Abstract
In this work, the inactivation kinetics of Alicyclobacillus acidoterrestris spores by temperature-assisted high hydrostatic pressure was assessed by means of the Weibull model. Spores from two A. acidoterrestris strains (a wild-type strain and a reference strain) were inoculated in commercial orange juice and subjected to high pressure levels (500 and 600 MPa) combined with four temperature regimes (25, 45, 60 and 70 °C) for time up to 30 min. Results showed that for a given high-pressure level spore inactivation was higher as temperature progressively increased. Furthermore, the Weibull model consistently produced satisfactory fit to the inactivation data based on the values of the root mean squared error (RMSE < 0.54 log colony-forming units (CFU)/mL) and the coefficient of determination (R2 > 0.90 in most cases). The shape of inactivation curves was concave upward (p < 1) for all temperature/high pressure levels tested, indicating rapid inactivation of the sensitive cells of the bacterium whereas the remaining ones adapted to high hydrostatic pressure (HHP) treatment. The values of the shape (p) and scale (δ) parameters of the Weibull model were dependent on the applied temperature for a given high pressure level and they were further described in a secondary model using first-order fitting curves to provide predictions of the surviving spore population at 55 and 65 °C. Results revealed a systematic over-prediction for the wild-type strain regardless of temperature and high pressure applied, whereas for the reference strain under-prediction was evident after 3 log-cycles reduction of the surviving bacteria spores. Overall, the results obtained indicate that the effectiveness of high hydrostatic pressure against A. acidoterrestris spores is strain-dependent and also underline the need for temperature-assisted HPP for effective spore inactivation during orange juice processing.
1. Introduction
In the early 1980s the fruit juice industry had to deal with spoilage incidents that were caused by a bacterium later on named Alicyclobacillus [1,2,3]. This bacterium is difficult to be detected since no acid or gas production is apparent in the product when spoiled. Only after consumption an off-flavour described as “medicinal, phenolic and antiseptic” is the evident sign of spoilage [4,5] due to the production of the compounds 2-methoxyphenol (guaiacol), 2, 6 dibromophenol and 2,6 dichlorophenol [3,6,7,8]. Alicyclobacillus acidoterrestris is an aerobic, rod shaped Gram-positive, endospore-forming and non-pathogenic spoilage microorganism [2,8]. It has the ability to grow in a wide pH range (2.0–7.0) with optimal pH values between 3.5–4.0 and at a temperature range of 25–60 °C with optimal between 40–45 °C [9,10,11]. A. acidoterrestris spores have the ability to survive pasteurization procedures of fruit juice and because of their acidophilic nature they can germinate and result in spoilage after favourable conditions [4,12]. The maximum concentration of Alicyclobacillus spores that is accepted in the raw material by the fruit industry is 100 colony-forming units (CFU)/mL [13]. Part of the spore resistance is due to the presence of an external protein coat with strong hydrophobic bonds that stabilizes and reduces membrane permeability in extreme acidic and high-temperature environments [14].
Acidothermophilic bacteria like A. acidoterrestris are considered to be important target microorganisms in quality control of heat-processed acidic foods. Therefore, it has been suggested as a reference microorganism for designing pasteurization processes in acidic fruit products [15,16]. Results concerning thermal inactivation of A. acidoterrestris spores in orange juice showed a D-value at 90 °C between 10 and 23 min and at 95 °C between 2.5 and 8.7 min, depending on bacterial strain and the conditions of the experiment [17,18]. Consequently, typical juice pasteurization processes employed by the fruit juice industry today (i.e., 86 to 96 °C for 2 min) cannot ensure spore inactivation especially for products with longer shelf life [19]. The inactivation of A. acidoterrestris has been investigated by a number of challenging non thermal-based technologies, such as electric fields [20], ultrasounds [21], pulsed light [22], hyperbaric storage [23], ultraviolet radiation [24] natural antimicrobials [25], high hydrostatic pressure (HHP) combined with mild heat treatment [5], supercritical carbon dioxide assisted HHP [26].
HHP is a modern method of non-thermal food pasteurization, commercially employed by many food industries with an increasing number of HHP-treated foods placed in the market. It relies on the application of high pressures (400–600 MPa) on food and beverages so as to inactivate spoilage or pathogenic microorganisms and therefore extend the shelf life of the product [27,28]. It needs to be noted however that bacterial spores are resistant to HHP, so it has been proposed that this process must be combined with mild heat treatment for effective spore inactivation [29,30,31,32,33]. HHP processing is a challenging alternative to thermal processing in orange juice production. With HHP treatment flavour, colour and taste can be hardly affected since most of the molecules such as amino acids, vitamins, nutrients and functional properties are retained [18]. HHP can inactivate microorganisms at lower temperatures compared to conventional pasteurization while maintaining a maximum degree of sensory and nutritional quality [34,35].
The objectives of this study were: (a) to investigate the effect of temperature-assisted high pressure processing on the inactivation of the spores of two strains of A. acidoterrestris in orange juice and (b) to describe the inactivation kinetics by means of the Weibull model.
2. Materials and Methods
2.1. Bacterial Strains and Spore Suspension
Two strains of A. acidoterrestris were used in this study: a wild-type strain isolated previously from apple juice that was kindly provided by the Laboratory of Food Microbiology and Hygiene of the Aristotle University of Thessaloniki (denoted strain A). The GenBank accession number of 16S rRNA sequence of strain A is MW142406. A reference strain of A. acidoterrestris DSMZ2498 (denoted strain B) obtained from DSMZ (Deutsche Sammlung von Mikroorganismen and Zellkuturen, Braunschweig, Germany) culture collection. Both strains were maintained at −80 °C in Yeast Extract Starch Glucose (YSG) broth (Yeast Extract 2.0 g; Glucose 1.0 g; Soluble starch 2.0 g; 1000 mL H20) with pH adjusted to 3.7 using 1N HCl and supplemented with 20% (v/v) glycerol (APPLICHEM, Darmstadt, Germany). The strains were pre-cultured in the same medium at 45 °C for 48 h to obtain a stock culture.
The cells from the culture were inoculated on acidified Bacillus acidoterrestris medium (BAT) agar plates (pH 3.7) (BTA20500, Biolab, Budapest, Hungary) and incubated at 45 °C for 7 days to sporulate. The sporulation of the cells was confirmed by phase contrast microscopy. When at least 80% of the cells had sporulated, the spores were harvested by adding 2.5 mL of cold sterile distilled water to the plates and scraping gently the surface with a sterile glass rod. The process was repeated twice. The spores obtained were centrifuged at 5000 rpm for 20 min at 4 °C. The supernatant was then discarded and the spores were washed three times by centrifugation (5000 rpm for 20 min at 4 °C) using cold sterile distilled water. The spores of 15 plates were re-suspended in 10 mL of sterile phosphate buffer (pH 7.2) and stored at 4 °C until use. The concentration of the spore suspension was determined by plating and it was ca. 107 spores/mL (see Section 2.2) [18,19].
2.2. Enumeration of A. acidoterrestris Spores
For the determination of spore concentration of A. acidoterrestris in non-treated and HPP-treated orange juice samples, a volume of 2 mL of each sample was heat shocked at 80 °C for 10 min [18]. Colony counting for each sample was performed from the appropriate decimal dilutions followed by spread plating on duplicate acidified BAT agar plates (pH 3.7) after incubation of the plates at 45 °C for 3 days and the results are expressed as log CFU/mL. Moreover, in order to estimate the difference in the amount of spores and vegetative cells, non heat-shocked juice was also evaluated following serial dilutions and spread plating as described above (data not shown).
2.3. Orange Juice Samples
Experiments were undertaken using commercially available pasteurized orange juice (pH 3.7; 11.45 °Brix) purchased from a local supermarket, with no initial A. acidoterrestris contamination (assessed experimentally, (see Section 2.2). A volume of 0.4 mL of spore suspension was added to 4 mL of orange juice in plastic film pouches (45 mm wide × 95 mm long × 90 μm thickness) (Flexo-Pack SA., Athens, Greece) to achieve a final spore concentration of ca. 106 spores/mL [18,19]. The pouches were heat-sealed using a HenkoVac 1700 machine (Howden Food Equipment B.V., The Netherlands) taking care to expel most of the air.
2.4. High Pressure Treatment of Orange Juice Samples
The inactivation of A. acidoterresris spores for both strains A and B was undertaken at 500 and 600 MPa in combination with different temperature regimes (25, 45, 60, and 70 °C) for pressurization time up to 30 min (1, 3, 5, 15, 30 min). The HHP processing was carried out with a Food Pressure Unit (FPU) 1.01 (Resato International BV, Roden, Holland). The system consists of a high-pressure intensifier for the buildup of pressure, an electric motor to drive a hydraulic pump and a block of 6 small vessels (42 mL) measuring 2.5 cm in diameter and 10 cm in length each. The vessels are closed with a unique Resato thread connection on the top of the vessel. The pressure transmitting fluid is polyglycol ISO viscosity class VG 15 (Resato International BV, Roden, Holland) and the maximum operating pressure and temperature of the system is 1000 MPa and 90 °C, respectively, with pressure adjustable in steps of 20 MPa. Pressure transducers are used to monitor the pressure and temperature transmitters are mounted in each vessel to monitor the temperature. The come-up rate was approximately 100 MPa per 7 s and the pressure release time was less than 3 s. Pressurization time reported in this work does not include the pressure come-up and release times. Right after high-pressure treatment, the pouches were immediately cooled in an ice bath. Overall, the experiment was repeated twice with duplicate samples from different pouches analyzed for each combination of pressure, temperature and time.
2.5. Determination of Inactivation Kinetics
Log-survival data of A. acidoterresris spores from strains A and B obtained in temperature-assisted high-pressure processing were described by the Weibull model [36]:
where Nt is the surviving load of spores (CFU/mL) at a given treatment time (min), N0 is the initial load of spores in the juice (CFU/mL), p is the shape parameter of the curve (dimensionless) showing upward (p < 1) and downward (p > 1) concavity and δ is the scale parameter (min) corresponding to the time of the first decimal reduction (1D). The Weibull model was fitted to the experimental data using the GInaFiT ver. 1.7 software [37] and the goodness-of-fit of survivor curves was assessed using the coefficient of determination (R2) and the root mean squared error (RMSE). In addition, the z values were determined at each high pressure level separately (500 and 600 MPa) and strain type of the bacterium by calculating the reciprocal of the slope of the straight line between log(δ) and temperature to compare with previous results.
The Weibull model was further validated with independent experiments to find out whether survival spore populations from both strains A and B obtained from different temperatures at the same pressurization levels could be effectively predicted. For this purpose, two additional temperatures (55 and 65 °C) within the range used to develop the model were selected for the same pressure levels (500 and 600 MPa). Additional orange juice samples were prepared, inoculated, pressurized (1, 3, 5, 15, and 30 min) and enumerated as mentioned above. The performance of validation procedure was assessed graphically by plotting the observed vs. predicted values of A. acidoterrestris for each strain individually.
3. Results and Discussion
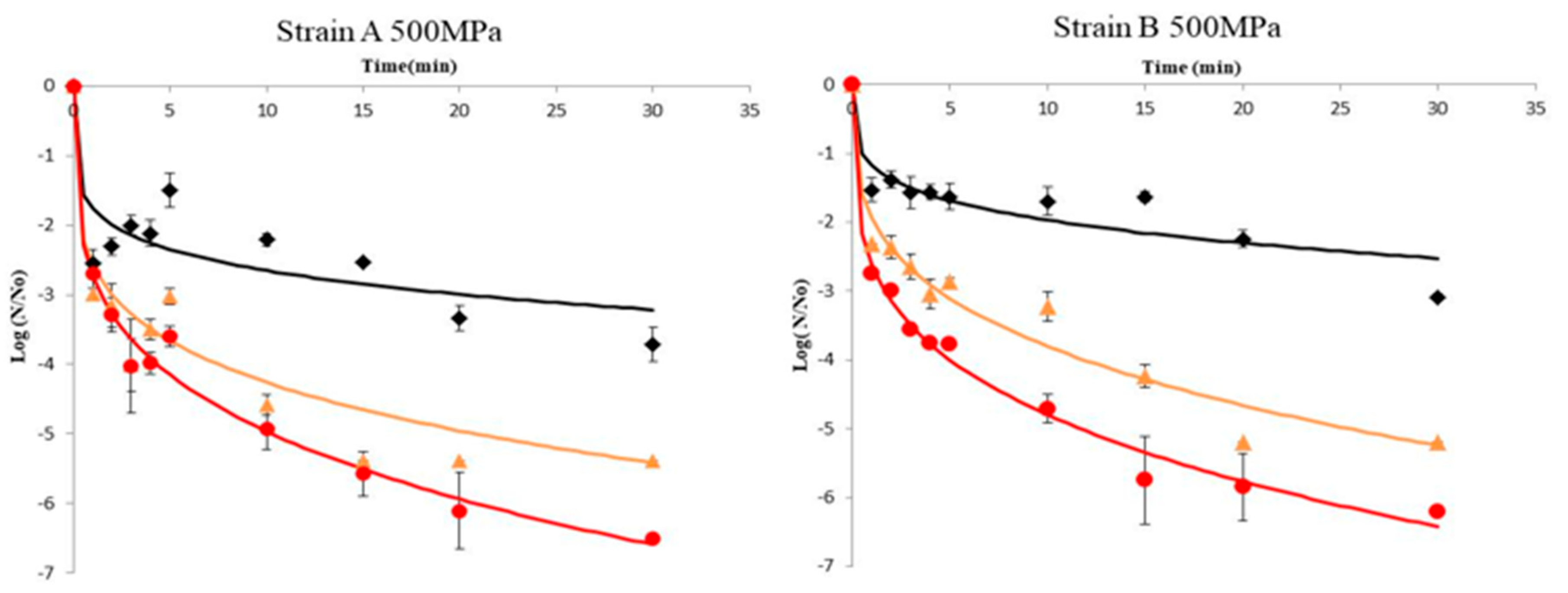
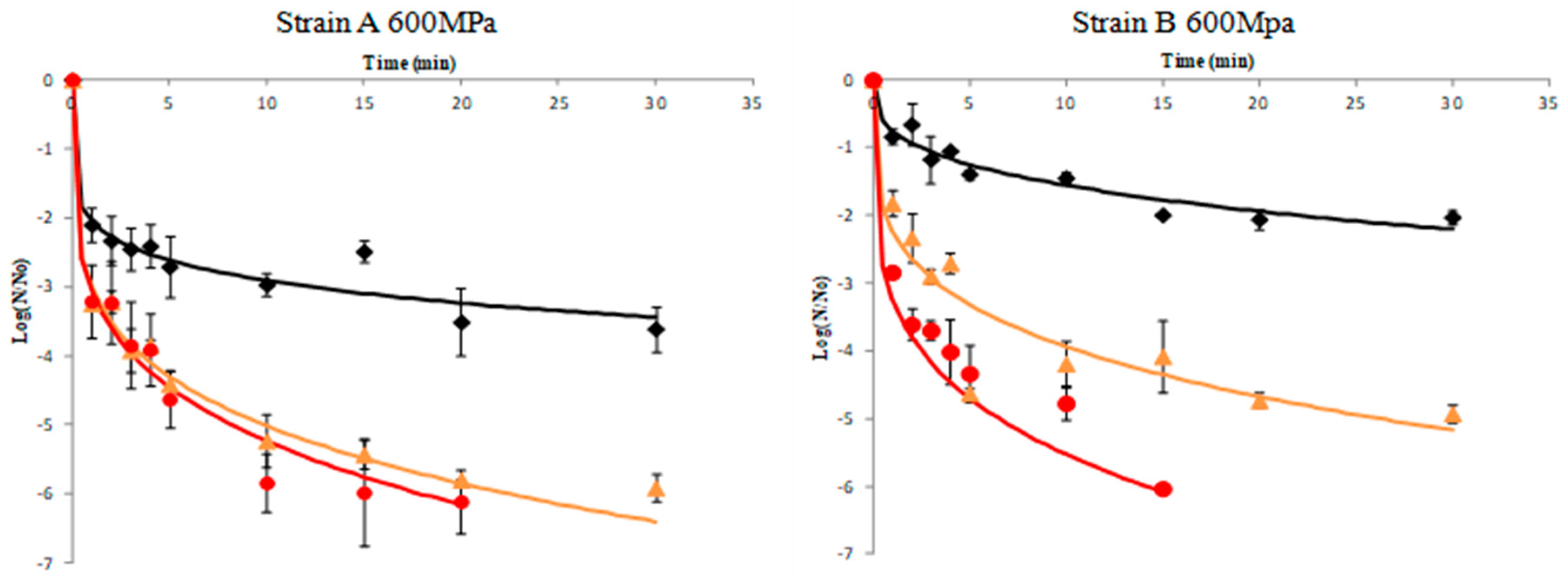
Results of A. acidoterrestris spores’ inactivation of strains A and B in orange juice pressurized at 500 and 600 MPa in combination with different temperature regimes (45, 60, and 70 °C) are illustrated in Figure 1 and Figure 2, respectively. To prevent the effect of the initial load of spores (N0), data are presented in terms of log (N/N0), where N0 was ca. 106 CFU/mL in all experiments. Inactivation kinetics did not follow a first-order pattern but an upward concavity was noticeable at all temperatures assayed. A possible explanation for this inactivation pattern is that the microorganism population is composed by several subpopulations (spores and vegetative cells in different physiological state), each one presenting a distinct inactivation pattern, which causes the non-linear curves [37,38]. In other words the presence of subpopulations have symmetric or asymmetric heat resistance distributions [35,39]. Thus, upward concavity could be considered as evidence of quick inactivation of the sensitive cells of the population, whereas the remaining survivors were more resistant to the lethal agent. Similar upward inactivation curves were obtained for A. acidoterrestris during temperature-assisted HHP processing of fruit juices with different soluble solids content (up to 20 °Brix) [5], as well as in orange juice where high pressure processing was combined with mild heat treatment (45–65 °C), although inactivation kinetics were simulated by the Bigelow model (first order) despite the fact that deviation from linearity was evident. The results obtained in this work demonstrated that temperature was a major parameter in the inactivation of A. acidoterrestris spores. Thus for a given high pressure level, the higher the temperature the higher the spore inactivation. It needs to be noted that when high-pressure processing was undertaken at 500 MPa/25 °C and 600 MPa/25 °C the reduction in spore population was less than 1.0–1.5 log cycles throughout the process for both strains of the bacterium (Supplementary Table S1). These results are in agreement with previous researchers [19,28,32] who reported little or no inactivation of A. acidoterrestris spores when HHP was applied at ambient temperature.
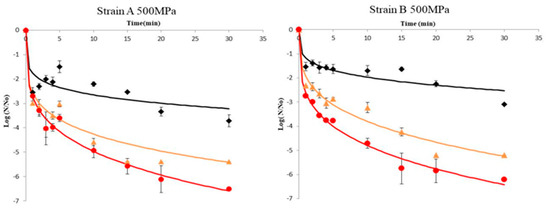
Figure 1.
Inactivation curves of A. acidoterrestris spores at 500 MPa and 45 °C (◆), 60 °C (▲) and 70 °C (●). Solid lines represent data fitting with the Weibull model. Data are mean values of two replications ± standard deviation.
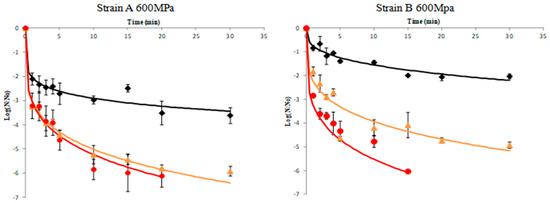
Figure 2.
Inactivation curves of A. acidoterrestris spores at 600 MPa and 45 °C (◆), 60 °C (▲) and 70 °C (●). Solid lines represent data fitting with the Weibull model. Data are mean values of two replications ± standard deviation.
For the wild-type strain A of A. acidoterrestris, results indicated that at 45 °C there was ca. 3 log-cycles reduction of spore counts after 30 min processing time at both 500 and 600 MPa, whereas at 60 °C the same reduction magnitude was achieved in 1–2 min in both high pressures employed. For complete inactivation of the spores the required time was 15 min at 600 MPa/70 °C and 30 min at 500 MPa/70 °C. It is worth noting that for strain A an initial decrease of ca. 2.5 log-cycles was observed within the first minute of processing at 500 MPa followed by an increase thereafter until 5 min (Figure 1 and Figure 2). This could be attributed to the dormancy of spores that was interrupted by the implementation of HHP treatment, since HHP has proven to induce the germination of spores [40]. A two-step HHP treatment has been proposed to inactivate alicyclobacilli spores. In the first step, high pressure induces spore germination whereas in the second step high pressure causes the inactivation of germinated spores [27].
For the reference strain B of A. acidoterrestris, results revealed ca. 1.5 log-cycles reduction after treatment at 500 MPa/45 °C for 10 min and ca. 1.3 log-cycles reduction under 600 MPa/45 °C for the same time (Figure 1 and Figure 2). This is in agreement with Vercammen et al. [28] who inoculated A. acidoterrestris spores in tomato sauce at various pH values and applied high-pressure treatment at 600 MPa/40 °C for 10 min and reported spores reduction of ca. 1.5 log-cycles for tomato sauce at pH 4.2. Moreover, other researchers [5] applied high-pressure treatment at 600 MPa/45 °C for 10 min using a 10 °Brix broth medium (close to 11.45 °Brix of the orange juice employed in this study) and reported 1.2 log-cycles reduction of A. acidoterrestris spores. Complete spore inactivation was achieved at 600 MPa/70 °C and 500 MPa/70 °C after 20 and 30 min, respectively (Figure 1 and Figure 2).
Comparing the two strains, it can be underlined that the wild-type strain A was less resistant to high-pressure treatment compared to the reference strain B at all temperatures assayed (45 and 60 °C) with the exception of 70 °C where stain A was proved more resistant (Figure 1 and Figure 2).
Specifically, strain A presented 5 log-cycles’ reduction when treated at 500 MPa/60 °C and 600 MPa/60 °C for 15 min, whereas the respective reduction for strain B under the same conditions was 3 log-cycles. For complete spore inactivation, a treatment at 600 MPa/70 °C for 15 and 20 min was necessary for strains B and A, respectively (Figure 1 and Figure 2). This difference in high-pressure tolerance could be attributed to the fact that the effectiveness of HHP against A. acidoterrestris spores is strain-dependent [9,41], possibly due to the different distribution of fatty acids in the cytoplasmatic membrane of the bacterium [9,42]. Furthermore, other factors that may affect HPP thermal resistance include the number and age of spores, protoplast dehydration and sporulation temperature [43].
The inactivation curves of both strains A and B of A. acidoterrestris spores fitted with the Weibull model are also depicted in Figure 1 and Figure 2, respectively. The model provided a good fit enabling the study of inactivation of A. acidoterrestris spores through the variation of δ and p parameters (Table 1). Concave upward (p < 1) inactivation curves were observed at all temperature/high-pressure combinations tested (Table 1). From this type of concavity we can assume that spores presented a mixed resistance to inactivation treatment [38,39] and the remaining spores were more resistant or maybe they could adapt better to stressful conditions and therefore have higher possibility to survive. The fitting capacity of the model was evaluated by estimating the RMSE and the R2 values. The mean values of RMSE were 0.421 log CFU/mL and 0.281 log CFU/mL for strain A at 500 and 600 MPa, respectively, whereas for strain B the RMSE mean values were 0.281 log CFU/mL and 0.418 log CFU/mL at the same high-pressure levels. In addition, the R2 values for most curves were higher than 0.940 indicating that the Weibull model fitted the experimental data closely, justifying thus its use to describe the inactivation kinetics of A. acidoterrestris (Table 1). The Weibull model has been extensively used in previous works to model the inactivation of A. acidoterrestris due to its simplicity and flexibility. Specifically, Uchida and Silva [5] reported the successful use of the Weibull model during HHP treatment at 600 MPa combined with different temperatures (35, 45, 55, and 65 °C) in malt extract broth adjusted to 10, 20 and 30 °Brix for the inactivation of A. acidoterrestris spores. In addition, it has been successfully employed to model the inactivation kinetics of A. acidoterrestris in BAM broth when treated at 350 and 450 MPa combined with different temperatures (35, 45, and 50 °C) [35]. Moreover, apart from HHP treatment, the Weibull model could effectively describe the inactivation of A. acidoterrestris vegetative cells and spores by other emerging technologies such as ultrasound [21], UV-C light irradiation [44] and pulsed light [22].

Table 1.
Weibull model-estimated kinetic parameters and goodness-of-fit indices for the inactivation of A. acidoterrestris spores for Strains A and B using temperature-assisted high-pressure processing.
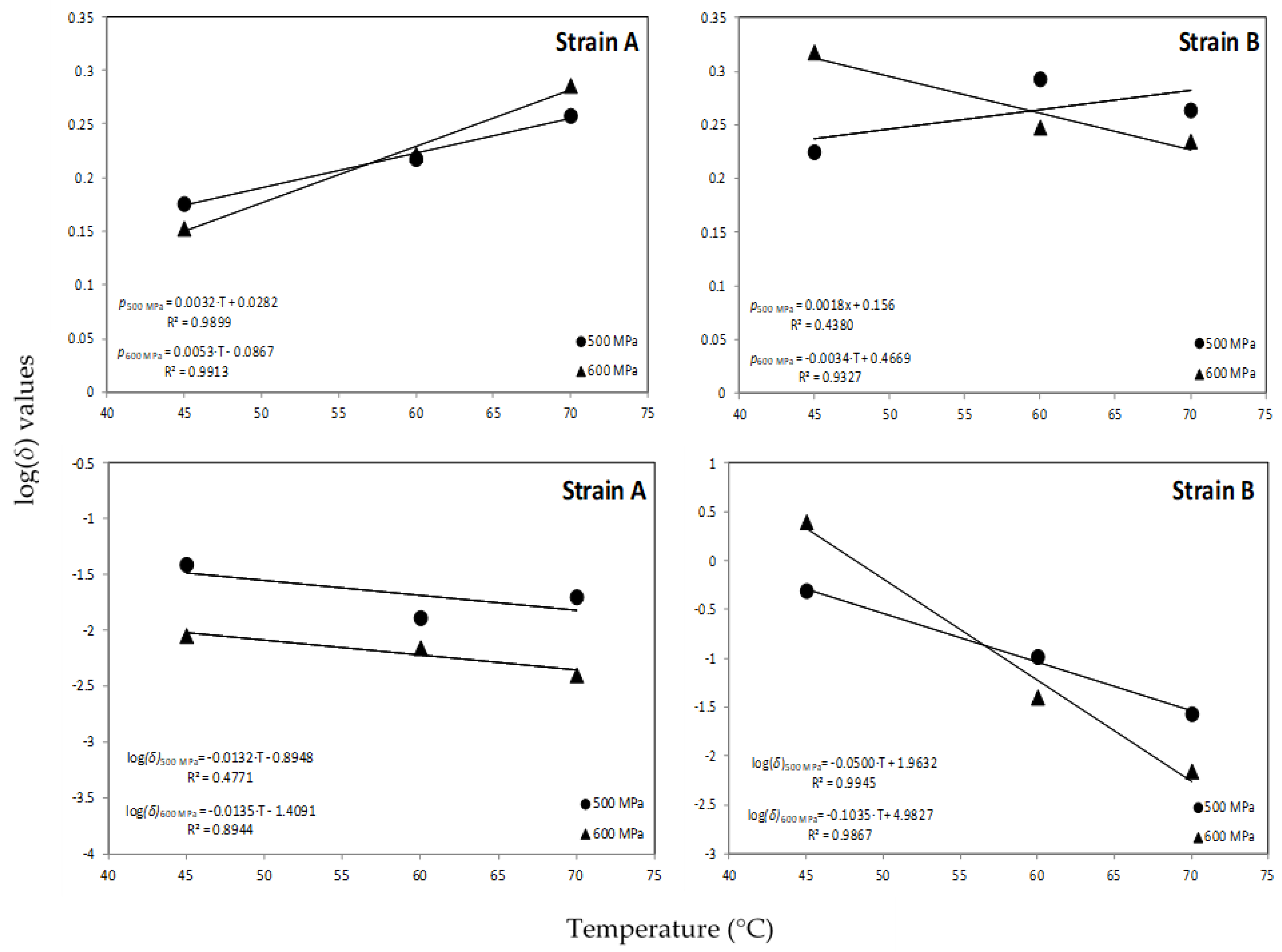
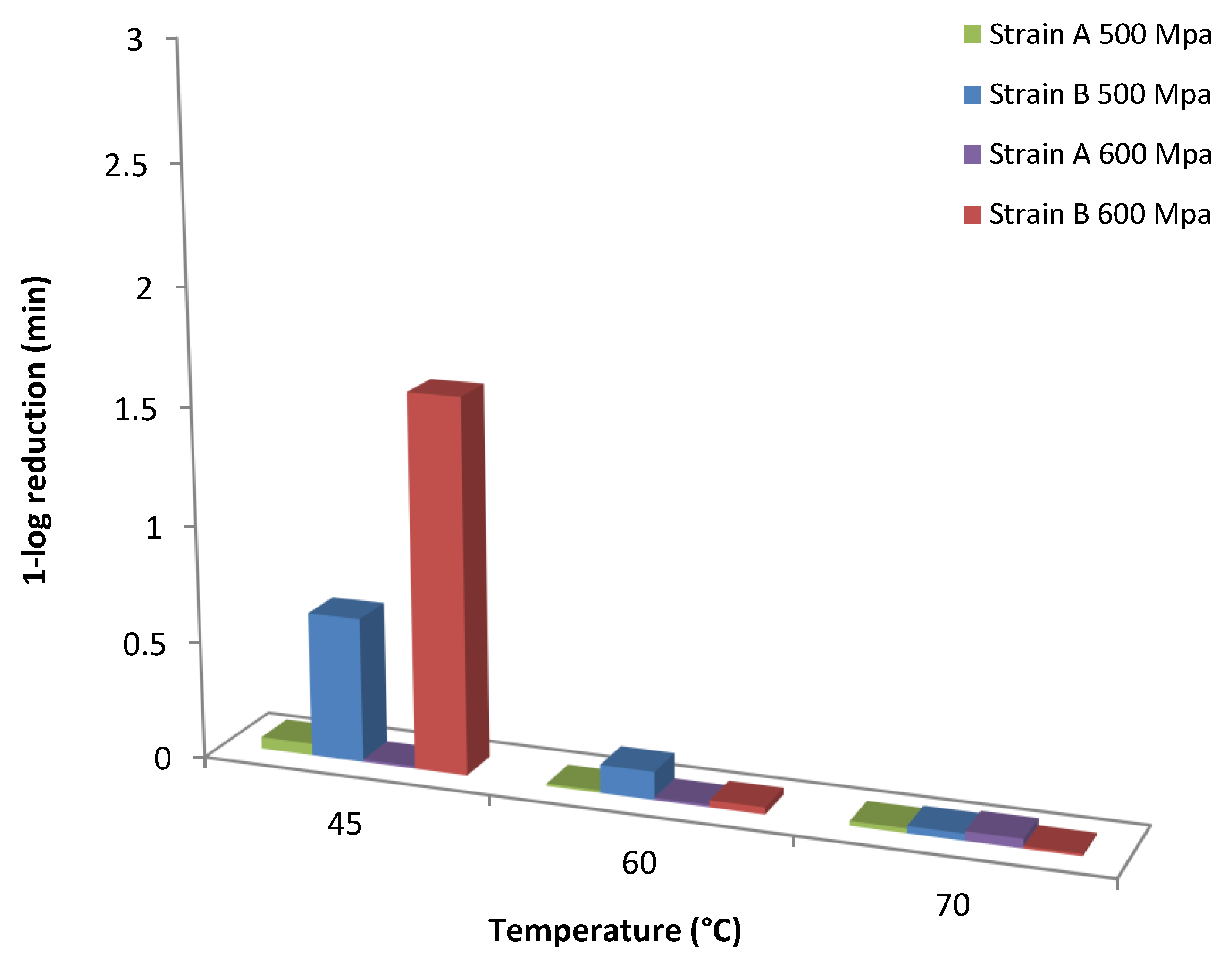
An increase trend in the shape factor (p) with the HHP treatment was observed at all temperatures assayed with the exception of 600 MPa for strain B where a decrease in the values of p was evident with increasing temperature level (Table 1; Figure 3). This trend in shape factor values is in agreement with previous researchers [35,45] who reported that the shape parameter was dependent on temperature at certain pressures. However, other reports noted that the shape factor indicates the kinetic pattern that describes the inactivation process and thus it should be independent of the external factor (i.e., temperature) [5,46], whereas van Boekel [38] reported that in only 7 out of 55 studies the shape factor seemed to be dependent from temperature. The scale parameter δ in this model equals the first decimal reduction time (1D, min) that results in 1-log CFU/mL reduction of the surviving spores’ population and could be used as an indication of how rapidly the spores are inactivated [47]. Based on the estimated values of this parameter (δ), the longest spore survival in orange juice was observed for the reference strain B at 600 MPa/45 °C, whereas the wild-type strain A presented lower survival at the same temperature (Figure 4). The differences for the 1-log CFU/mL reduction in the surviving population of spores between the two strains were practically diminished as processing temperature increased. It could thus be suggested that at the selected high pressure levels, the temperature should be higher than 60 °C for effective spore inactivation. Regarding the scale parameter (δ), a decrease of log(δ) with temperature was noted at all high pressure levels applied (Figure 3).
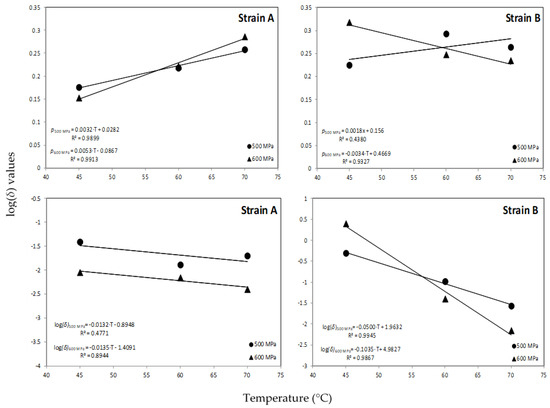
Figure 3.
Effect of HPP temperature on the Weibull log (δ) and p values for A. acidoterrestris spore inactivation at 500 and 600 MPa.
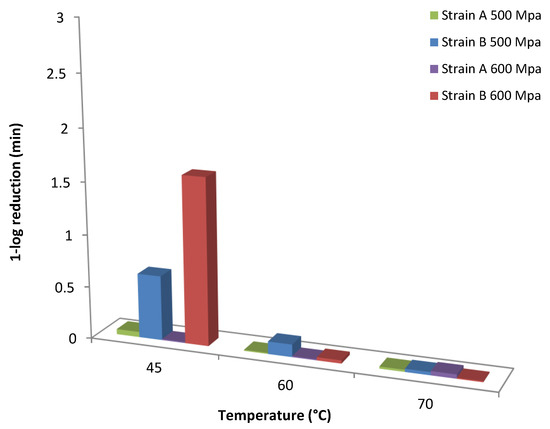
Figure 4.
Model based comparison of 1 log reduction (1D value) time of the spores of A. acidoterrestris strains A and B when treated with high pressure levels of 500 s and 600 MPa at 45, 60 and 70 °C.
The data points were fitted with a linear function as shown in Figure 3 providing information on the temperature sensitivity of δ values for a given high pressure level. Thus, for the wild strain A the z values were 76 and 74 °C for HPP treatment at 500 and 600 MPa, respectively, indicating that temperature changes have minor effect on δ values for this strain. However, the respective z values for strain B were 20 and 10 °C for the same pressurization levels, indicating a larger effect of temperature changes on δ values for this strain of the bacterium. The results obtained in this work for the z values of the reference strain B are comparable with previous researchers [5] who reported z values between 20.07 and 21.43 °C for A. acidoterrestris spore inactivation at 600 MPa combined with mild heat in malt extract broth adjusted to 10, 20 and 30 °Brix. In another work [18], a z value of 34.4 °C was reported for spore inactivation of A. acidoterrestris in orange juice during HPP processing at 600 MPa in the temperature range of 45–65 °C.
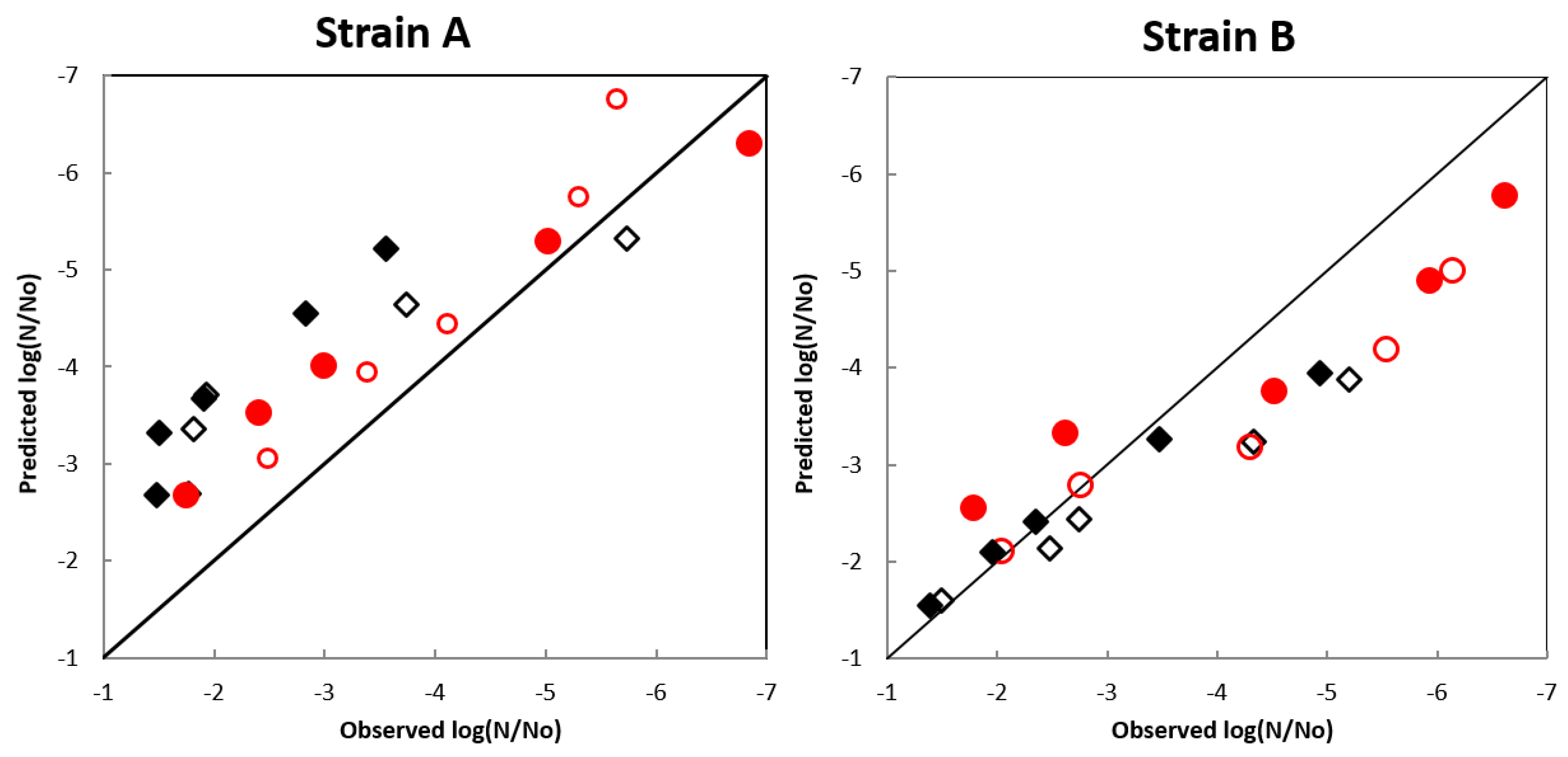
In model validation, two different temperatures were assayed (55 and 65 °C) for each HHP treatment (500 and 600 MPa) for both strains of A. acidoterrestris. The results are shown in Figure 5 in terms of observed vs. predicted log (N/N0) of surviving spores of the bacterium. For the wild-type strain A, over-prediction was observed (i.e., the predicted surviving population of the spores was higher than the experimentally measured) regardless of temperature and pressure level applied. However, for the reference strain B satisfactory performance of the model was observed until −3 log reduction cycles, but after this value a systematic under-prediction was evident (Figure 5). No data could be found in the literature to compare our results because the majority of published reports on A. acidoterrestris spore inactivation do not include external validation of the developed models. In a recent work [5], validation results were presented for the inactivation of A. acidoterrestris spores by high pressure (600 MPa) combined with mild heat (45 °C) in fruit concentrates adjusted to different soluble solids concentrations (10, 20 and 30 °Brix) compared to malt extract broth. The authors also used the Weibull model to describe inactivation kinetics and reported close estimates of δ and p values between the laboratory medium and the fruit concentrates.

Figure 5.
External validation for Weibull model correlating the observed and predicted values of Alicyclobacillus acidoterrestris spores when treated with the combination of 500 MPa at 55 °C (◇) and 65 °C (◆) and 600 MPa at 55 °C (○) and 65 °C (●).
4. Conclusions
Results obtained in this study revealed increased inactivation of the spores by increasing high pressure and temperature levels. Complete spore inactivation was achieved at treatments with the highest pressure (600 MPa) and temperature (70 °C) for both strains. Therefore, HHP presents promising perspectives for the juice industry to be employed for the inactivation of A. acidoterrestris spores and thus increase the shelf life of fruit juices. Furthermore the Weibull model could be successfully used to describe the inactivation of A. acidoterrestris spores when treated at different temperature and high pressure levels. However, strain variability is an important factor affecting the performance of the model and thus future work should be undertaken to include more strains and processing conditions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/21/7542/s1, Table S1: Population of A. acidoterrestris spores (strains A and B) when treated at 500 and 600 MPa combined with 25 °C.
Author Contributions
Conceptualization, P.S., A.A.A., G.-J.E.N. and C.C.T.; methodology, P.S., A.A.A., G.-J.E.N. and C.C.T.; validation, P.S. and E.Z.P.; formal analysis, P.S. and E.Z.P.; investigation, P.S., A.A.A. and C.C.T.; resources, C.C.T.; data curation, P.S. and E.Z.P.; writing—original draft preparation, P.S. and E.Z.P.; writing—review and editing, P.S. and E.Z.P.; visualization, P.S. and E.Z.P.; supervision, A.A.A., C.C.T., G.-J.E.N. and E.Z.P.; project administration, C.C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank E. Beletsiotis and QACS/Erganal company for providing the sequencing of strain A. Sourri would like to thank the Institute of Technology of Agricultural Products of the Hellenic Agricultural Organization “DEMETER” for supporting this research as part of her Ph.D. thesis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cenry, G.; Hennlich, W.; Porolla, K. Spoilage of fruit juices by bacilli: Isolation and characterization of the spoiling microorganisms. Z. Lebensm.-Unters. Forsch. 1984, 179, 224–227. [Google Scholar]
- Silva FVM, Gibbs P Alicyclobacillus acidoterrestris spores in fruit products and design of pasteurization processes. Trends Food Sci. Technol. 2001, 12, 68–74. [CrossRef]
- Hartyáni, P.; Dalmadi, I.; Knorr, D. Electronic nose investigation of Alicyclobacillus acidoterrestris inoculated apple and orange juice treated by high hydrostatic pressure. Food Control. 2013, 32, 262–269. [Google Scholar] [CrossRef]
- Smit, Y.; Camron, M.; Venter, P.; Witthuhn, R.C. Alicyclobacillus spoilage and isolation—A review. Food Microbiol. 2011, 28, 331–349. [Google Scholar] [CrossRef]
- Uchida, R.; Silva, F.V.M. Alicyclobacillus acidoterrestris spore inactivation by high pressure combined with mild heat: Modeling the effects of temperature and soluble solids. Food Control 2017, 73, 426–432. [Google Scholar] [CrossRef]
- Yamazaki, K.; Teduka, H.; Inoue, N.; Shinano, H. Specific primers for detection of Alicyclobacillus acidoterrestris by RT-PCR. Lett. Appl. Microbiol. 1996, 23, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.C.; Guo, C.F.; Yuan, Y.H.; Luo, X.X.; Yue, T.L. Detection of medicinal off-flavor in apple juice with artificial sensing system and comparison with test panel evaluation and GC-MS. Food Control 2015, 51, 270–277. [Google Scholar] [CrossRef]
- Molva, C.; Baysal, A.H. Evaluation of bioactivity of pomegranate fruit extract against Alicyclobacillus acidoterrestris DSM 3922 vegetative cells and spores in apple juice. Food Sci. Technol. 2015, 62, 989–995. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Cibelli, F.; Corbo, M.R.; Sinigaglia, M. Effects of high-pressure homogenization on the survival of Alicyclobacillus acidoterrestris in a laboratory medium. Lett. Appl. Microbiol. 2007, 45, 382–386. [Google Scholar] [CrossRef]
- Silva, F.V.M. High pressure processing pretreatment enhanced the thermosonication inactivation of Alicyclobacillus acidoterrestris spores in orange juice. Food Control 2016, 62, 365–372. [Google Scholar]
- Kakagianni, M.; Kalantzi, K.; Beletsiotis, E.; Ghikas, D.; Lianou, A.; Koutsoumanis, K. Development and validation of predictive models for the effect of storage temperature and pH on the growth boundaries and kinetics of Alicyclobacillus acidoterrestris ATCC49025 in fruit drinks. Food Microbiol. 2018, 74, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Gouws, P.A.; Gie, L.; Pretorius, A.; Dhansay, N. Isolation and identification of Alicyclobacillus acidocaldarius by 16S rDNA from mango juice and concentrate. Int. J. Food Sci. Technol. 2005, 40, 789–792. [Google Scholar] [CrossRef]
- Casas, J.; Valverde, M.T.; Marín-Iniesta, F.; Calvo, L. Inactivation of Alicyclobacillus acidoterrestris spores by high pressure CO2 in apple cream. Int. J. Food Microbiol. 2012, 156, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Steyn, C.E.; Cameron, M.; Witthuhn, R.C. Occurrence of Alicyclobacillus in the fruit processing environment—A review. Int. J. Food Microbiol. 2012, 147, 1–11. [Google Scholar] [CrossRef]
- Silva, F.V.M.; Gibbs, P. Target selection in designing pasteurization processes for shelf-stable high-acid fruit products. Crit. Rev. Food Sci. Nutr. 2004, 44, 353–360. [Google Scholar] [CrossRef]
- Silva, F.V.M.; Gibbs, P. Principles of thermal processing: Pasteurization. In Engineering Aspects of Thermal Food Processing; Simpson, R., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 13–48. [Google Scholar]
- Eiroa, M.N.U.; Junqueira, C.A.; Schmidt, F.L. Alicyclobacillus in orange juice: Occurrence and heat resistance of spores. J. Food Prot. 1999, 62, 883–886. [Google Scholar] [CrossRef]
- Silva, F.V.M.; Tan, E.K.; Farid, M. Bacterial spore inactivation at 45–65 °C using high pressure processing: Study of Alicyclobacillus acidoterrestris in orange juice. Food Microbiol. 2012, 32, 206–211. [Google Scholar] [CrossRef]
- Lee, S.Y.; Dougherty, R.H.; Kang, D.H. Inhibitory effects of high pressure and heat on Alicyclobacillus acidoterrestris spores in apple juice. Appl. Environ. Microbiol. 2002, 68, 4158–4161. [Google Scholar] [CrossRef]
- Kim, N.H.; Ryang, J.H.; Lee, B.S.; Kim, C.T.; Rhee, M.S. Continuous ohmic heating of commercially processed apple juice using five sequential electric fields results in rapid inactivation of Alicyclobacillus acidoterrestris spores. Int. J. Food Microbiol. 2017, 246, 80–84. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Wang, Z. Kinetic models for the inactivation of Alicyclobacillus acidiphilus DSM14558 and Alicyclobacillus acidoterrestris DSM 3922 in apple juice by ultrasound. Int. J. Food Microbiol. 2010, 139, 177–181. [Google Scholar] [CrossRef]
- Ferrario, M.I.; Guerrero, S.N. Inactivation of Alicyclobacillus acidoterrestris ATCC 49025 spores in apple juice by pulsed light. Influence of initial contamination and required reduction levels. Rev. Argent. Microbiol. 2018, 50, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.A.; Martins, A.P.; Santos, M.D.; Fidalgo, L.G.; Delgadillo, I. Growth inhibition and inactivation of Alicyclobacillus acidoterrestris endospores in apple juice by hyperbaric storage at ambient temperature. Innov. Food Sci. Emerg. Technol. 2019, 52, 232–236. [Google Scholar] [CrossRef]
- Keyser, M.; Muller, I.A.; Cilliers, F.P.; Nel, W.; Gouws, P.A. Ultraviolet radiation as a non-thermal treatment for the inactivation of microorganisms in fruit juice. Innov. Food Sci. Emerg. Technol. 2008, 9, 348–354. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Sinigaglia, M.; Corbo, M.R. Control of Alicyclobacillus acidoterrestris in apple juice by citrus extracts and a mild heat treatment. Food Control 2013, 31, 553–559. [Google Scholar] [CrossRef]
- Porębska, I.; Sokolowska, B.; Skapska, S.; Rzoska, S. Treatment with high hydrostatic pressure and supercritical carbon dioxide to control Alicyclobacillus acidoterrestris spores in apple juice. Food Control 2017, 73, 24–30. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Alicyclobacillus acidoterrestris: New methods for inhibiting spore germination. Int. J. Food Microbiol. 2008, 125, 103–110. [Google Scholar] [CrossRef]
- Vercammen, A.; Vivijs, B.; Lurquin, I.; Michiels, C.W. Germination and inactivation of Bacillus coagulans and Alicyclobacillus acidoterrestris spores by high hydrostatic pressure treatment in buffer and tomato sauce. Int. J. Food Microbiol. 2012, 152, 162–167. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chung, H.J.; Kang, D.Y. Combined treatment of high pressure and heat on killing spores of Alicyclobacillus acidoterrestris in apple juice concentrate. J. Food Prot. 2006, 69, 1056–1060. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. High pressure processing of milk: Modeling the inactivation of psychotrophic Bacillus cereus spores at 38–70 °C. J. Food Eng. 2015, 165, 141–148. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. Inactivation of Byssochlamys nivea ascospores in strawberry puree by high pressure, power ultrasound and thermal processing. Int. J. Food Microbiol. 2015, 14, 129–136. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. High pressure thermal processing for the inactivation of Clostridium perfringens spores in beef slurry. Innov. Food Sci. Emerg. Technol. 2016, 33, 26–31. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. Modelling the inactivation of psychotrophic Bacillus cereus spores in beef slurry by 600 MPa HPP combined with 38–70 °C: Comparing with thermal processing and estimating the energy requirements. Food Bioprod. Process. 2016, 99, 179–187. [Google Scholar] [CrossRef]
- Alpas, H.; Alma, L.; Bozoglu, F. Inactivation of Alicyclobacillus acidoterrestris vegetative cells in model system, apple, orange and tomato juices by high hydrostatic pressure. World J. Microbiol. Biotechnol. 2003, 19, 619–623. [Google Scholar] [CrossRef]
- Buzrul, S.; Alpas, H.; Bozoglu, F. Use of Weibull frequency distribution model to describe the inactivation of Alicyclobacillus acidoterrestris by high pressure at different temperatures. Food Res. Int. 2005, 38, 151–157. [Google Scholar] [CrossRef]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguerinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef]
- Geeraerd, A.H.; Valdramidis, V.P.; Van Impre, J.F. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2004, 102, 95–105. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int. J. Food Microbiol. 2002, 74, 139–159. [Google Scholar] [CrossRef]
- Peleg, M.; Cole, M.B. Reinterpretation of microbial survival curves. Crit. Rev. Food Sci. 2010, 38, 353–380. [Google Scholar] [CrossRef]
- Wuytack, E.; Boven, S.; Michiels, C.W. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Appl. Environ. Microbiol. 1998, 64, 3220–3224. [Google Scholar] [CrossRef] [PubMed]
- Solokowska, B.; Skapska, S.; Fonberg-Broczek, M.; Niezgoda, J.; Porebska, I.; Dekowska, A.; Rzoska, S.J. Germination and inactivation of Alicyclobacillus acidoterrestris spores induced by moderate hydrostatic pressure. Pol. J. Microbiol. 2015, 64, 351–359. [Google Scholar] [CrossRef]
- Goto, K.; Mochida, K.; Asahara, M.; Suzuki, M.; Kasai, H.; Yokota, A. Alicyclobacillus pomorum sp. Nov., a novel thermoacidophilic, endospore-forming bacterium that does not possess ω alicyclic fatty acids, and emended description of the genus Alicyclobacillus. Int. J. Syst. Evol. Microbiol. 2003, 53, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Kang, D.H. Alicyclobacillus spp. in the fruit juice industry: Histrory, characteristics, and current isolation/detection procedures. Crit. Rev. Microbiol. 2004, 30, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Baysal, A.H.; Molva, C.; Unluturk, S. UV-C light inactivation and modeling kinetics of Alicyclobacillus acidoterrestris spores in white grape and apple juices. Int. J. Food Microbiol. 2013, 166, 494–498. [Google Scholar] [CrossRef]
- Tassou, C.C.; Panagou, E.Z.; Samaras, F.J.; Galiatsatou, P.; Mallidis, C.G. Temperature-assisted high hydrostatic pressure inactivation of Staphylococcus aureus in a ham model system: Evaluation in selective and nonselective medium. J. Appl. Microbiol. 2008, 104, 1764–1773. [Google Scholar] [CrossRef]
- Cunha, L.M.; Oliveira, F.A.R.; Oliveira, J.C. Optimal experimental design for estimating the kinetic parameters of processes described by the Weibull probability distribution function. J. Food Eng. 1998, 37, 175–191. [Google Scholar] [CrossRef]
- Albert, I.; Mafart, P. A modified Weibull model for bacterial inactivation. Int. J. Food Microbiol. 2005, 100, 197–211. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).