Effect of Acetone Content on the Preparation Period and Curing/Pyrolysis Behavior of Liquid Polycarbosilane
Abstract
1. Introduction
2. Experimental Procedures
2.1. Raw Materials
2.2. Materials Preparation
2.3. Characterization
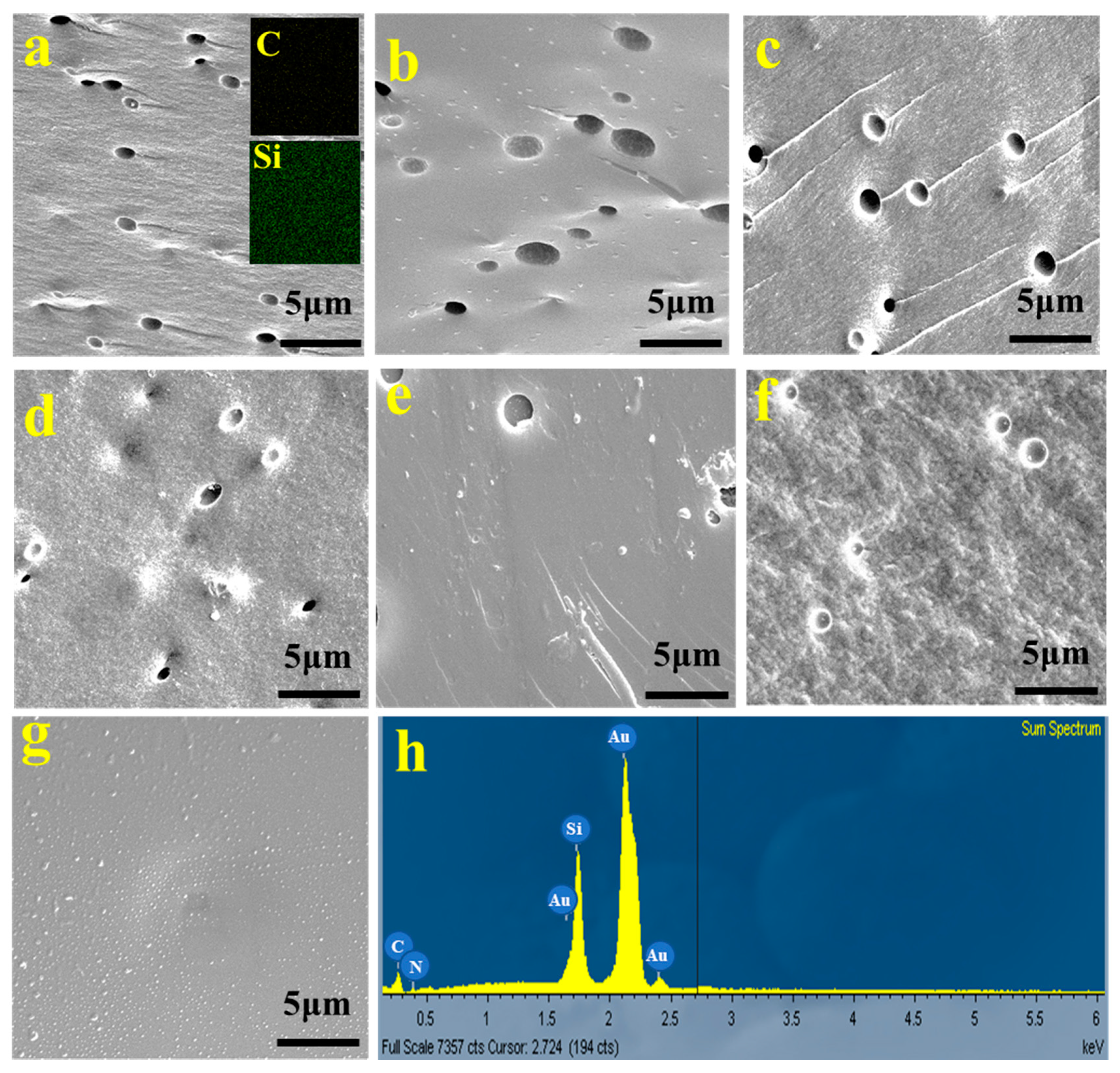
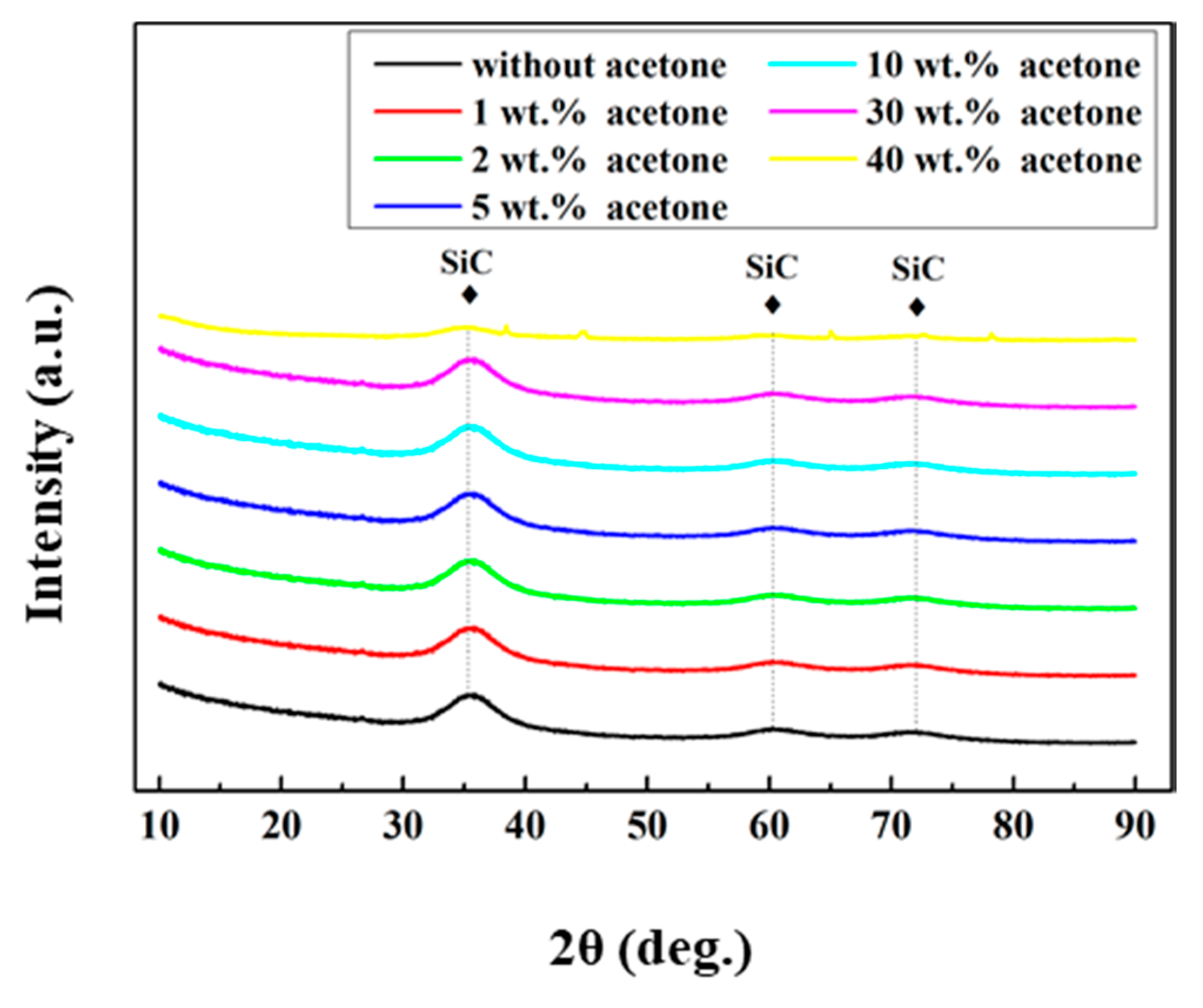
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fan, S.W.; Sun, H.D.; Ma, X.; Deng, J.L.; Yang, C.; Cheng, L.F.; Zhang, L.T. Microstructure and properties of a new structure-function integrated C/C-SiC brake material. J. Alloys Comp. 2018, 769, 239–249. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Cheng, L.F.; Zhang, H.J.; Yin, X.W.; Yin, H.F.; Yan, G.Z. Effects of graphite fillers on the thermophysical properties of 3D C/SiC composites. J. Alloys Comp. 2019, 770, 989–994. [Google Scholar] [CrossRef]
- Omrani, E.; Barari, B.; Moghadam, A.D.; Rohatgi, P.K.; Pillai, K.M. Mechanical and tribo-logical properties of self-lubricating bio-based carbon fabric epoxy composites made using liquid composite molding. Tribol. Int. 2015, 92, 222–232. [Google Scholar] [CrossRef]
- Fan, S.W.; Zhang, L.T.; Cheng, L.F.; Tian, G.L.; Yang, S.J. Effect of braking pressure and braking speed on the tribological properties of C/SiC aircraft brake materials. Compos. Sci. Technol. 2010, 70, 959–965. [Google Scholar] [CrossRef]
- Lv, X.Y.; Ye, F.; Cheng, L.F.; Fan, S.W.; Liu, Y.S. Fabrication of SiC whisker-reinforced SiC ceramic matrix composites based on 3D printing and chemical vapor infiltration technology. J. Eur. Ceram. Soc. 2019, 39, 3380–3386. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, D.Y.; Dong, S.; Qiang, Q.; Zhang, X.H. A novel vibration-assisted slurry impregnation to fabricate Cf/ZrB2-SiC composite with enhanced mechanical properties. J. Eur. Ceram. Soc. 2019, 39, 798–805. [Google Scholar] [CrossRef]
- Baker, B.; Rubio, V.; Ramanujam, P.; Binner, J.; Hussain, A.; Ackerman, T.; Brown, P.; Dautremont, I. Development of a slurry injection technique for continuous fiber ultra-high temperature ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 3927–3937. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, H.; Lu, X. Fabrication and properties of Cf/SiC porous ceramics by grinding-mould pressing-sintering process. J. Eur. Ceram. Soc. 2019, 39, 1775–1780. [Google Scholar] [CrossRef]
- Hu, C.L.; Tang, S.F.; Pang, S.Y.; Cheng, H.M. Long-term oxidation behaviors of C/SiC composites with a SiC/UHTC/SiC three-layer coating in a wide temperature range. Corros. Sci. 2019, 147, 1–8. [Google Scholar] [CrossRef]
- Ma, X.K.; Yin, X.W.; Fan, X.M.; Cao, X.Y.; Yang, L.W.; Sun, X.N.; Cheng, L.F. Improved tensile strength and toughness of dense Cf/SiC-SiBC with tailored PyC interphase. J. Eur. Ceram. Soc. 2019, 39, 1766–1774. [Google Scholar] [CrossRef]
- Vinci, A.; Zoli, L.; Sciti, D. Influence of fiber content on the strength of carbon fiber reinforced HfCf/SiC composites up to 2100 °C. J. Eur. Ceram. Soc. 2019, 39, 3594–3603. [Google Scholar] [CrossRef]
- Kashyap, S.K.; Mitra, R. Effect of LaB6 additions on densification, microstructure, and creep with oxide scale formation in ZrB2-SiC composites sintered by spark plasma sintering. J. Eur. Ceram. Soc. 2019, 39, 2782–2793. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Hu, P.; Dong, S.; Liu, X.; Wang, C.L.; Nan, Z.Z.; Zhang, X.H. Oxidation behavior and ablation mechanism of Cf/ZrB2-SiC composite fabricated by vibration-assisted slurry impregnation combined with low-temperature hot pressing. Corros. Sci. 2019, 161, 108181. [Google Scholar] [CrossRef]
- Rubio, V.; Binner, J.; Cousinet, S.; Page, G.L.; Ackerman, T.; Hussain, A.; Brown, P.; Dautremont, I. Materials characterization and mechanical properties of Cf-UHTC powder composites. J. Eur. Ceram. Soc. 2019, 39, 813–824. [Google Scholar] [CrossRef]
- Yan, C.L.; Liu, R.J.; Cao, Y.B.; Zhang, C.R. Fabrication and properties of PIP 3D Cf/ZrC-SiC composites. Mater. Sci. Eng. A 2014, 591, 105–110. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, F.; Duan, S.C.; Zhou, W.C.; Zhu, D.M. Mechanical and dielectric properties of SiCf/SiC composites fabricated by PIP combined with CIP process. Ceram. Int. 2016, 42, 6800–6806. [Google Scholar] [CrossRef]
- Garcia, B.W. Preceramic Resin Formulations, Ceramic Materials Comprising the Preceramic Resin Formulations, and Related Articles and Methods. U.S. Patent Application No. 10,731,036, 4 August 2020. [Google Scholar]
- Xiong, H.W.; Chen, H.H.; Zhao, L.Z.; Huang, Y.J.; Zhou, K.C.; Zhang, D. SiCw/SiCp reinforced 3D-SiC ceramics using direct ink writing of polycarbosilane-based solution: Microstructure, composition and mechanical properties. J. Eur. Ceram. Soc. 2019, 39, 2648–2657. [Google Scholar] [CrossRef]
- He, J.B.; Gao, Y.; Wang, Y.G.; Fang, J.Y.; An, L.N. Synthesis of ZrB2-SiC nanocomposite powder via polymeric precursor route. Ceram. Int. 2017, 43, 1602–1607. [Google Scholar] [CrossRef]
- Zheng, G.B.; Sano, H.; Uchiyama, Y.; Kobayashi, K.; Cheng, H.M. Effect of boron addition on oxidation resistance of carbon fiber polycarbosilane-derived SiC composites. J. Mater. Sci. Lett. 1998, 17, 2047–2049. [Google Scholar] [CrossRef]
- Jian, K.; Chen, Z.H.; Ma, Q.S.; Hu, H.F.; Zheng, W.W. Effects of polycarbosilane infiltration processes on the microstructure and mechanical properties of 3D-Cf/SiC composites. Ceram. Int. 2007, 33, 905–909. [Google Scholar] [CrossRef]
- Bae, J.C.; Cho, K.Y.; Yoon, D.H.; Baek, S.S.; Park, J.K.; Kim, J.L.; Im, D.W.; Riu, D.H. Highly efficient densification of carbon fiber-reinforced SiC-matrix composites by melting infiltration and pyrolysis using polycarbosilane. Ceram. Int. 2013, 39, 5623–5629. [Google Scholar] [CrossRef]
- Gupta, R.K.; Mishra, R.; Tiwari, R.K.; Ranjan, A.; Saxena, A.K. Studies on the rheological behavior of polycarbosilane part I: Effect of time, temperature and atmosphere. Silicon 2011, 3, 27–35. [Google Scholar] [CrossRef]
- Tian, Q.; Wu, N.; Wang, B.; Wang, Y.D. Fabrication of hollow SiC ultrafine fibers by single-nozzle electrospinning for high-temperature thermal insulation application. Mater. Lett. 2019, 239, 109–112. [Google Scholar] [CrossRef]
- Zhou, H.J.; Yang, J.S.; Le, G.; Ni, D.W.; Wang, H.D.; Dong, S.M. Effect of ZrC amount and distribution on the thermomechanical properties of Cf/SiC-ZrC composites. Int. J. Appl. Ceram. Technol. 2019, 16, 1321–1328. [Google Scholar] [CrossRef]
- Usevičiūtė, L.; Baltrėnaitė, E. Methods for determining lignocellulosic biochar wettability. Waste Biomass Valorization 2019, 11, 4457–4468. [Google Scholar] [CrossRef]
- Bouillon, E.; Langlais, F.; Pailler, R.; Naslain, R.; Cruege, F.; Huong, P.V.; Sarthou, J.C.; Delpuech, A.; Laffon, C.; Lagarde, P. Conversion mechanisms of a polycarbosilane precursor into a SiC-based ceramic material. J. Mater. Sci. 1991, 26, 1333–1345. [Google Scholar] [CrossRef]
- Fang, Y.H.; Huang, M.H.; Yu, Z.J.; Xia, H.P.; Chen, L.F.; Zhang, Y.; Zhang, L.T. Synthesis, characterization, and pyrolytic conversion of a novel liquid polycarbosilane. J. Am. Ceram. Soc. 2008, 91, 3298–3302. [Google Scholar] [CrossRef]
- Rajinder, P. Shear viscosity behavior of emulsions of two immiscible liquids. J. Colloid Interface Sci. 2000, 225, 359–366. [Google Scholar]
- Eshgarf, H.; Afrand, M. An experimental study on rheological behavior of non-Newtonian hybrid nano-coolant for application in cooling and heating systems. Exp. Therm. Fluid Sci. 2016, 76, 221–227. [Google Scholar] [CrossRef]
- Sterczyńska, A.; Śliwińska-Bartkowiak, M.; Zienkiewicz-Strzałka, M.; Derylo-Marczewska, A. Surface properties of synthesized nanoporous carbon and silica matrices. J. Vis. Exp. 2019, 145. [Google Scholar] [CrossRef]
- Wang, K.; Ding, C.N.; Jiang, S.G.; Wu, Z.Y.; Shao, H.; Zhang, W.Q. Application of the addition of ionic liquids using a complex wetting agent to enhance dust control efficiency during coal mining. Process. Saf. Environ. Prot. 2019, 122, 13–22. [Google Scholar] [CrossRef]
- Zhao, G.J.; Yuan, Z.M.; Yin, J.G.; Ma, S.X. Thermophysical properties of fatty acid methyl and ethyl esters. J. Chem. Thermodyn. 2019, 134, 195–212. [Google Scholar] [CrossRef]
- Kim, K.R.; Choi, S.Y.; Kim, J.G.; Paek, S.; Goh, W.I. Electrochemical investigation of exchange current density of uranium and rare-earths couples (M3/M0) in LiCl-KCl eutectic electrolyte. Int. J. Electrochem. Sci. 2015, 10, 7660–7670. [Google Scholar]
- Ye, X.L.; Chen, Z.F.; Ai, S.F.; Hou, B.; Zhang, J.X.; Zhou, Q.B.; Liu, H.Z.; Cui, S. Effect of thickness of SiC films on compression and thermal properties of SiC/Cfcomposites. Ceram. Int. 2019, 45, 4674–4679. [Google Scholar] [CrossRef]
- Li, L.; Barnett, K.J.; McClelland, D.J.; Zhao, D.T.; Liu, G.Z.; Huber, G.W. Gas-phase dehydration of tetrahydrofurfuryl alcohol to dihydropyran over Υ-Al2O3. Appl. Catal. B 2019, 245, 62–70. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Fang, L.; Xiang, H.C.; Xu, M.Y.; Tang, Y.; Jantunen, H.L.; Li, C.C. Structural, infrared reflectivity spectra and microwave dielectric properties of the Li7Ti3O9F ceramic. Ceram. Int. 2019, 45, 10163–10169. [Google Scholar] [CrossRef]
- Lu, Z.Z.; Cao, J.W.; Bai, S.Z.; Wang, M.Y.; Li, D.C. Microstructure and mechanical properties of TiAl-based composites prepared by stereolithography and gel casting technologies. J. Alloys Compd. 2015, 633, 280–287. [Google Scholar] [CrossRef]
- Ashby, B.A. Addition reaction. U.S.S.R. 1964, 662, 159. [Google Scholar]
- Sangermano, M.; Marchi, S.; Meier, P.; Kornmann, X. UV-activated hydrosilation reaction for silicone polymer crosslinking. J. Appl. Polym. Sci. 2013, 128, 1521–1526. [Google Scholar] [CrossRef]
- Tang, X.Y.; Zhang, L.; Tu, H.B.; Gu, H.; Chen, L.F. Decarbonization mechanisms of polycarbosilane during pyrolysis in hydrogen for preparation of silicon carbide fibers. J. Mater. Sci. 2010, 45, 5749–5755. [Google Scholar] [CrossRef]
- Li, H.B.; Zhang, L.T.; Cheng, L.F.; Wang, Y.G.; Yu, Z.J.; Huang, M.H.; Tu, H.B.; Xia, H.P. Polymer-ceramic conversion of a highly branched liquid polycarbosilane for SiC-based ceramics. J. Mater. Sci. 2008, 43, 2806–2811. [Google Scholar] [CrossRef]
- Guo, W.J.; Bai, S.X.; Ye, Y.C.; Zhu, L.; Li, S. C/C-SiC composite fabricated via PCS-Si slurry reactive melt infiltration. Int. J. Appl. Ceram. Technol. 2019, 16, 88–96. [Google Scholar] [CrossRef]
- Hoffman, R.L. Explanations for the cause of shear thickening in concentrated colloidal suspensions. J. Rheol. 1998, 42, 111–123. [Google Scholar] [CrossRef]
- Hurwitz, F.I. Filler/Polycarbosilane Systems as CMC Matrix Precursors; The American Ceramic Society: Westerville, OH, USA, 1998. [Google Scholar]







| Acetone Introduction | PCS | PCS with 5% Acetone | PCS with 30% Acetone | PCS after 30% Acetone Volatilization |
|---|---|---|---|---|
| Number average molecular weight | 2093 | 2148 | 2056 | 2297 |
| Weight average molecular weight | 3409 | 3367 | 3301 | 3799 |
| PDI (molecular weight divergence) | 1.63 | 1.57 | 1.6 | 1.65 |
| Molecular weight >10000 | 3% | 3% | 3% | 5% |
| 5000~10000 | 17% | 17% | 16% | 19% |
| 3000~5000 | 20% | 20% | 21% | 20% |
| 1500~3000 | 33% | 34% | 33% | 33% |
| 0~1500 | 27% | 26% | 28% | 23% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, X.; Hu, P. Effect of Acetone Content on the Preparation Period and Curing/Pyrolysis Behavior of Liquid Polycarbosilane. Appl. Sci. 2020, 10, 7607. https://doi.org/10.3390/app10217607
Liu Y, Liu X, Hu P. Effect of Acetone Content on the Preparation Period and Curing/Pyrolysis Behavior of Liquid Polycarbosilane. Applied Sciences. 2020; 10(21):7607. https://doi.org/10.3390/app10217607
Chicago/Turabian StyleLiu, Yizhi, Xu Liu, and Ping Hu. 2020. "Effect of Acetone Content on the Preparation Period and Curing/Pyrolysis Behavior of Liquid Polycarbosilane" Applied Sciences 10, no. 21: 7607. https://doi.org/10.3390/app10217607
APA StyleLiu, Y., Liu, X., & Hu, P. (2020). Effect of Acetone Content on the Preparation Period and Curing/Pyrolysis Behavior of Liquid Polycarbosilane. Applied Sciences, 10(21), 7607. https://doi.org/10.3390/app10217607





