Hydrogen Recovery from Waste Gas Streams to Feed (High-Temperature PEM) Fuel Cells: Environmental Performance under a Life-Cycle Thinking Approach
Abstract
:1. Introduction
2. Methodology
2.1. Goal and Scope
2.1.1. Function and Functional Unit
- Function 1: Generation of one unit of mass of the hydrogen at the requested purity of 99.97%, imposed on feeding a PEM fuel cell for stationary applications (Type I, Grade D) [35]. Based on this function, 1 kg of hydrogen with a molar purity of 99.97% was selected as a functional unit. This FU enabled direct comparison versus conventional hydrogen production processes to evaluate the possible environmental benefits associated with the recovery of the compound from waste gases.
- Function 2: Generation of one unit of electrical energy in the PEM fuel cell. To evaluate this function, the FU was 1 kWh of energy obtained from the fuel cell, which provided further information about the environmental burdens associated with the complete process and its viability as an energy source.
- Function 3: Treatment of one mass unit of the residual gas stream. As a result, the FU chosen was 1 kg of COG or APG introduced to the system, which allowed the analysis of the influence of the type of residual gas stream selected on the entire process. This function and FU were considered to benchmark against no treatment for the leaving gas stream. Therefore, it can be guaranteed that the recovery and further electricity generation is better from a life-cycle perspective than the direct release to the atmosphere.
2.1.2. System Description and Boundaries
2.1.3. Assumptions, Hypotheses, and Limitations
2.1.4. Life-Cycle Impact Assessment Method
2.2. Life-Cycle Inventory
3. Results and Discussion
3.1. Life-Cycle Impact Assessment Based on Hydrogen Production
| Production Process | GWP (kg CO2 eq./kg H2) | Reference |
|---|---|---|
| Natural gas thermolysis | 37.11 | [62] |
| Steam methane reforming | 11.89 | [53] |
| Coal gasification | 11.29 | [53] |
| Electrolysis (wind energy) | 0.97 | [53] |
| Electrolysis (photovoltaic energy) | 2.41 | [5] |
| Steam methane reforming | 10.56 | [54] |
| Methane partial oxidation | 10.70 | [60] |
| Electrolysis (grid electricity) | 30.50 | [63] |
| Supercritical water reforming of glycerol | 3.77 | [64] |
| Electrolysis (wind energy) | 0.68 | [57] |
| Autothermal bioethanol reforming | 4.36 | [61] |
| Methane cracking (liquid-metal technology) | 1.90 | [59] |
| Steam methane reforming | 11.50 | [55] |
| Autothermal natural gas reforming | 10.80 | [55] |
| Syngas chemical looping | 12.30 | [55] |
| Chemical looping reforming | 9.10 | [55] |
| Chemical looping combustion thermally coupled steam reforming | 3.01 | [25] |
| Supercritical water gasification (photovoltaic energy) | 2.41 | [58] |
| Electrolysis (USA mix electricity) | 28.60 | [56] |
| Coal gasification | 23.70 | [56] |
| Biomass gasification | 4.40 | [56] |
| Ethanol reforming | 12.20 | [56] |
| Methanol reforming | 17.90 | [56] |
| Methane reforming | 13.80 | [56] |
| Biomass gasification with methane tri-reforming | 1.35 | [65] |
| Production Process | AP (kg SO2 eq./kg H2) | Reference |
|---|---|---|
| Steam methane reforming | 0.008 | [54] |
| Electrolysis (photovoltaic electricity) | 0.028 | [63] |
| Electrolysis (wind electricity) | 0.003 | [63] |
| Biomass gasification | 0.034 | [63] |
| Methane partial oxidation | 0.008 | [60] |
| Supercritical water reforming of glycerol | 0.012 | [62] |
| Autothermal bioethanol reforming | 0.028 | [61] |
| Supercritical water gasification (photovoltaic energy) | 0.190 | [58] |
| Electrolysis (USA mix electricity) | 0.069 | [56] |
| Coal gasification | 0.011 | [56] |
| Biomass gasification | 0.016 | [56] |
| Ethanol reforming | 0.032 | [56] |
| Methanol reforming | 0.017 | [56] |
| Methane reforming | 0.012 | [56] |
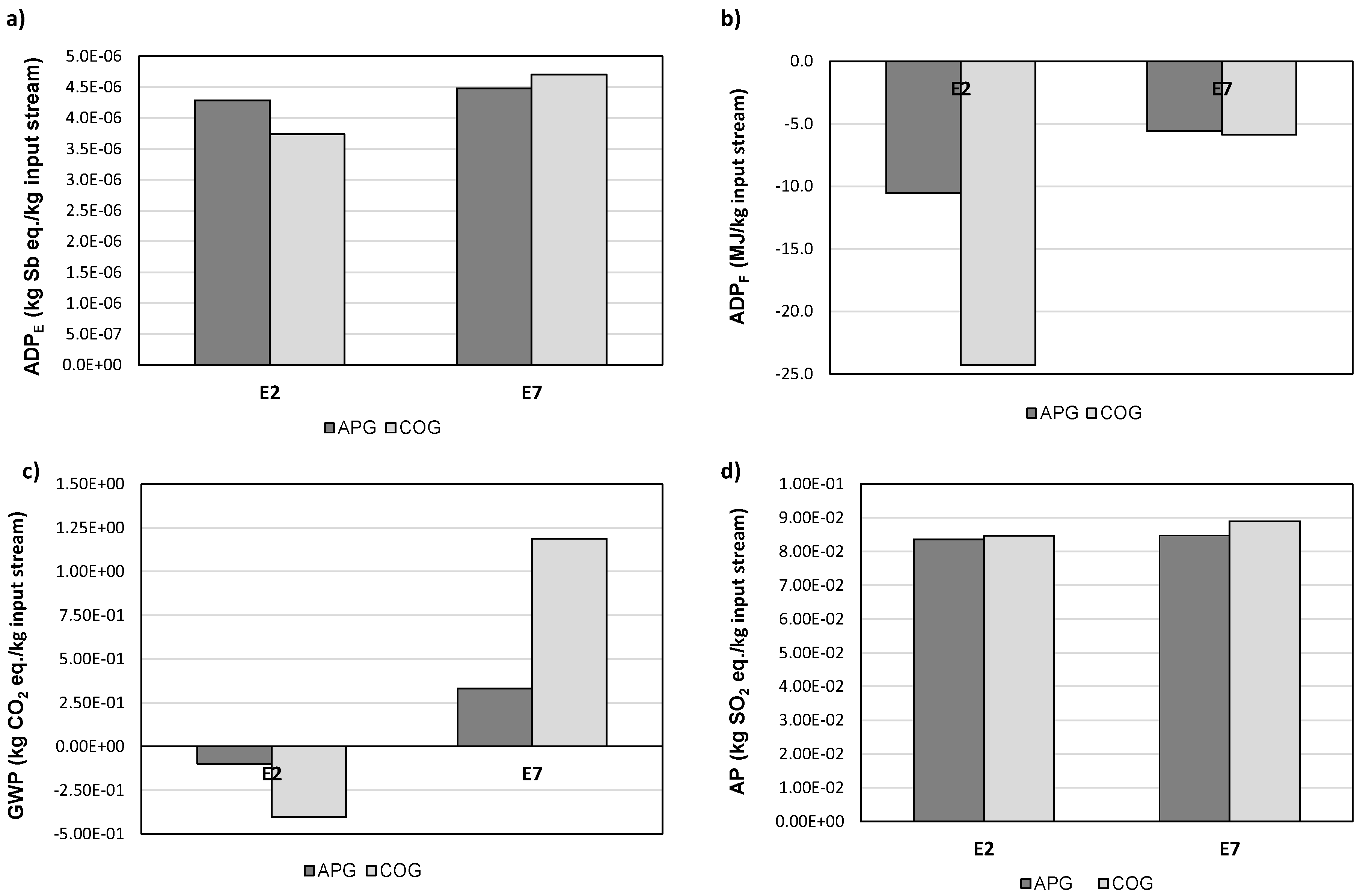
3.2. Life-Cycle Impact Assessment Based on Energy Production
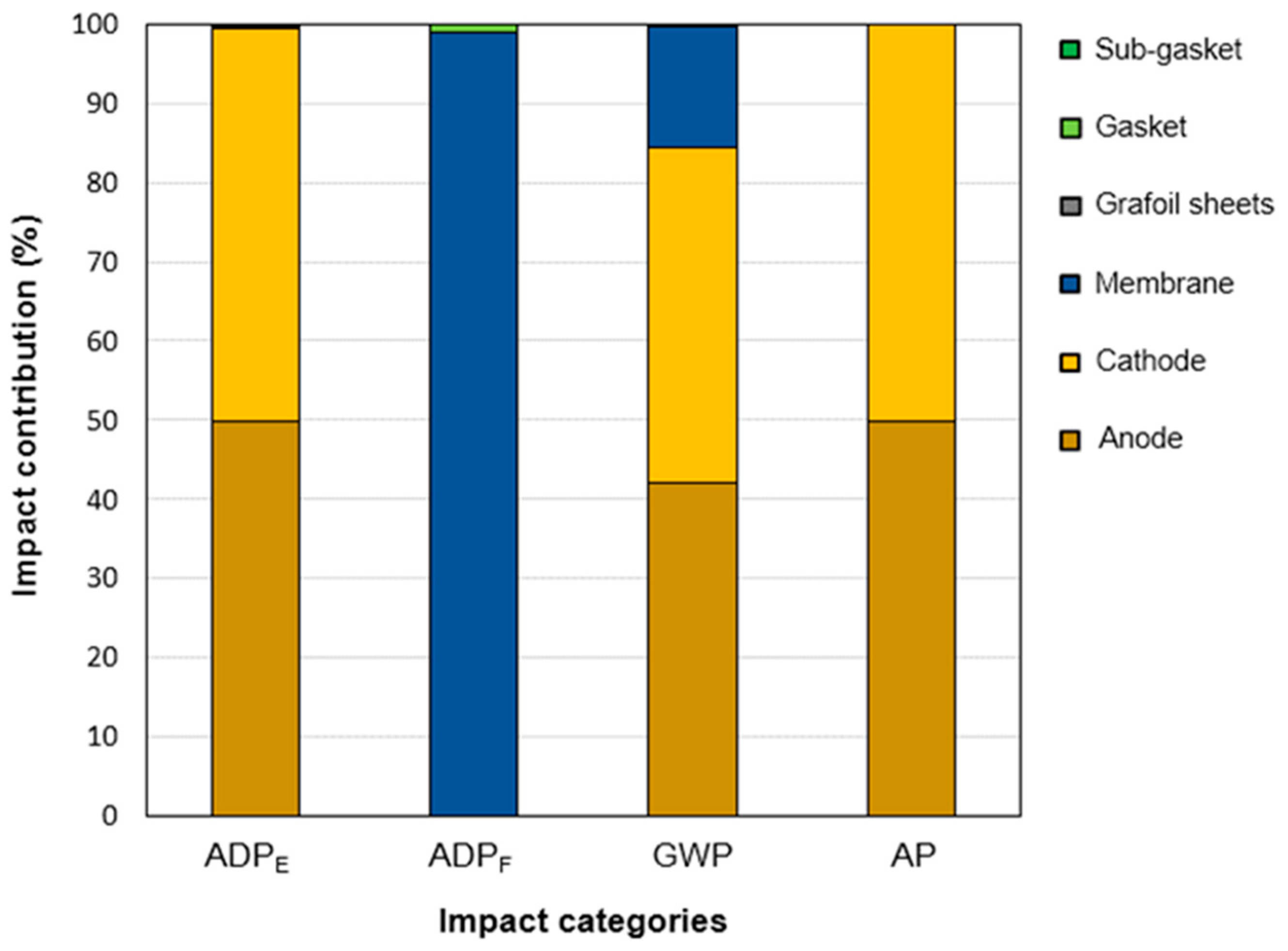
3.3. Life-Cycle Impact Assessment Based on Waste Gas Stream Management
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zalakeviciute, R.; Bastidas, M.; Buenaño, A.; Rybarczyk, Y. A Traffic-based method to predict and map urban air quality. Appl. Sci. 2020, 10, 2035. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zeng, X.; Huang, G.; Yu, L.; Li, Y. Robust planning of energy and environment systems through introducing traffic sector with cost minimization and emissions abatement under multiple uncertainties. Appl. Sci. 2019, 9, 928. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, K.A.; Foster, M.; Nieuwenhuijsen, M.J.; May, A.D.; Ramani, T.; Zietsman, J.; Khreis, H. Urban policy interventions to reduce traffic emissions and traffic-related air pollution: Protocol for a systematic evidence map. Environ. Int. 2020, 142. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Najafi, B.; Casalegno, A.; Rinaldi, F. Optimization of an HT-PEM fuel cell based residential micro combined heat and power system: A multi-objective approach. J. Clean. Prod. 2018, 180, 126–138. [Google Scholar] [CrossRef]
- Nomnqa, M.; Ikhu-Omoregbe, D.; Rabiu, A. Performance evaluation of a HT-PEM fuel cell micro-cogeneration system for domestic application. Energy Syst. 2019, 10, 185–210. [Google Scholar] [CrossRef]
- Testa, E.; Giammusso, C.; Bruno, M.; Maggiore, P. Analysis of environmental benefits resulting from use of hydrogen technology in handling operations at airports. Clean Technol. Environ. Policy 2014, 16, 875–890. [Google Scholar] [CrossRef]
- Saufi Sulaiman, M.; Singh, B.; Mohamed, W.A.N.W. Experimental and theoretical study of thermoelectric generator waste heat recovery model for an ultra-low temperature PEM fuel cell powered vehicle. Energy 2019, 179, 628–646. [Google Scholar] [CrossRef]
- Díaz, M.; Ortiz, A.; Ortiz, I. Progress in the use of ionic liquids as electrolyte membranes in fuel cells. J. Memb. Sci. 2014, 469, 379–396. [Google Scholar] [CrossRef] [Green Version]
- Yáñez, M.; Ortiz, A.; Gorri, D.; Ortiz, I. Comparative performance of commercial polymeric membranes in the recovery of industrial hydrogen waste gas streams. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Dincer, I.; Rosen, M.A. Sustainability aspects of hydrogen and fuel cell systems. Energy Sustain. Dev. 2011, 15, 137–146. [Google Scholar] [CrossRef]
- Protasova, L.; Snijkers, F. Recent developments in oxygen carrier materials for hydrogen production via chemical looping processes. Fuel 2016, 181, 75–93. [Google Scholar] [CrossRef]
- Chaubey, R.; Sahu, S.; James, O.O.; Maity, S. A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources. Renew. Sustain. Energy Rev. 2013, 23, 443–462. [Google Scholar] [CrossRef]
- Levalley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Kaiwen, L.; Bin, Y.; Tao, Z. Economic analysis of hydrogen production from steam reforming process: A literature review. Energy Sources Part B Econ. Plan. Policy 2018, 13, 109–115. [Google Scholar] [CrossRef]
- Ju, H.K.; Badwal, S.; Giddey, S. A comprehensive review of carbon and hydrocarbon assisted water electrolysis for hydrogen production. Appl. Energy 2018, 231, 502–533. [Google Scholar] [CrossRef]
- Maarefian, M.; Bandehali, S.; Azami, S.; Sanaeepur, H.; Moghadassi, A. Hydrogen recovery from ammonia purge gas by a membrane separator: A simulation study. Int. J. Energy Res. 2019, 43, 8217–8229. [Google Scholar] [CrossRef]
- Cho, S.H.; Chue, K.T.; Kim, J.N. A two stage PSA for argon and hydrogen recovery from ammonia purge gas. Chem. Eng. Commun. 1998, 163, 97–109. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Asgari, A. Production of hydrogen from purge gases of ammonia plants in a catalytic hydrogen-permselective membrane reactor. Int. J. Hydrogen Energy 2009, 34, 5795–5802. [Google Scholar] [CrossRef]
- Yáñez, M.; Relvas, F.; Ortiz, A.; Gorri, D.; Mendes, A.; Ortiz, I. PSA purification of waste hydrogen from ammonia plants to fuel cell grade. Sep. Purif. Technol. 2020, 240, 116334. [Google Scholar] [CrossRef]
- Razzaq, R.; Li, C.; Zhang, S. Coke oven gas: Availability, properties, purification, and utilization in China. Fuel 2013, 113, 287–299. [Google Scholar] [CrossRef]
- Xiang, D.; Zhou, Y. Concept design and techno-economic performance of hydrogen and ammonia co-generation by coke-oven gas-pressure swing adsorption integrated with chemical looping hydrogen process. Appl. Energy 2018, 229, 1024–1034. [Google Scholar] [CrossRef]
- Xie, H.; Yu, Q.; Zuo, Z.; Zhang, J.; Han, Z.; Qin, Q. Thermodynamic analysis of hydrogen production from raw coke oven gas via steam reforming. J. Therm. Anal. Calorim. 2016, 126, 1621–1631. [Google Scholar] [CrossRef]
- Xu, J.; Lin, W.; Xu, S. Hydrogen and LNG production from coke oven gas with multi-stage helium expansion refrigeration. Int. J. Hydrogen Energy 2018, 43, 12680–12687. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Zhang, G. Life cycle greenhouse gas assessment of hydrogen production via chemical looping combustion thermally coupled steam reforming. J. Clean. Prod. 2018, 179, 335–346. [Google Scholar] [CrossRef]
- Li, W.; He, S.; Li, S. Experimental study and thermodynamic analysis of hydrogen production through a two-step chemical regenerative coal gasification. Appl. Sci. 2019, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Piasecka, I.; Tomporowski, A.; Flizikowski, J.; Kruszelnicka, W.; Kasner, R.; Mroziński, A. Life cycle analysis of ecological impacts of an offshore and a land-basedwind power plant. Appl. Sci. 2019, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Asdrubali, F.; Baldinelli, G.; D’Alessandro, F.; Scrucca, F. Life cycle assessment of electricity production from renewable energies: Review and results harmonization. Renew. Sustain. Energy Rev. 2015, 42, 1113–1122. [Google Scholar] [CrossRef]
- Ketzer, F.; Skarka, J.; Rösch, C. Critical Review of Microalgae LCA Studies for Bioenergy Production. Bioenergy Res. 2018, 11, 95–105. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. Life cycle assessment of hydrogen energy systems: A review of methodological choices. Int. J. Life Cycle Assess. 2017, 22, 346–363. [Google Scholar] [CrossRef]
- Evangelisti, S.; Tagliaferri, C.; Brett, D.J.L.; Lettieri, P. Life cycle assessment of a polymer electrolyte membrane fuel cell system for passenger vehicles. J. Clean. Prod. 2017, 142, 4339–4355. [Google Scholar] [CrossRef]
- ISO. ISO 14040:2006. Environmental Management—Life Cycle Assessment—Principles and Framework; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- ISO. ISO 14044:2006. Environmental Management—Life Cycle Assessment—Requirements and Guidelines; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Margallo, M.; Cobo, S.; Laso, J.; Fernández, A.; Muñoz, E.; Santos, E.; Aldaco, R.; Irabien, A. Environmental performance of alternatives to treat fly ash from a waste to energy plant. J. Clean. Prod. 2019, 231, 1016–1026. [Google Scholar] [CrossRef]
- ISO. ISO 14687-2:2012. Hydrogen Fuel—Product Specification—Part 2: Proton Exchange Membrane (PEM) Fuel Cell Applications for Road Vehicles; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Wagner, U.; Eckl, R.; Tzscheutschler, P. Energetic life cycle assessment of fuel cell powertrain systems and alternative fuels in Germany. Energy 2006, 31, 2726–2739. [Google Scholar] [CrossRef]
- Hawkins, T.R.; Singh, B.; Majeau-Bettez, G.; Strümman, A.H. Comparative Environmental Life Cycle Assessment of Conventional and Electric Vehicles. J. Ind. Ecol. 2013, 17, 53–64. [Google Scholar] [CrossRef]
- Simons, A.; Bauer, C. A life-cycle perspective on automotive fuel cells. Appl. Energy 2015, 157, 884–896. [Google Scholar] [CrossRef]
- Ahmadi, P.; Kjeang, E. Comparative life cycle assessment of hydrogen fuel cell passenger vehicles in different Canadian provinces. Int. J. Hydrogen Energy 2015, 40, 12905–12917. [Google Scholar] [CrossRef]
- Sørensen, B. Total life-cycle assessment of PEM fuel cell car. In Proceedings of the 15th World Hydrogen Energy Conference, Yokohama, Japan, 27 June–2 July 2004. [Google Scholar]
- Duclos, L.; Lupsea, M.; Mandil, G.; Svecova, L.; Thivel, P.X.; Laforest, V. Environmental assessment of proton exchange membrane fuel cell platinum catalyst recycling. J. Clean. Prod. 2017, 142, 2618–2628. [Google Scholar] [CrossRef]
- Hayashi, K.; Gaillard, G.; Nemecek, T. Life cycle assessment of agricultural production systems: Current issues and future perspectives. In Proceedings of the International Seminar on Technology Development for Good Agricultural Practice in Asia and Oceania, Tsukuba, Japan, 24–28 October 2005. [Google Scholar]
- Iribarren, D.; Moreira, M.T.; Feijoo, G. Implementing by-product management into the Life Cycle Assessment of the mussel sector. Resour. Conserv. Recycl. 2010, 54, 1219–1230. [Google Scholar] [CrossRef]
- Freon, P.; Durand, H.; Avadí, A.; Huaranca, S.; Orozco Moreyra, R. Life cycle assessment of three Peruvian fishmeal plants: Toward a cleaner production. J. Clean. Prod. 2017, 145, 50–63. [Google Scholar] [CrossRef]
- Vázquez-Rowe, I.; Villanueva-Rey, P.; Hospido, A.; Moreira, M.T.; Feijoo, G. Life cycle assessment of European pilchard (Sardina pilchardus) consumption. A case study for Galicia (NW Spain). Sci. Total Environ. 2014, 475, 48–60. [Google Scholar] [CrossRef]
- Ross, S.A.; Topp, C.F.E.; Ennos, R.A.; Chagunda, M.G.G. Relative emissions intensity of dairy production systems: Employing different functional units in life-cycle assessment. Animal 2017, 11, 1381–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Liu, Q.; Ji, N.; Kansha, Y.; Tsutsumi, A. Optimization of steam methane reforming coupled with pressure swing adsorption hydrogen production process by heat integration. Appl. Energy 2015, 154, 392–401. [Google Scholar] [CrossRef]
- Dominguez-Ramos, A.; Irabien, A. The carbon footprint of Power-to-Synthetic Natural Gas by Photovoltaic solar powered Electrochemical Reduction of CO2. Sustain. Prod. Consum. 2019, 17, 229–240. [Google Scholar] [CrossRef]
- Sphera Gabi 9.2 Software-System and Databases for Life Cycle Engineering; Sphera: Stuttgart, Echterdingen, Germany, 2019.
- Heijungs, R.; Guinée, J.B.; Huppes, G.; Lankreijer, R.M.; Udo de Haes, H.A.; Wegener Sleeswijk, A.; Ansems, A.M.M.; Eggels, P.G.; van Diun, R.; de Goede, H.P. Environmental Life Cycle Assessment of Products: Guide and Backgrounds; Centre of Environmental Science (CML), Leiden University: Leiden, The Netherlands, 1992; ISBN 9051910649. [Google Scholar]
- Ecoinvent. Ecoinvent v3 Database; Ecoinvent: Zurich, Switzerland, 2016. [Google Scholar]
- Latunussa, C.E.L.; Ardente, F.; Blengini, G.A.; Mancini, L. Life Cycle Assessment of an innovative recycling process for crystalline silicon photovoltaic panels. Sol. Energy Mater. Sol. Cells 2016, 156, 101–111. [Google Scholar] [CrossRef]
- Cetinkaya, E.; Dincer, I.; Naterer, G.F. Life cycle assessment of various hydrogen production methods. Int. J. Hydrogen Energy 2012, 37, 2071–2080. [Google Scholar] [CrossRef]
- Hajjaji, N.; Pons, M.N.; Renaudin, V.; Houas, A. Comparative life cycle assessment of eight alternatives for hydrogen production from renewable and fossil feedstock. J. Clean. Prod. 2013, 44, 177–189. [Google Scholar] [CrossRef]
- Khojasteh Salkuyeh, Y.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from natural gas using current and emerging technologies. Int. J. Hydrogen Energy 2017, 42, 18894–18909. [Google Scholar] [CrossRef]
- Siddiqui, O.; Dincer, I. A well to pump life cycle environmental impact assessment of some hydrogen production routes. Int. J. Hydrogen Energy 2019, 44, 5773–5786. [Google Scholar] [CrossRef]
- Ghandehariun, S.; Kumar, A. Life cycle assessment of wind-based hydrogen production in Western Canada. Int. J. Hydrogen Energy 2016, 41, 9696–9704. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Zuo, H.; Wu, X.; Jiaqiang, E.; Wang, T.; Zhang, F.; Lu, N. System development and environmental performance analysis of a solar-driven supercritical water gasification pilot plant for hydrogen production using life cycle assessment approach. Energy Convers. Manag. 2019, 184, 60–73. [Google Scholar] [CrossRef]
- Postels, S.; Abánades, A.; von der Assen, N.; Rathnam, R.K.; Stückrad, S.; Bardow, A. Life cycle assessment of hydrogen production by thermal cracking of methane based on liquid-metal technology. Int. J. Hydrogen Energy 2016, 41, 23204–23212. [Google Scholar] [CrossRef]
- Susmozas, A.; Iribarren, D.; Dufour, J. Life-cycle performance of indirect biomass gasification as a green alternative to steam methane reforming for hydrogen production. Int. J. Hydrogen Energy 2013, 38, 9961–9972. [Google Scholar] [CrossRef]
- Khila, Z.; Baccar, I.; Jemel, I.; Houas, A.; Hajjaji, N. Energetic, exergetic and environmental life cycle assessment analyses as tools for optimization of hydrogen production by autothermal reforming of bioethanol. Int. J. Hydrogen Energy 2016, 41, 17723–17739. [Google Scholar] [CrossRef]
- Hauck, M.; Steinmann, Z.J.N.; Schipper, A.M.; Gorrissen, F.; Venkatesh, A.; Huijbregts, M.A.J. Estimating the Greenhouse Gas Balance of Individual Gas-Fired and Oil-Fired Electricity Plants on a Global Scale. J. Ind. Ecol. 2017, 21, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Aksyutin, O.E.; Ishkov, A.G.; Romanov, K.V.; Grachev, V.A. The carbon footprint of natural gas and its role in the carbon footprint of energy production. Int. J. Geomate 2018, 15, 155–160. [Google Scholar] [CrossRef]
- Naterer, G.F.; Suppiah, S.; Stolberg, L.; Lewis, M.; Ferrandon, M.; Wang, Z.; Dincer, I.; Gabriel, K.; Rosen, M.A.; Secnik, E.; et al. Clean hydrogen production with the Cu-Cl cycle-Progress of international consortium, II: Simulations, thermochemical data and materials. Int. J. Hydrogen Energy 2011, 36, 15486–15501. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis—A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Galera, S.; Gutiérrez Ortiz, F.J. Life cycle assessment of hydrogen and power production by supercritical water reforming of glycerol. Energy Convers. Manag. 2015, 96, 637–645. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Zhao, J.; Qi, H.; Ma, Z.; Cui, P.; Zhu, Z.; Gao, J.; Wang, Y. Life cycle assessment and techno-economic analysis of biomass-to-hydrogen production with methane tri-reforming. Energy 2020, 199, 117488. [Google Scholar] [CrossRef]
- Rooijen, V. A Life Cycle Assessment of the PureCell Stationary Fuel Cell System: Providing a Guide for Environmental Improvement; No. CSS06-09; Center for Sustainable Systems: Ann Arbor, MI, USA, 2006. [Google Scholar]
- Stropnik, R.; Lotric, A.; Montenegro, A.; Sekavcnik, M.; Mori, M. Critical materials in PEMFC systems and a LCA analysis for the potential reduction of environmental impacts with EoL strategies. Energy Sci Eng. 2019, 7, 2519–2539. [Google Scholar] [CrossRef]
- Gilbert, P.; Walsh, C.; Traut, M.; Kesieme, U.; Pazouki, K.; Murphy, A. Assessment of full life-cycle air emmisions of alternative shipping fuels. J. Clean. Prod. 2018, 172, 855–866. [Google Scholar] [CrossRef]

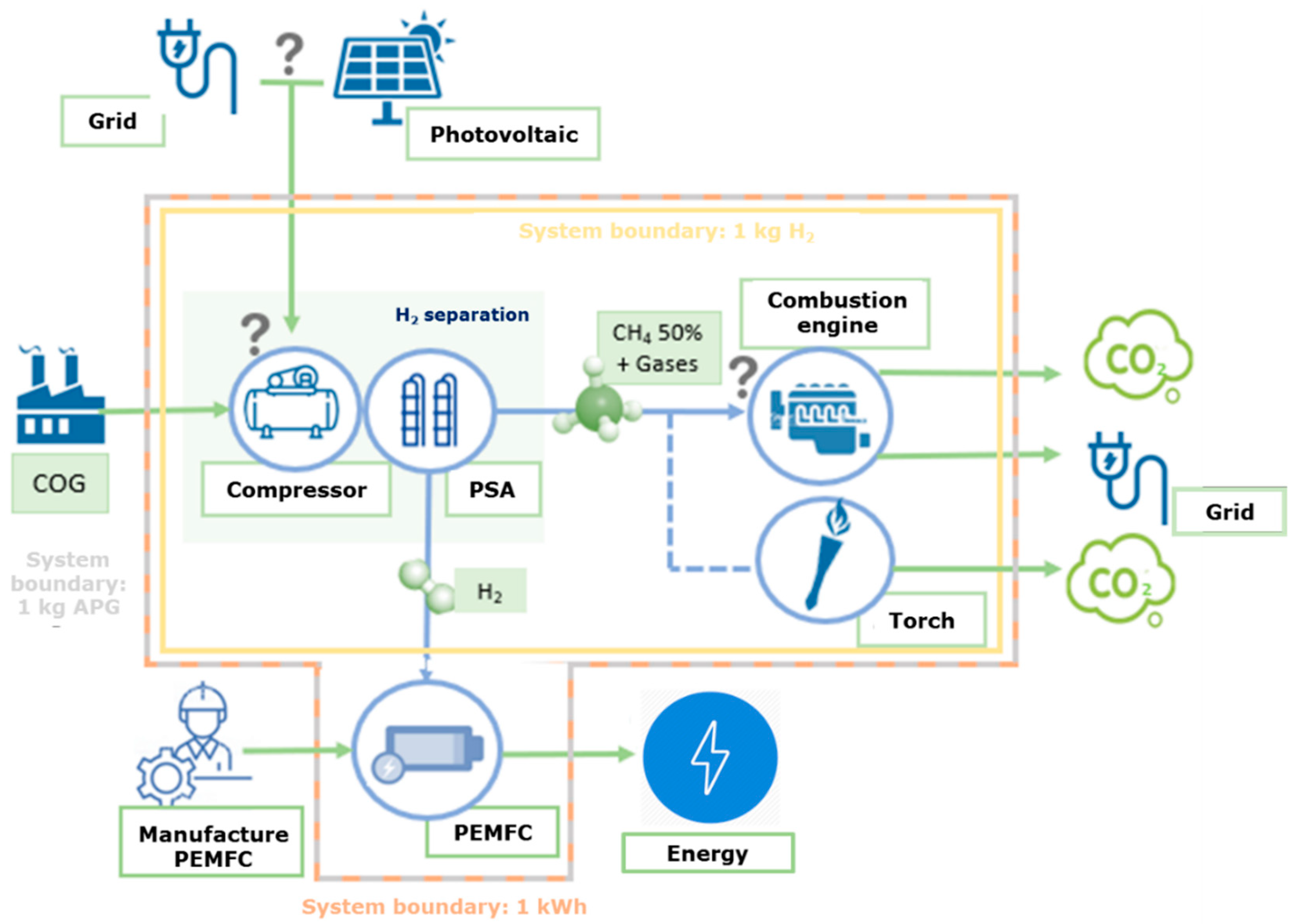
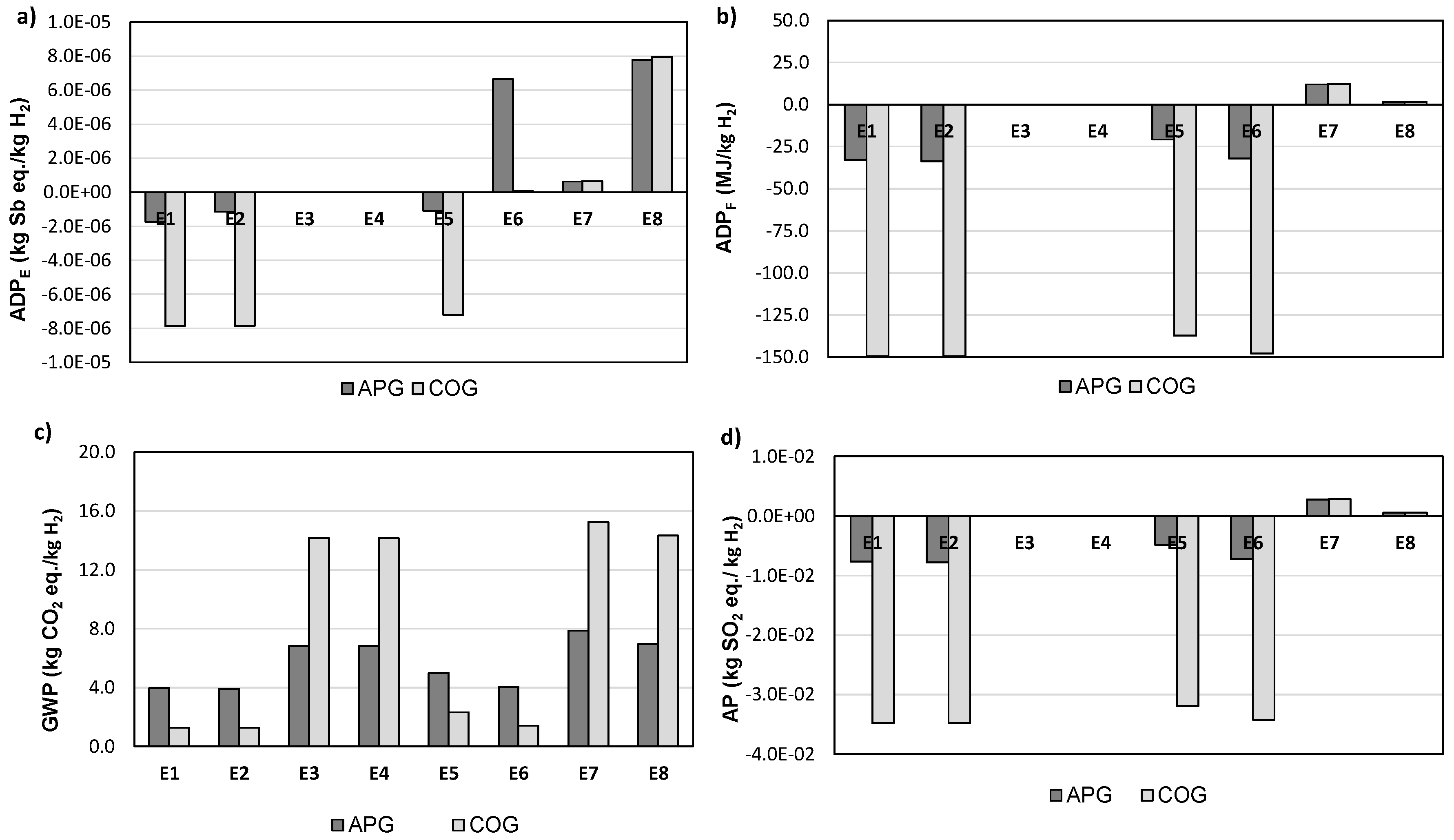
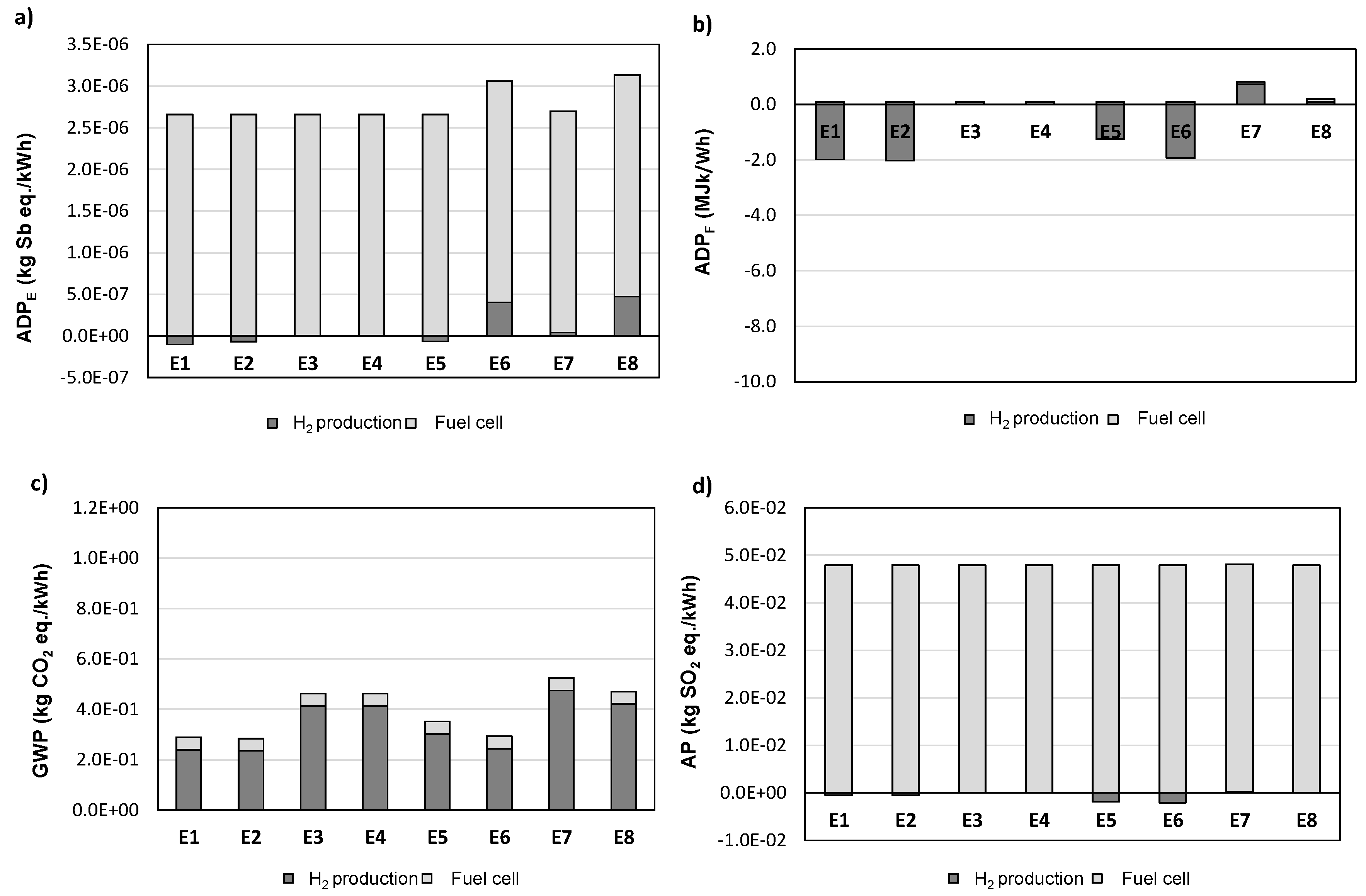
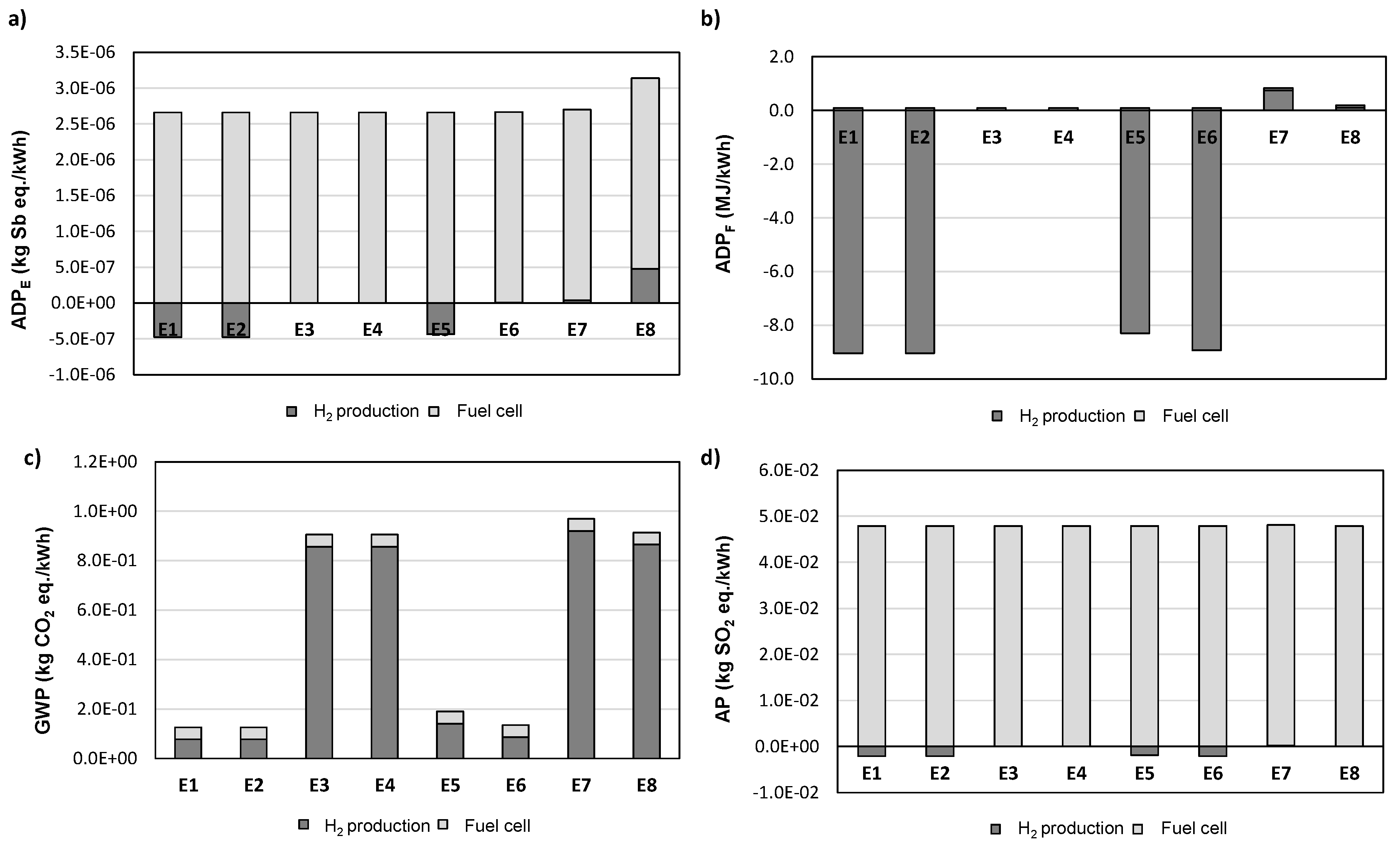
| Scenarios | E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 |
|---|---|---|---|---|---|---|---|---|
| Compression (Yes/No) | Y | Y | Y | Y | N | N | N | N |
| Electricity Source (Grid/Photovoltaic) | G | P | G | P | G | P | G | P |
| Methane recovery (Yes/No) | Y | Y | N | N | Y | Y | N | N |
| Gas Stream | Flow Rate (kg/h) | Molar Composition | ||||
|---|---|---|---|---|---|---|
| H2 | N2 | CH4 | CO2 | CO | ||
| COG | 1566 | 0.602 | 0.047 | 0.262 | 0.021 | 0.068 |
| APG | 137 | 0.610 | 0.220 | 0.170 | 0.000 | 0.000 |
| Gas Stream | Scenarios | Inputs | Outputs | ||||
|---|---|---|---|---|---|---|---|
| Feed Flow (kg/h) | N2 Separation Energy (kWh) | CH4 Separation Energy (kWh) | Fuel Cell (Units) | Fuel Cell Energy (kWh) | Engine Energy (kWh) | ||
| COG | E1/E2 | 1566 | 0 | 0 | 1 | 2927 | 6789 |
| APG | 137 | 0 | 2.7 | 1 | 245 | 128 | |
| COG | E3/E4 | 1566 | 0 | 0 | 1 | 2927 | 0 |
| APG | 137 | 0 | 0 | 1 | 245 | 0 | |
| COG | E5/E6 | 1566 | 555 | 0 | 1 | 2927 | 6789 |
| APG | 137 | 44 | 2.7 | 1 | 245 | 128 | |
| COG | E7/E8 | 1566 | 555 | 0 | 1 | 2927 | 0 |
| APG | 137 | 44 | 0 | 1 | 245 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abejón, R.; Fernández-Ríos, A.; Domínguez-Ramos, A.; Laso, J.; Ruiz-Salmón, I.; Yáñez, M.; Ortiz, A.; Gorri, D.; Donzel, N.; Jones, D.; et al. Hydrogen Recovery from Waste Gas Streams to Feed (High-Temperature PEM) Fuel Cells: Environmental Performance under a Life-Cycle Thinking Approach. Appl. Sci. 2020, 10, 7461. https://doi.org/10.3390/app10217461
Abejón R, Fernández-Ríos A, Domínguez-Ramos A, Laso J, Ruiz-Salmón I, Yáñez M, Ortiz A, Gorri D, Donzel N, Jones D, et al. Hydrogen Recovery from Waste Gas Streams to Feed (High-Temperature PEM) Fuel Cells: Environmental Performance under a Life-Cycle Thinking Approach. Applied Sciences. 2020; 10(21):7461. https://doi.org/10.3390/app10217461
Chicago/Turabian StyleAbejón, Ricardo, Ana Fernández-Ríos, Antonio Domínguez-Ramos, Jara Laso, Israel Ruiz-Salmón, María Yáñez, Alfredo Ortiz, Daniel Gorri, Nicolas Donzel, Deborah Jones, and et al. 2020. "Hydrogen Recovery from Waste Gas Streams to Feed (High-Temperature PEM) Fuel Cells: Environmental Performance under a Life-Cycle Thinking Approach" Applied Sciences 10, no. 21: 7461. https://doi.org/10.3390/app10217461







