Ultrasonic Regeneration Studies on Activated Carbon Loaded with Isopropyl Alcohol
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Pretreatment and Analysis of Activated Carbon
2.2. Adsorption of IPA by Activated Carbon
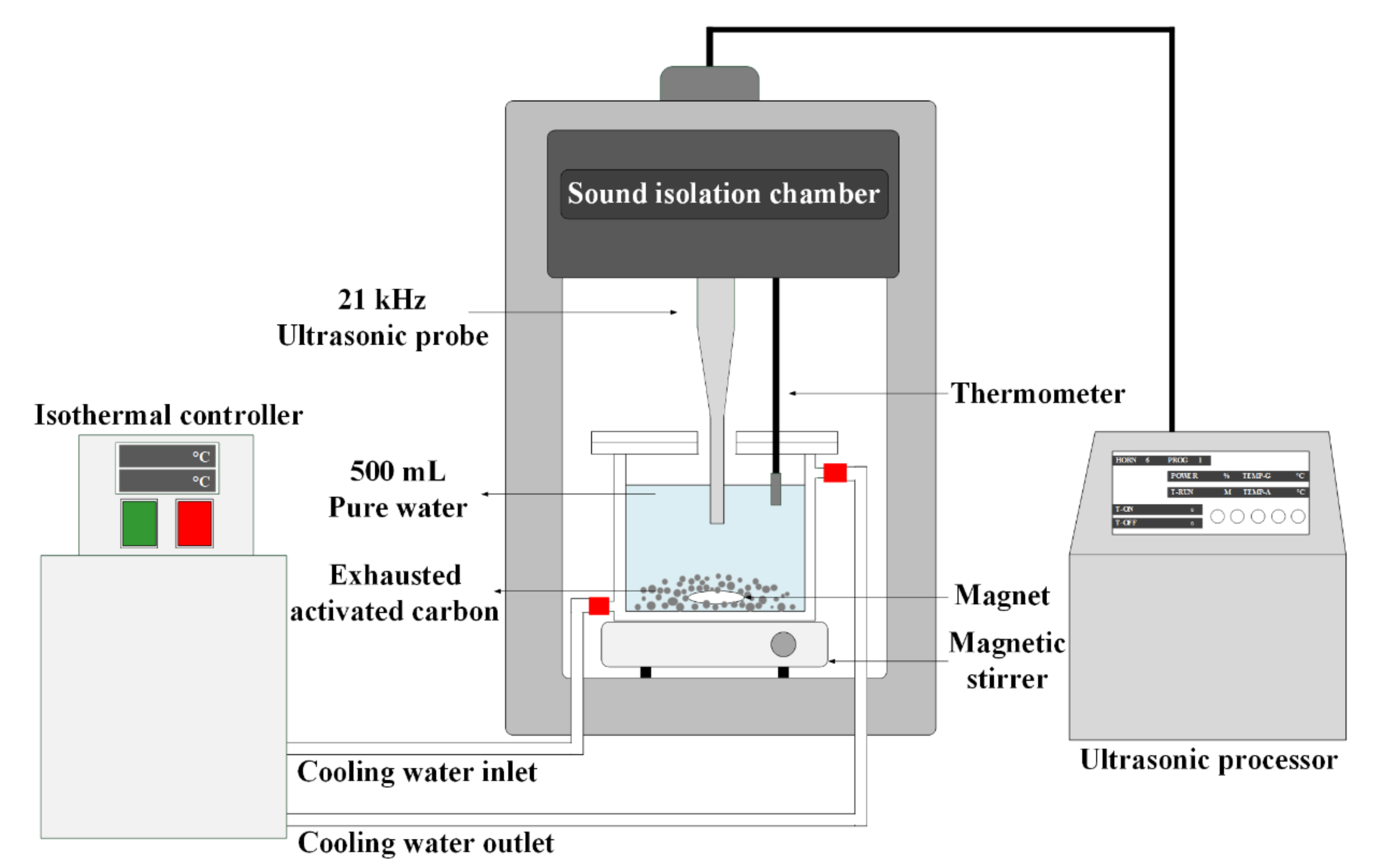
2.3. Ultrasonic Regeneration of IPA-Saturated Activated Carbon
3. Results
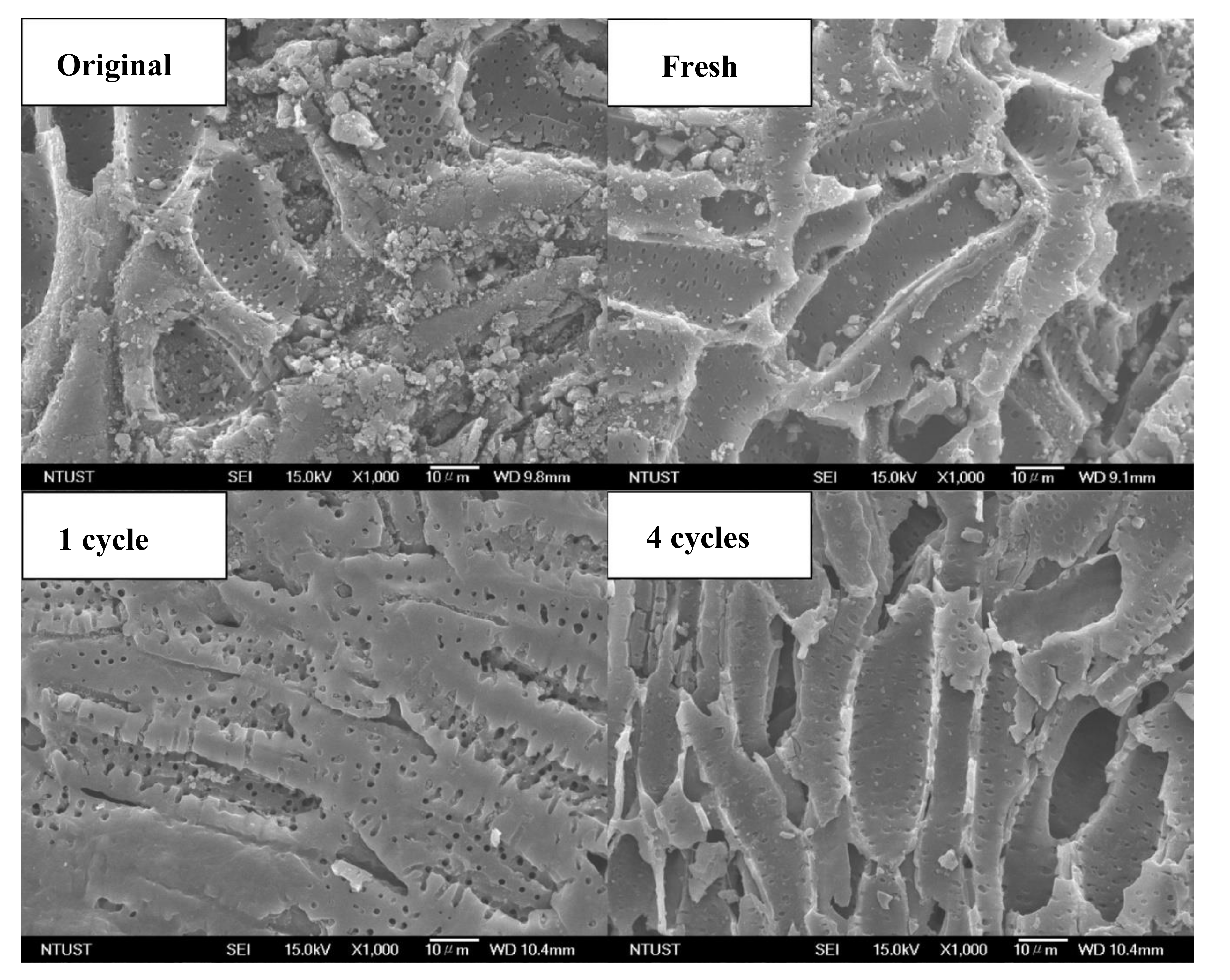
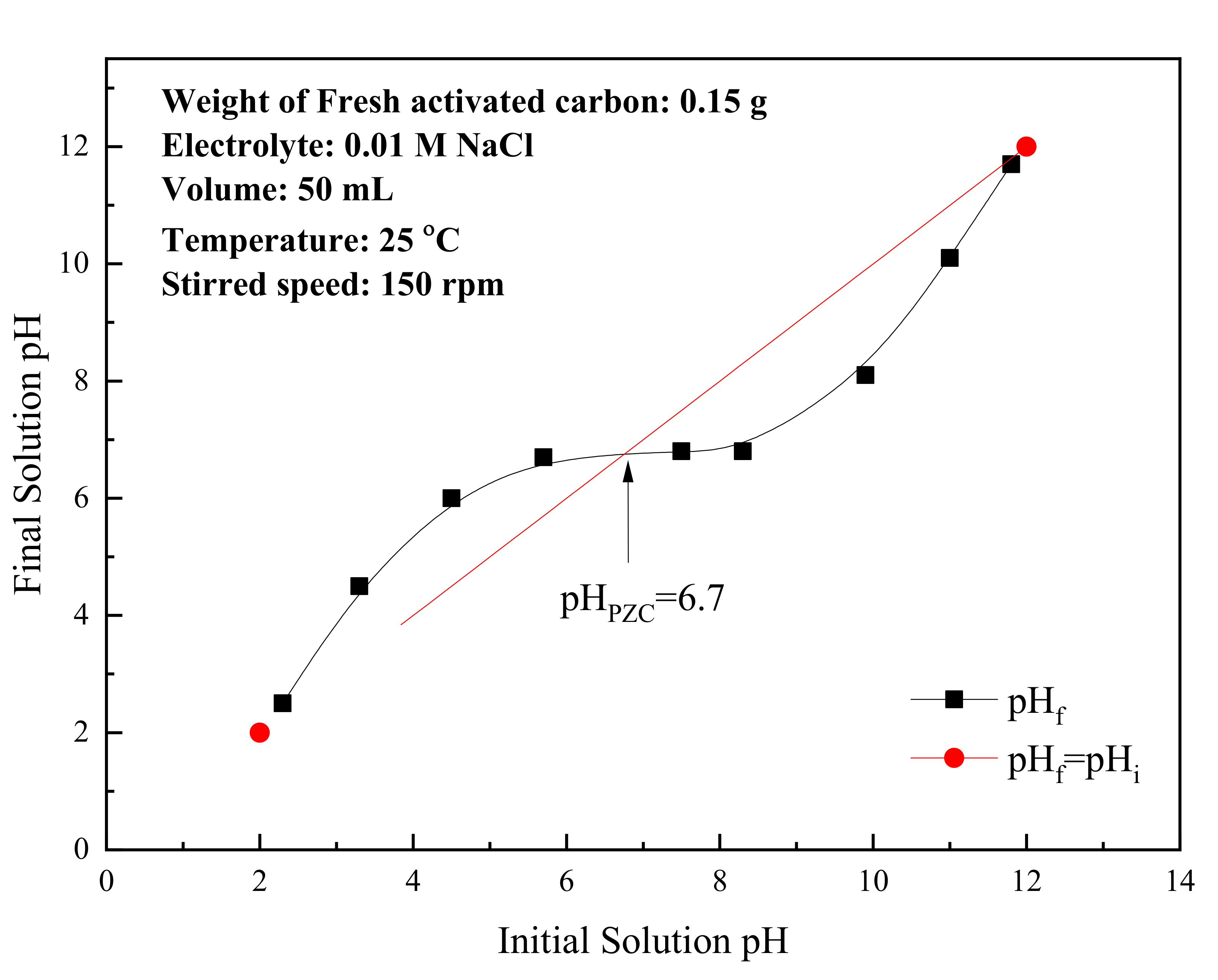
3.1. Properties of the Activated Carbon
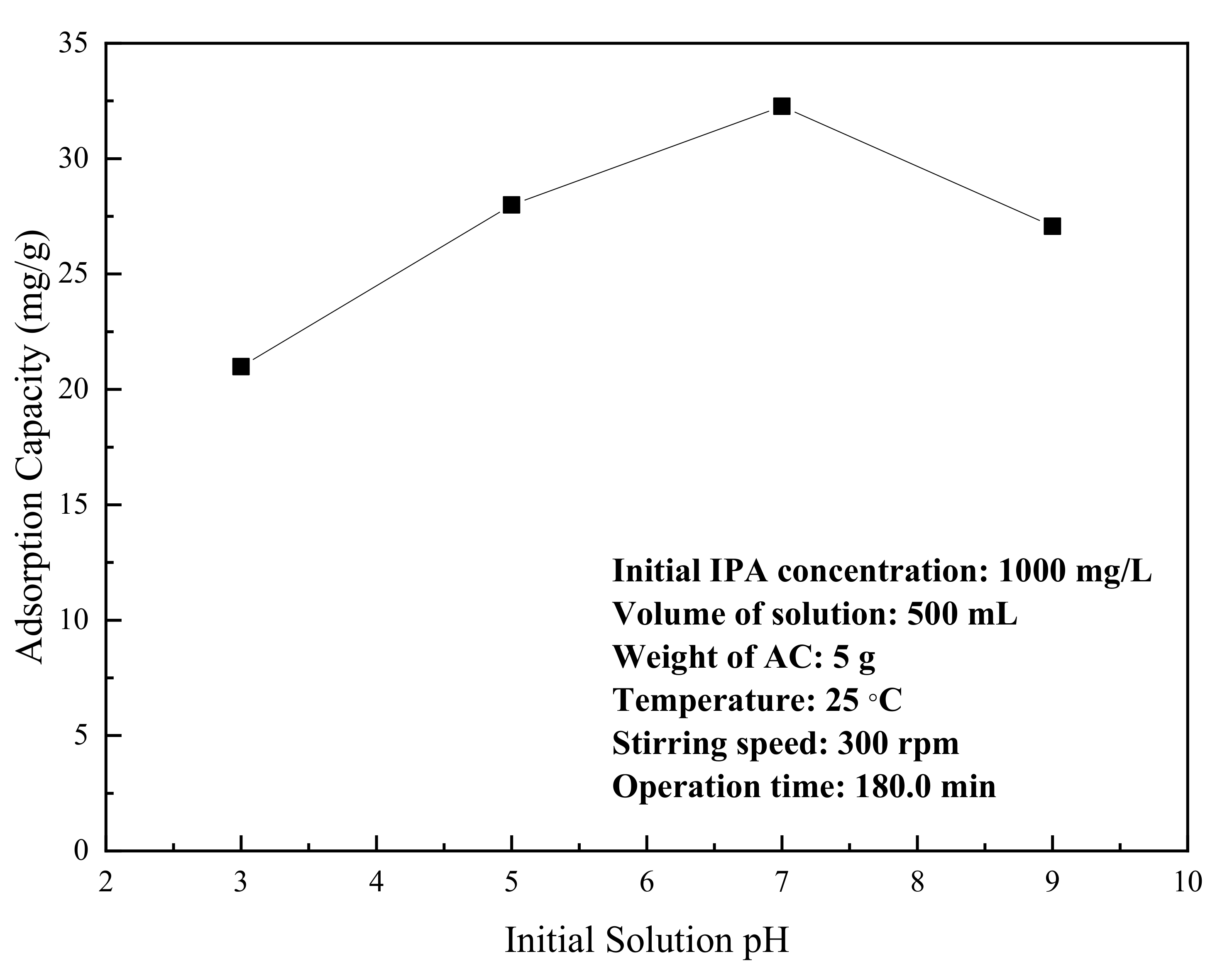
3.2. Adsorption of Isopropyl Alcohol
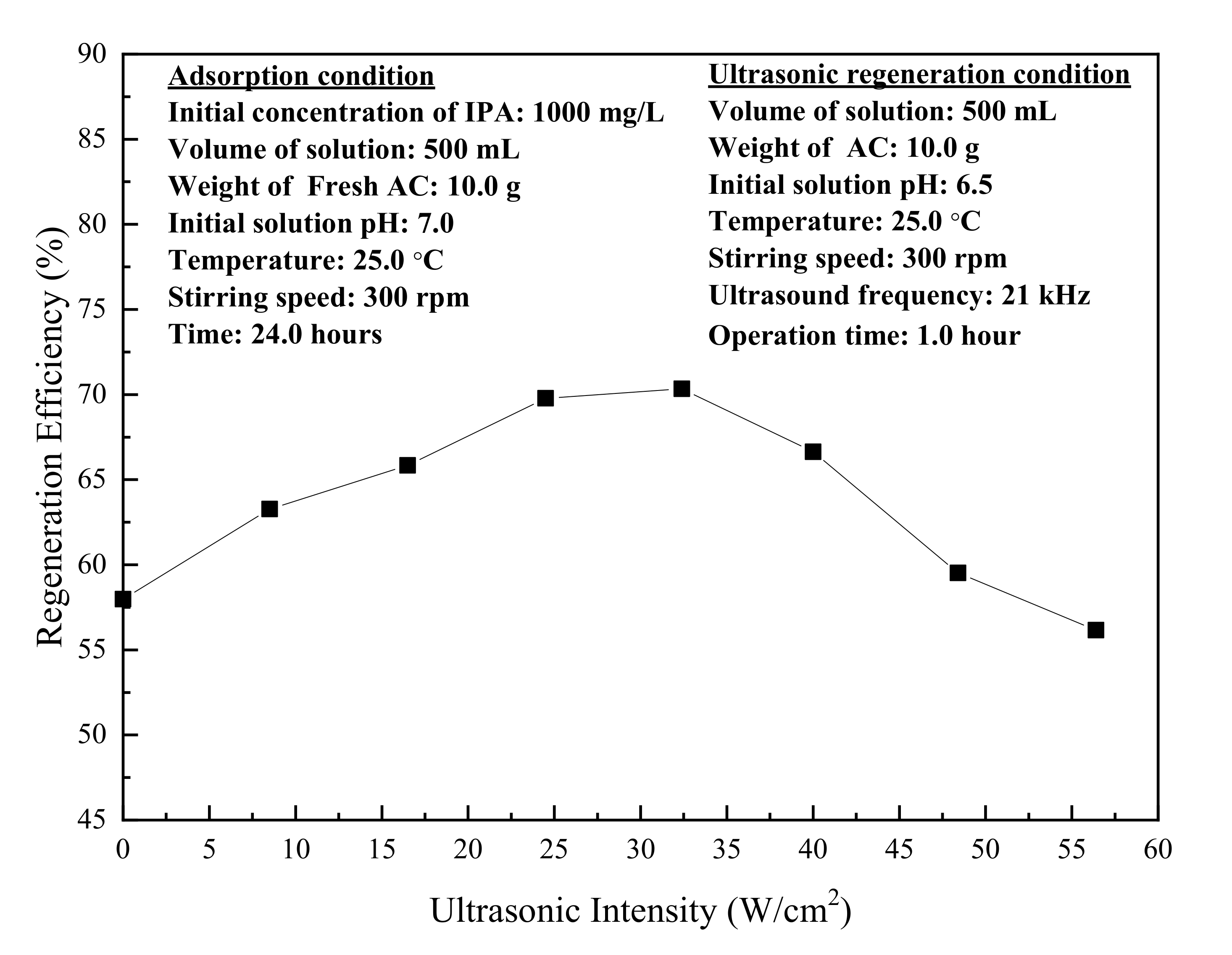
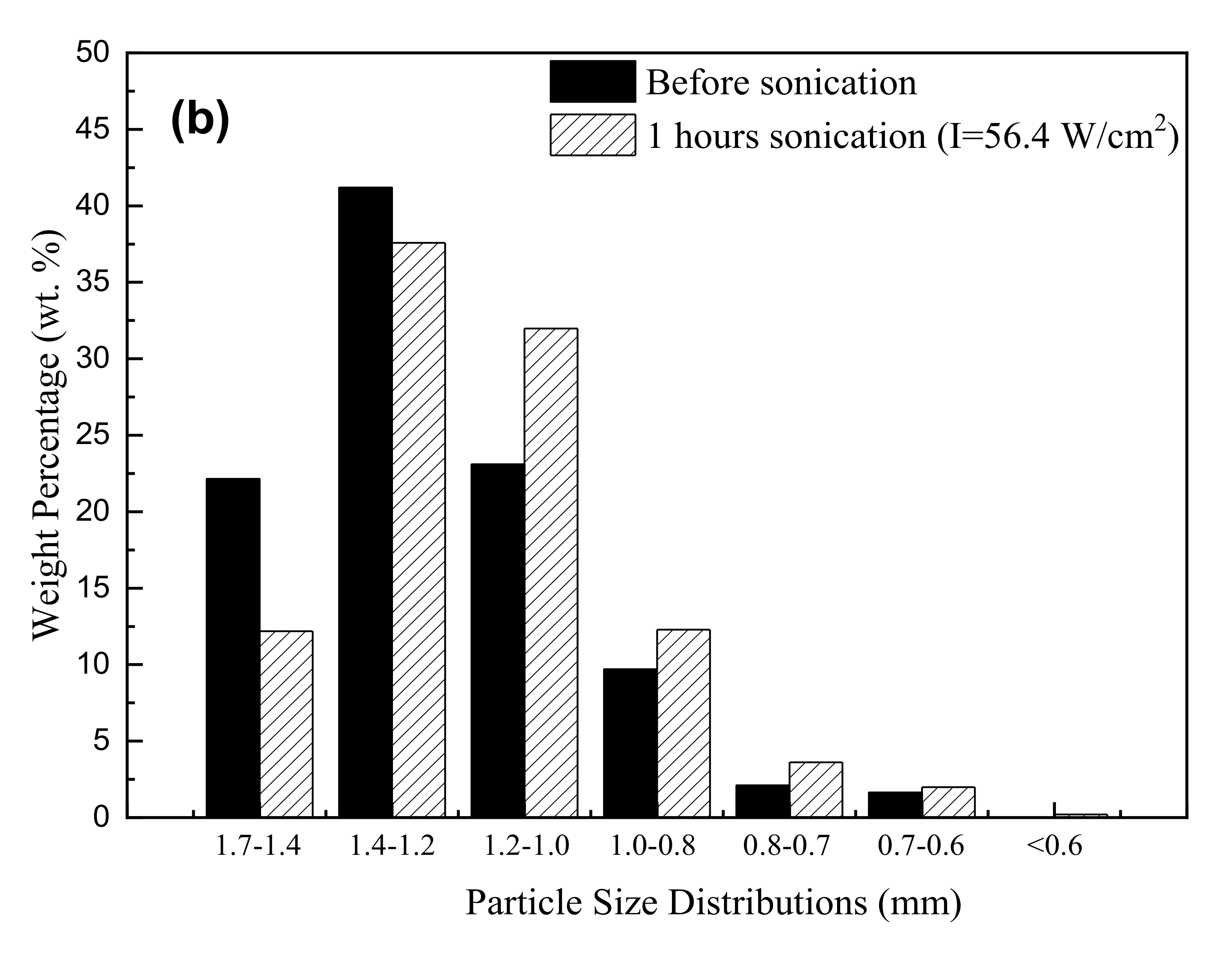
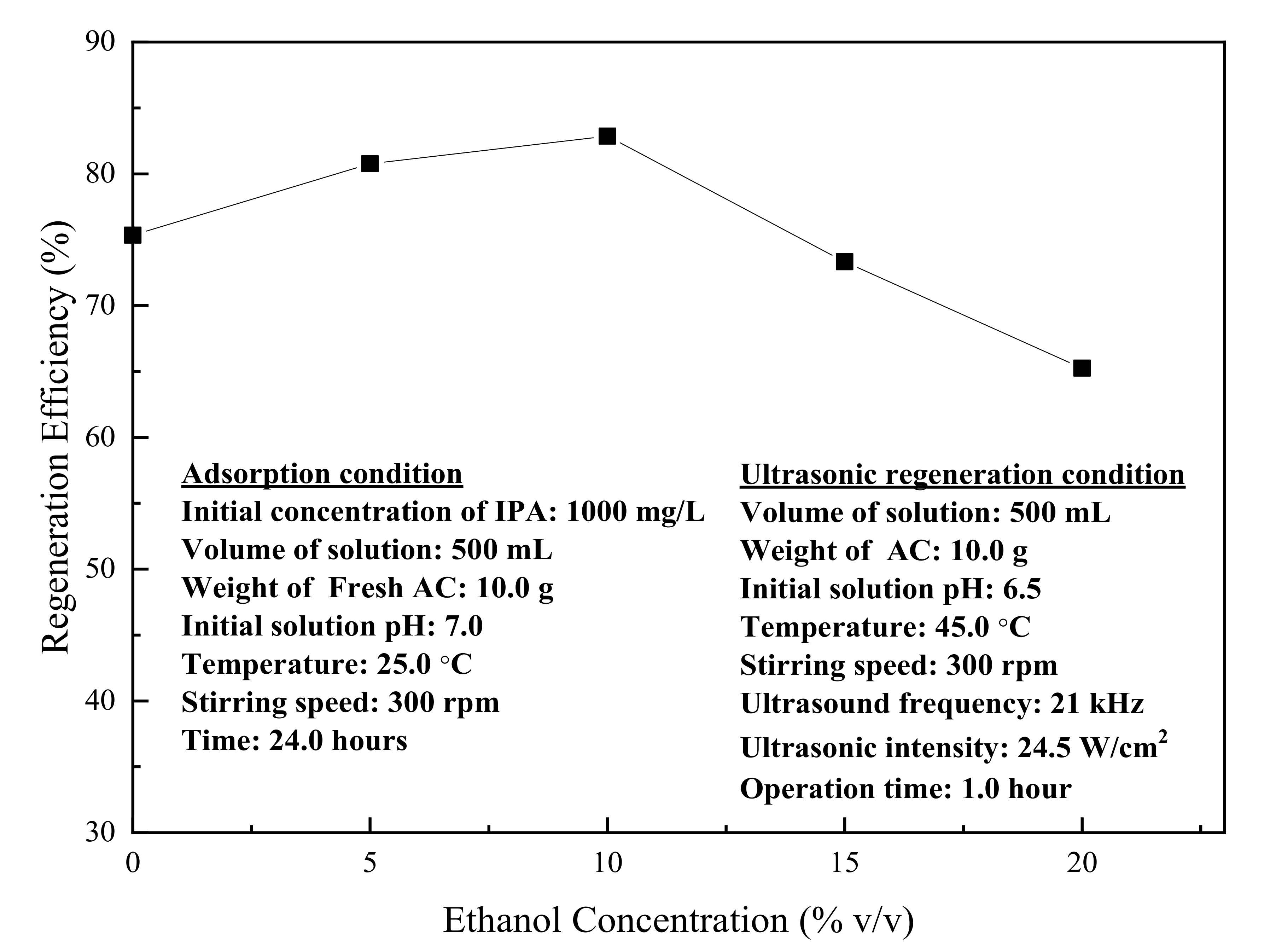
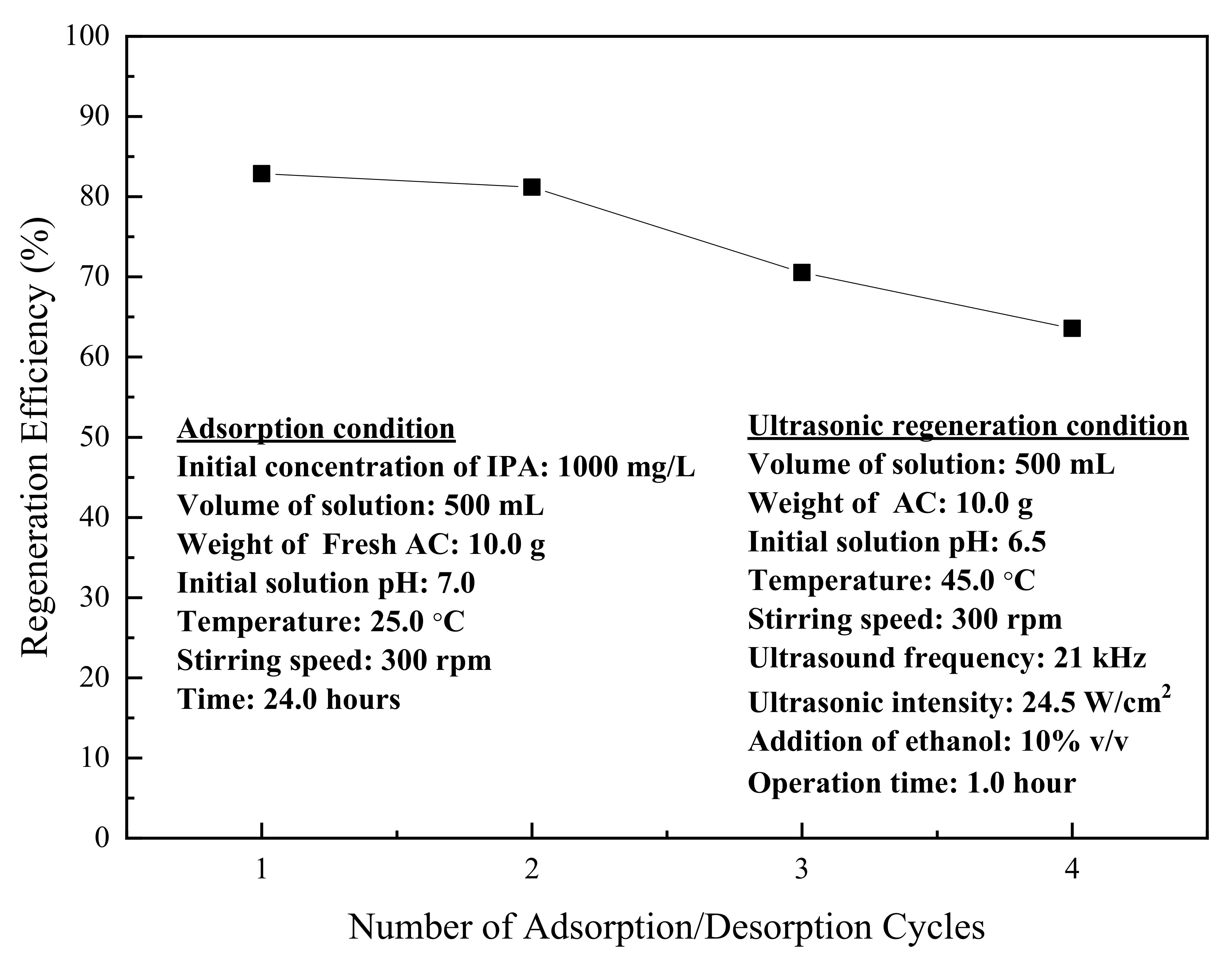
3.3. Ultrasonic Regeneration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McGuire, G.E. Semiconductor Materials and Process Technology Handbook; Noyes Publications: Park Ridge, NJ, USA, 1988. [Google Scholar]
- Wu, J.J.; Yang, J.S.; Muruganandham, M.; Wu, C.C. The Oxidation Study of 2-propanol using Ozone-based Advanced Oxidation Processes. Sep. Purif. Technol. 2008, 62, 39–46. [Google Scholar] [CrossRef]
- Sudaryanto, Y.; Hartono, S.B.; Irawaty, W.; Hindarso, H.; Ismadji, S. High Surface Area Activated Carbon Prepared from Cassava Peel by Chemical Activation. Bioresour. Technol. 2009, 97, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Marketandmarket. Activated Carbon Market by Type (Powdered, Granular, Others (Pelletized, Bead)), Application (Liquid Phase (Water Treatment, Foods & Beverages, Pharmaceutical & Medical), Gaseous Phase (Industrial, Automotive)), Region—Global Forecast to 2021. 2017. Available online: https://www.marketsandmarkets.com/Market-Reports/activated-carbon-362.html (accessed on 23 October 2020).
- Arena, N.; Lee, J.; Clift, R. Life Cycle Assessment of Activated Carbon Production from Coconut Shells. J. Clean. Prod. 2016, 125, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Zanella, O.; Tssaro, I.C.; Féris, L.A. Desorption-and Decomposition-Based Techniques for the Regeneration of Activated Carbon. Chem. Eng. Technol. 2014, 37, 1447–1459. [Google Scholar] [CrossRef]
- Álvarez, P.M.; Beltrán, F.J.; Gómez-Serrano, V.; Jaramillo, J.; Rodríguez, E.M. Comparison between Thermal and Ozone Regenerations of Spent Activated Carbon Exhausted with Phenol. Water Res. 2004, 38, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, Ö.; Cecen, F. Effect of Activation Type on Bioregeneration of Various Activated Carbons Loaded with Phenol. Chem. Technol. Biotechnol. 2006, 81, 1081–1092. [Google Scholar] [CrossRef]
- Zhang, H.P.; Ye, L.Y.; Zhong, H. Regeneration of Phenol-saturated Activated Carbon in an Electrochemical Reactor. Chem. Technol. Biotechnol. 2002, 77, 1246–1250. [Google Scholar] [CrossRef]
- Berenguer, R.; Marco-Lozar, J.P.; Quijada, C.; Cazorla-Amorós, D.; Morallón, E. Electrochemical Regeneration and Porosity Recovery of Phenol-saturated Granular Activated Carbon in an Alkaline Medium. Carbon 2010, 48, 2734–2745. [Google Scholar] [CrossRef]
- Liu, S.X.; Sun, C.L.; Zhang, S.R. Photocatalytic Regeneration of Exhausted Activated Carbon Saturated with Phenol. Bull. Environ. Contam. Toxicol. 2004, 73, 1017–1024. [Google Scholar] [CrossRef]
- Juang, R.S.; Lin, S.H.; Cheng, C.H. Liquid-phase Adsorption and Desorption of Phenol onto Activated Carbons with Ultrasound. Ultrason. Sonochem. 2006, 13, 251–260. [Google Scholar] [CrossRef]
- Hao, H.W.; Wu, M.S.; Chen, Y.F.; Yin, Y.W.; Lu, Z.L. Cavitation-Induced Pyrolysis of Toxic Chlorophenol by High-Frequency Ultrasonic Irradiation. Environ. Toxicol. 2003, 18, 413–417. [Google Scholar] [CrossRef]
- Teo, K.C.; Xu, Y.R.; Yang, C. Sonochemical Degradation for Toxic Halogenated Organic Compounds. Ultrason. Sonochem. 2001, 8, 241–246. [Google Scholar] [CrossRef]
- Lim, J.L.; Okada, M. Regeneration of Granular Activated Carbon Using Ultrasound. Ultrason. Sonochem. 2005, 12, 277–282. [Google Scholar] [CrossRef]
- Jing, G.H.; Zhou, Z.M.; Song, L.; Dong, M.X. Ultrasound Enhanced Adsorption and Desorption of Chromium (VI) on Activated Carbon and Polymeric Resin. Desalination. 2011, 279, 423–427. [Google Scholar] [CrossRef]
- Ince, N.H.; Tezcanli, G.; Belen, R.K.; Apikyan, I.G. Ultrasound as a Catalyzer of Aqueous Reaction Systems: The State of the Art and Environmental Applications. Appl. Catal. B-Environ. 2001, 29, 167–176. [Google Scholar] [CrossRef]
- Breitbach, M.; Bathen, D. Influence of Ultrasound on Adsorption Processes. Ultrason. Sonochem. 2001, 8, 277–283. [Google Scholar] [CrossRef]
- Adewuyi, Y.G. Sonochemistry: Environmental Science and Engineering Applications. Ind. Eng. Chem. Res. 2001, 40, 4681–4715. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Djeribi, R.; Naffrechoux, E. Desorption of Metal Ions from Activated Carbon in the Presence of Ulreasound. Ind. Eng. Chem. Res. 2005, 44, 4737–4744. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E.; Suptil, J.; Fachinger, C. Ultrasonic Desorption of p-Chlorophenol from Granular Activated Carbon. Chem. Eng. J. 2005, 106, 153–161. [Google Scholar] [CrossRef]
- Saoudi, F.; Hamdaoui, O. Innovative Technique for 4-chlorophenol Desorption from Granular Activated Carbon by Low Frequency Ultrasound: Influence of Operational Parameters. Micropor. Mesopor. Mater. 2011, 141, 69–76. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E.; Tifouti, L.; Pétrier, C. Effects of Ultrasound on Adsorption-Desorption of P-chlorophenol on Granular Activated Carbon. Ultrason. Sonochem. 2003, 10, 109–114. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the Characterization of Acidic and Basic Surface Sites on Carbons by Various Techniques. Carbon 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated Carbon Surface Modifications by Adsorption of Bacteria and Their Effect on Aqueous Lead Adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.S.; Zheng, T.; Wang, P.; Jiang, J.P.; Li, N. Adsorption Isotherm, Kinetic and Mechanism Studies of Some Substituted Phenols on Activated Carbon Fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, B.; Lin, Y.; Guan, Y. Aromatic and Hydrophobic Surfaces of Wood-derived Biochar Enhance Perchlorate Adsorption via Hydrogen Bonding to Oxygen-containing Organic Groups. Environ. Sci. Technol. 2014, 48, 279–288. [Google Scholar] [CrossRef]
- Horn, R.S.; Tseng, I.C. Regeneration of Granular Activated Carbon Saturated with Acetone and Isopropyl Alcohol Via a Recirculation Process Under H2O2/UV Oxidation. J. Hazard. Mater. 2008, 154, 366–372. [Google Scholar] [CrossRef]
- Lin, S.H.; Wang, C.S. Recovery of Isopropyl Alcohol from Waste Solvent of a Semiconductor Plant. J. Hazard. Mater. 2004, 106B, 161–168. [Google Scholar] [CrossRef]
- Liu, C.; Sun, Y.; Wang, D.; Sun, Z.; Chen, M.; Zhou, Z.; Chen, W. Performance and Mechanism of Low-frequency Ultrasound to Regenerate the Biological Activated Carbon. Ultrason. Sonochem. 2017, 34, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Hamdaoui, O.; Naffrechoux, E. An Investigation of the Mechanisms of Ultrasonically Enhanced Desorption. AIChE J. 2007, 53, 363–373. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T.; Cain, C.A. Desorption by Ultrasound: Phenol on Activated Carbon and Polymeric Resin. AIChE J. 1998, 44, 1519–1527. [Google Scholar] [CrossRef] [Green Version]
- Hinoue, M.; Ishimatsu, S.; Fueta, Y.; Hori, H. A New Desorption Method for Removing Organic Solvents from Activated Carbon using Surfactant. J. Occup. Health 2017, 59, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Type of Activated Carbon | Element Content (wt. %) | ||||||
|---|---|---|---|---|---|---|---|
| C | O | Mg | P | Si | K | Cl | |
| Original Activated Carbon | 89.60 ± 1.66 | 9.26 ± 2.23 | 0.18 ± 0.17 | 0.24 ± 0.25 | 0.24 ± 0.24 | 0.34 ± 0.22 | 0.14 ± 0.12 |
| Fresh Activated Carbon | 92.80 ± 1.15 | 6.40 ± 0.90 | - a | - a | 0.08 ± 0.14 | 0.56 ± 0.50 | 0.08 ± 0.14 |
| Activated Carbon | Micropore Volume (cm3/g) | BET Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Mean Pore Diameter (nm) |
|---|---|---|---|---|
| Original Activated Carbon | 243.06 ± 0.36 | 1057.9 ± 1.59 | 0.423 ± 0.001 | 1.6 ± 0.002 |
| Fresh Activated Carbon | 250.57 ± 0.38 | 1090.6 ± 1.64 | 0.435 ± 0.001 | 1.6 ± 0.002 |
| Activated Carbon after First Regeneration Cycle | 227.65 ± 0.34 | 990.9 ± 1.49 | 0.395 ± 0.001 | 1.6 ± 0.002 |
| Activated Carbon after Second Regeneration Cycle | 227.88 ± 0.34 | 991.9 ± 1.49 | 0.394 ± 0.001 | 1.6 ± 0.002 |
| Activated Carbon after Third Regeneration Cycle | 223.86 ± 0.34 | 974.4 ± 1.46 | 0.385 ± 0.001 | 1.6 ± 0.002 |
| Activated Carbon after Fourth Regeneration Cycle | 217.93 ± 0.32 | 948.5 ± 1.42 | 0.377 ± 0.001 | 1.6 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, H.-Y.; Moed, N.M.; Ku, Y.; Lee, H.-Y. Ultrasonic Regeneration Studies on Activated Carbon Loaded with Isopropyl Alcohol. Appl. Sci. 2020, 10, 7596. https://doi.org/10.3390/app10217596
Hong H-Y, Moed NM, Ku Y, Lee H-Y. Ultrasonic Regeneration Studies on Activated Carbon Loaded with Isopropyl Alcohol. Applied Sciences. 2020; 10(21):7596. https://doi.org/10.3390/app10217596
Chicago/Turabian StyleHong, Hsuan-Yi, Niels Michiel Moed, Young Ku, and Hao-Yeh Lee. 2020. "Ultrasonic Regeneration Studies on Activated Carbon Loaded with Isopropyl Alcohol" Applied Sciences 10, no. 21: 7596. https://doi.org/10.3390/app10217596
APA StyleHong, H.-Y., Moed, N. M., Ku, Y., & Lee, H.-Y. (2020). Ultrasonic Regeneration Studies on Activated Carbon Loaded with Isopropyl Alcohol. Applied Sciences, 10(21), 7596. https://doi.org/10.3390/app10217596







