 | - | 2.5 | 15 | - | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 0 | - | - | - | - | [50] |
| Rhodosporidium fluviale | 0.3 | 75 | 87 | 2 | 75 | 0 | 0.5 | 92 | 0 | - | - | - | - | - | - | 0.5 | 100 | 100 | 1 | 72 | 97 | - | - | - | [104] |
| Microorganism | Strain | Biomass | Pretreatment | System | ULH/DLH | S(g/L) | X (g/L) | L% (w/w) | L (g/L) | L (g/g) | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yeasts | ||||||||||||
| Trichosporon dematis | CH007 | Corncob | DA | batch | flask | DLH | 42 | 17 | 40 | 7 | 0.16 | [258] |
| Cryptococcus curvatus | ATCC 20509 | Wheat straw | DA | batch | ULH | 29 | 17 | 34 | 5.8 | [201] | ||
| Cryptococcus curvatus | ATCC 20509 | Wheat straw | DA | batch | DLH | 21 | 16 | 27 | 4.2 | [201] | ||
| Lipomyces starkeyi | ATCC 12659 | Wheat straw | DA | batch | ULH | 29 | 15 | 31 | 4.6 | [201] | ||
| Lipomyces starkeyi | ATCC 12659 | Wheat straw | DA | batch | DLH | 21 | 13 | 29 | 3.7 | [201] | ||
| Rhodosporidium toruloides | ATCC 10788 | Wheat straw | DA | batch | DLH | 21 | 9.9 | 25 | 2.4 | [201] | ||
| Rhodotorula glutinis | ATCC 204091 | Wheat straw | DA | batch | ULH | 29 | 14 | 25 | 3.5 | [201] | ||
| Rhodotorula glutinis | ATCC 204091 | Wheat straw | DA | batch | DLH | 21 | 12 | 21 | 2.4 | [201] | ||
| Trichosporon dematis | 32903 | Corncob | DA | batch | ULH | 71 | 24 | 7.5 | 0.1 | [73] | ||
| Trichosporon dematis | 32903 | Corncob | DA | batch | DLH | 73 | 45 | 11 | 0.16 | [73] | ||
| Cryptococcus sp. | SM5S05 | Corncob | DA | batch | flask | ULH | 40 | 13 | 60 | 7.6 | 0.13 | [259] |
| Trichosporon cutaneum | Ch002 | Corncob | DA | batch | flask | DLH | 46 | 22 | 36 | 7.9 | [260] | |
| Trichosporon cutaneum | ACCC 20271 | Corncob | DA | batch | flask | ULH | 49 | 38 | 32 | 12 | 0.1 | [261] |
| Cryptococcus curvatus | ATCC 20509 | Corn stover | DA | batch | flask | ULH | 53 | 11 | 61 | 6.9 | [262] | |
| Trichosporon cutaneum | AS 2.571 | Corn stover | DA | batch | Bior 3L | DLH | 60 | 19 | 39 | 7.6 | 0.15 | [110] |
| Trichosporon dematis | CH007 | Corncob | DA | batch | flask | ULH | 60 | 24 | 40 | 9.8 | 0.16 | [263] |
| Trichosporon fermentans | CICC 1368 | Rice straw | DA | batch | flask | DLH | 35 | 29 | 40 | 12 | [228] | |
| Lipomyces tetrasporus | NRRL Y-11562 | Cornstover | DA | batch | flask | ULH | 123 | 54 | 53 | 29 | 0.15 | [40] |
| Lipomyces kononenkoae | NRRL Y-7042 | Cornstover | DA | batch | flask | ULH | 123 | 48 | 59 | 28 | 0.22 | [40] |
| Rhodosporidium toruloides | NRRL Y-1091 | Cornstover | DA | batch | flask | ULH | 123 | 43 | 61 | 26 | 0.19 | [40] |
| Yarrowia lipolytica | Po1g | Sugarcane bagasse | DA | batch | flask | DLH | 21 | 11 | 59 | 6.7 | [118] | |
| Yarrowia lipolytica | Po1g | Rice bran | DA | batch | flask | DLH | 30 | 11 | 48 | 5.2 | [56] | |
| Trichosporon cutaneum | ACCC 20271 | Corn stover | DA | batch | Bior 20L | DLH | 73–130 | 46 | 8.1 | [146] | ||
| Rhodosporidiobolus fluvialis | DMKU-SP314 | Sugar cane | DA/OX | batch | flask | ULH | 36 | 21 | 67 | 14 | [113] | |
| Rhodosporidiobolus fluvialis | DMKU-SP314 | Sugar cane | DA/OX | batch | Bior 2L | ULH | 36 | 24 | 75 | 18 | [113] | |
| Trichosporon mycotoxinivorans | S 2 | Paddy straw | Alk | ULH | 35 | 14 | 35 | 7.3 | [50] | |||
| Rhodosporidium paludigenum | KM281510 | Corncob | Alk | batch | flask | ULH | 100 | 23 | 70 | 16 | [47] | |
| Rhodosporidium paludigenum | KM281510 | Corncob | Alk | batch | Bior 3L | ULH | 100 | 28 | 70 | 20 | 0.21 | [47] |
| Rhodosporidium paludigenum | KM281510 | Corncob | Alk | fed batch | Bior 3L | ULH | 100 | 36 | 70 | 25 | 0.28 | [47] |
| Trichosporon dematis | 32903 | Corncob | Alk | batch | ULH | 67 | 28 | 6.8 | 0.1 | [73] | ||
| Trichosporon dematis | 32903 | Corncob | Alk | batch | DLH | 131 | 56 | 20 | 0.19 | [73] | ||
| Rhodosporidium toruloides | DSMZ 4444 | Corn stover | A/A | batch | ULH | 110 | 36 | 59 | 0.19 | [264] | ||
| Rhodosporidium toruloides | DSMZ 4445 | Corn stover | A/A | Fed batch | ULH | 110 | 54 | 59 | 0.29 | [264] | ||
| Rhodotorula glutinis | CGMCC 2.703 | Corncob | A/A | batch | Bior 5L | ULH | 42 | 15 | 36 | 5.5 | 0.13 | [265] |
| Rhodotorula glutinis | CGMCC 2.703 | Corncob | A/A | fed batch | Bior 5L | ULH | 42 | 75 | 47 | 34 | 0.15 | [265] |
| Meyerozyma guilliermondii | G5-MK414782 | Sugarcane bagasse | SE | batch | ULH | 60 | 6.1 | 38 | 2.3 | 0.05 | [48] | |
| Meyerozyma guilliermondii | G5-MK414782 | Rice husk | SE | batch | ULH | 60 | 6.5 | 37 | 2.4 | 0.04 | [48] | |
| Pichia kudriavzevii | G9-MH000699 | Sugarcane bagasse | SE | batch | ULH | 20 | 6.2 | 31 | 1.9 | 0.1 | [48] | |
| Pichia kudriavzevii | G9-MH000699 | Rice husk | SE | batch | ULH | 20 | 8.2 | 24 | 1.9 | 0.1 | [48] | |
| Pichia manshurica | G10-MH279643 | Sugarcane bagasse | SE | batch | ULH | 20 | 8.2 | 24 | 1.9 | 0.09 | [48] | |
| Pichia manshurica | G10-MH279643 | Rice husk | SE | batch | ULH | 21 | 6.5 | 28 | 1.8 | 0.09 | [48] | |
| Pichia kudriavzevii | SY2-MF926445 | Sugarcane bagasse | SE | batch | ULH | 60 | 6.1 | 30 | 1.9 | 0.04 | [48] | |
| Pichia kudriavzevii | SY2-MF926445 | Rice husk | SE | batch | ULH | 51 | 8.4 | 29 | 2.4 | 0.04 | [48] | |
| Candida albicans | SY3-MG996750 | Sugarcane bagasse | SE | batch | ULH | 20 | 6.2 | 31 | 1.9 | 0.1 | [48] | |
| Candida albicans | SY3-MG996750 | Rice husk | SE | batch | ULH | 21 | 8.3 | 22 | 1.8 | 0.09 | [48] | |
| Rhodotorula mucilaginosa | SY4-MH279637 | Sugarcane bagasse | SE | batch | ULH | 20 | 6.7 | 30 | 2 | 0.1 | [48] | |
| Rhodotorula mucilaginosa | SY4-MH279637 | Rice husk | SE | batch | ULH | 20 | 8 | 24 | 2 | 0.1 | [48] | |
| Filamentous fungi | ||||||||||||
| Umbelopsis (Mortierella) isabellina | ATHUM 2935 | Rice hull | DA | batch | flask | ULH | 26 | 5.6 | 64 | 3.6 | 0.21 | [69] |
| Umbelopsis (Mortierella) isabellina | ATCC42613 | Corn stover | DA | batch | flask | ULH | 31 | 14 | 34 | 4.8 | [38] | |
| Umbelopsis (Mortierella) isabellina | ATCC42613 | Corn stover | Alk | batch | flask | ULH | 30 | 11 | 29 | 2.5 | [38] | |
| Microalgae | ||||||||||||
| Auxenochlorella (Chlorella) protothecoides | Birch | OS/SE | batch | flask | ULH | 77 | 8.6 | 66 | 5.7 | [257] | ||
| Auxenochlorella (Chlorella) protothecoides | Spruce | OS/SE | batch | flask | ULH | 65 | 8.6 | 63 | 5.3 | [257] | ||
| Bacteria | ||||||||||||
| Rhodococcus opacus | DSM 1069 | Loblolly pine | OS | batch | flask | 27 | [266] | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés, G.; Mendonça, R.T.; Aggelis, G. Lignocellulosic Biomass as a Substrate for Oleaginous Microorganisms: A Review. Appl. Sci. 2020, 10, 7698. https://doi.org/10.3390/app10217698
Valdés G, Mendonça RT, Aggelis G. Lignocellulosic Biomass as a Substrate for Oleaginous Microorganisms: A Review. Applied Sciences. 2020; 10(21):7698. https://doi.org/10.3390/app10217698
Chicago/Turabian StyleValdés, Gabriela, Regis Teixeira Mendonça, and George Aggelis. 2020. "Lignocellulosic Biomass as a Substrate for Oleaginous Microorganisms: A Review" Applied Sciences 10, no. 21: 7698. https://doi.org/10.3390/app10217698
APA StyleValdés, G., Mendonça, R. T., & Aggelis, G. (2020). Lignocellulosic Biomass as a Substrate for Oleaginous Microorganisms: A Review. Applied Sciences, 10(21), 7698. https://doi.org/10.3390/app10217698







