Rabbit as an Aging Model in Reproduction: Advanced Maternal Age Alters GLO1 Expression in the Endometrium at the Time of Implantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Treatment and Recovery of Reproductive Tissues and Preimplantation Embryos
2.2. RNA Isolation and cDNA Synthesis of Reproductive Tissues
2.3. RNA Isolation and cDNA Synthesis of Single Blastocysts
2.4. Measurement of mRNA Levels by Quantitative PCR
2.5. Protein Preparation and Immunoblotting
2.6. GLO1 Enzymatic Assay
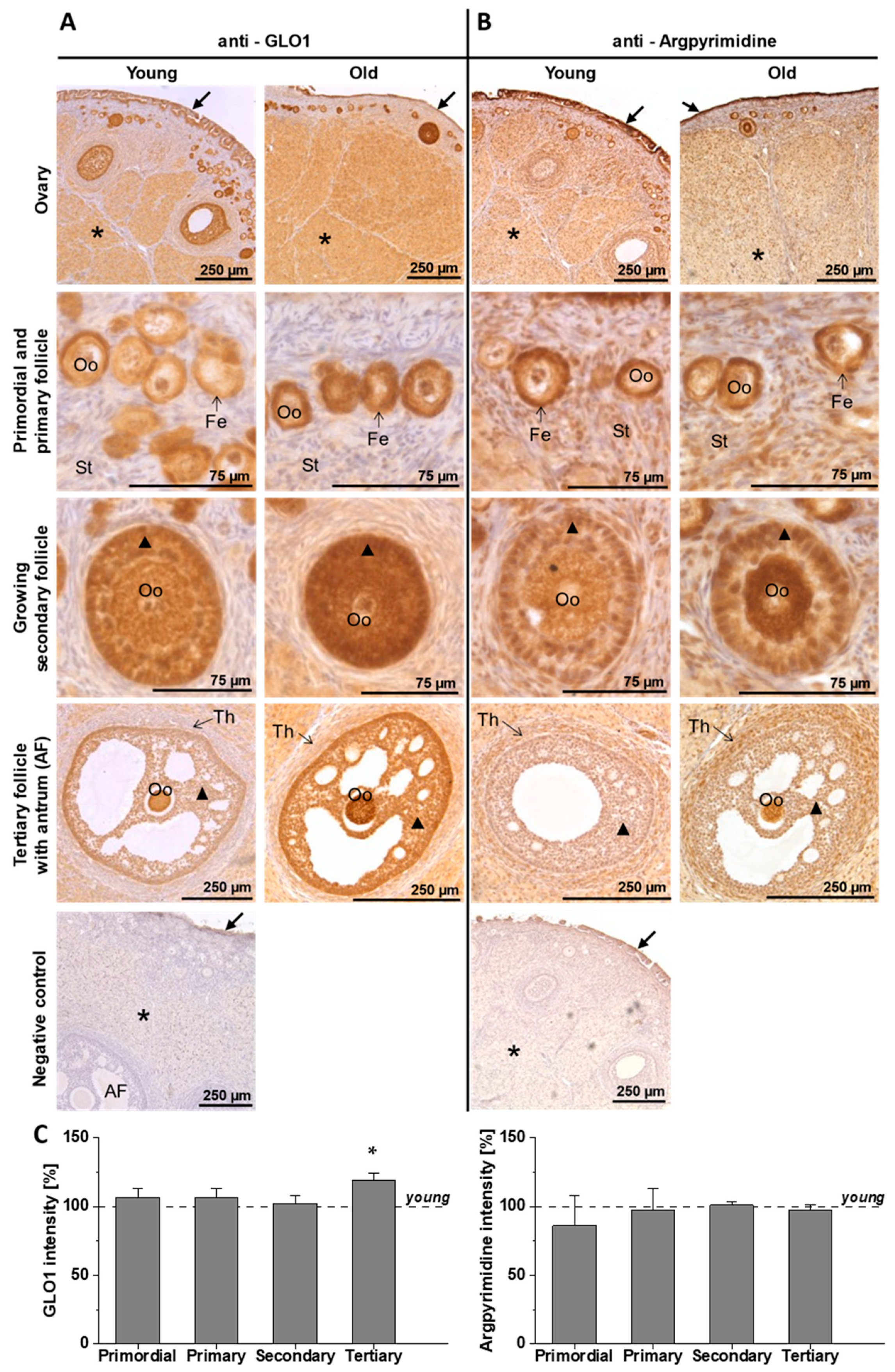
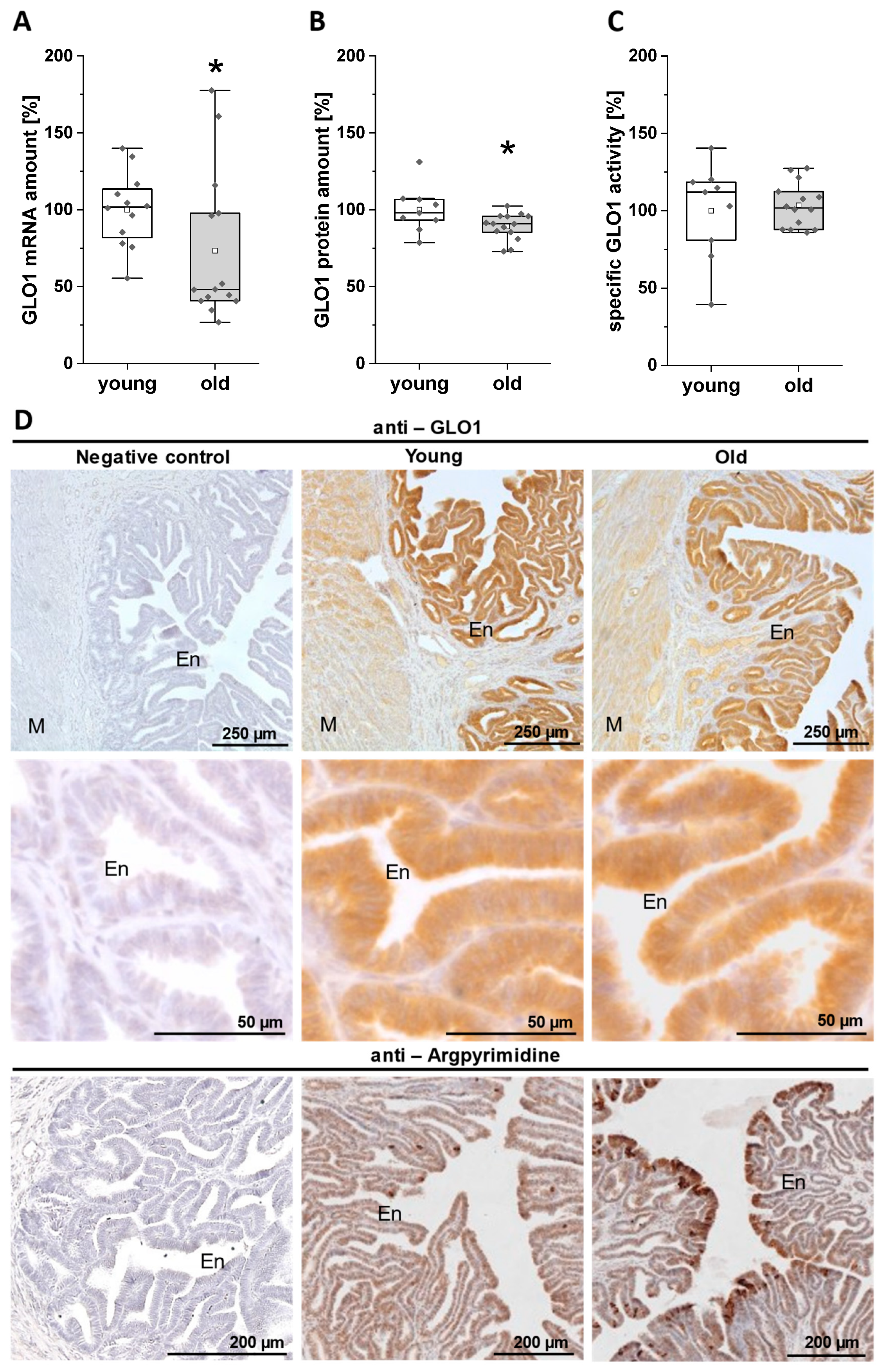
2.7. Immunohistochemical Localisation of GLO1 and Argpyrimidine in the Reproductive Tract Organs
2.8. Statistics
3. Results
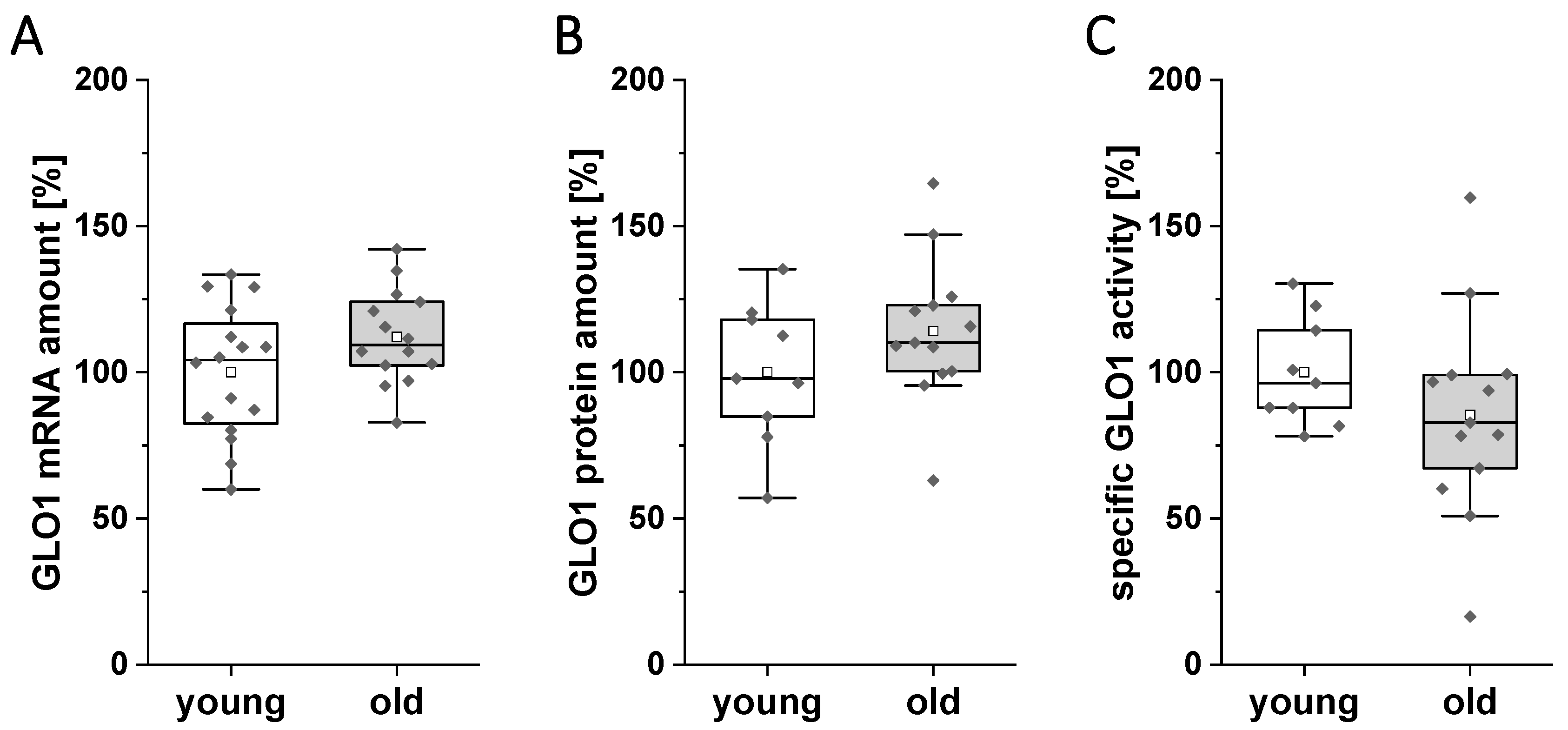
3.1. Expression and Activity of GLO1 in the Ovary of Young and Old Rabbits on Day 6 of Pregnancy
3.2. Expression and Activity of GLO1 in the Oviduct of Young and Old Rabbits on Day 6 of Pregnancy
3.3. Expression and Activity of GLO1 in the Uterus of Young and Old Rabbits on Day 6 of Pregnancy
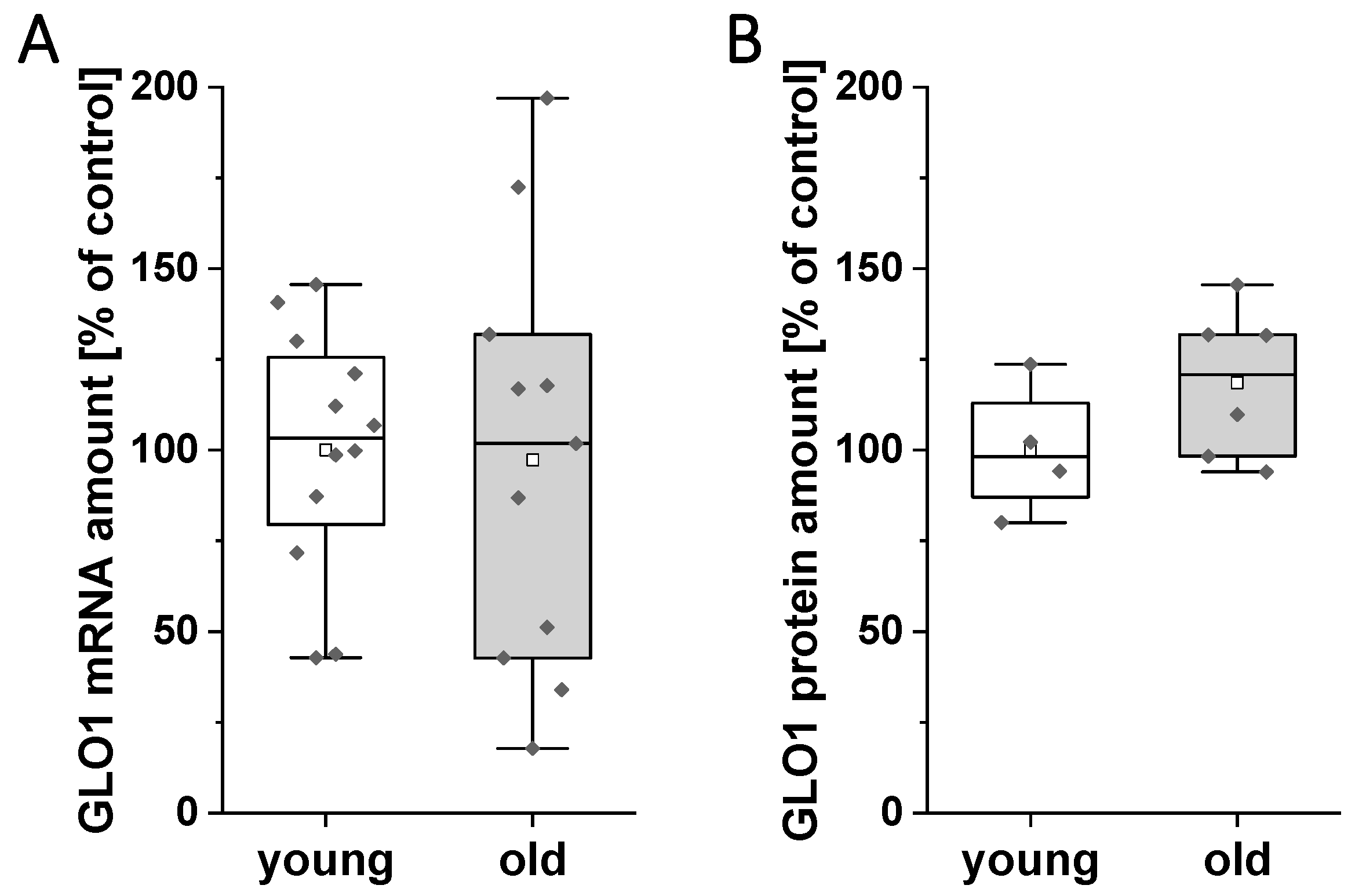
3.4. mRNA and Protein Amount of GLO1 in Blastocysts of Young and Old Rabbits on Day 6 of Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, L.; Sauve, R.; Birkett, N.; Fergusson, D.; van Walraven, C. Maternal age and risk of stillbirth: A systematic review. CMAJ 2008, 178, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathews, T.J.; Hamilton, B.E. Mean Age of Mothers is on the Rise: United States, 2000–2014. NCHS Data Brief 2016, 232, 1–8. [Google Scholar]
- OECD Family Database. The Structure of Families 2.3: Age of Mothers at Childbirth and Age-Specific Fertility. 2019. Available online: http://www.oecd.org/social/family/database.htm (accessed on 18 September 2020).
- Eurostat. Total Fertility Rate and Age of Women at Birth of First Child. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Fertility_statistics (accessed on 23 September 2020).
- Maurer, R.R.; Foote, R.H. Maternal ageing and embryonic mortality in the rabbit. I. Repeated superovulation, embryo culture and transfer. J. Reprod. Fertil. 1971, 25, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.B.; Charleston, J.S.; Battaglia, D.E.; Klein, N.A.; Soules, M.R. Morphometric analysis of primordial follicle number in pigtailed monkey ovaries: Symmetry and relationship with age. Biol. Reprod. 1999, 61, 553–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, K.P.; Walker, L.C.; Anderson, D.; Lacreuse, A.; Robson, S.L.; Hawkes, K. Depletion of ovarian follicles with age in chimpanzees: Similarities to humans. Biol. Reprod. 2007, 77, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.; Sebire, N.; Harris, J.; Robinson, S.; Regan, L. The risks associated with pregnancy in women aged 35 years or older. Hum. Reprod. 2000, 15, 2433–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, K.S.; Allen, A.C.; Dodds, L.; Turner, L.A.; Scott, H.; Liston, R. The perinatal effects of delayed childbearing. Obstet. Gynecol. 2005, 105, 1410–1418. [Google Scholar] [CrossRef]
- Kenny, L.C.; Lavender, T.; McNamee, R.; O’Neill, S.M.; Mills, T.; Khashan, A.S. Advanced maternal age and adverse pregnancy outcome: Evidence from a large contemporary cohort. PLoS ONE 2013, 8, e56583. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.C.; Jeffers, S.; Carter, J.; Duthely, L.; Cotter, A.; González-Quintero, V.H. Pregnancy at or beyond age 40 years is associated with an increased risk of fetal death and other adverse outcomes. Am. J. Obstet. Gynecol. 2007, 196, e11–e13. [Google Scholar] [CrossRef]
- Tatone, C. Oocyte senescence:A firm link to age-related female subfertility. Gynecol. Endocrinol. 2008, 24, 59–63. [Google Scholar] [CrossRef]
- Tatone, C.; Amicarelli, F.; Carbone, M.C.; Monteleone, P.; Caserta, D.; Marci, R.; Artini, P.G.; Piomboni, P.; Focarelli, R. Cellular and molecular aspects of ovarian follicle ageing. Hum. Reprod. Update 2008, 14, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Byun, K.; Yoo, Y.; Son, M.; Lee, J.; Jeong, G.-B.; Park, Y.M.; Salekdeh, G.H.; Lee, B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017, 177, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Bellier, J.; Nokin, M.-J.; Lardé, E.; Karoyan, P.; Peulen, O.; Castronovo, V.; Bellahcène, A. Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. Diabetes Res. Clin. Pract. 2019, 148, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Muronetz, V.I.; Melnikova, A.K.; Seferbekova, Z.N.; Barinova, K.V.; Schmalhausen, E.V. Glycation, Glycolysis, and Neurodegenerative Diseases: Is There Any Connection? Biochem. Mosc. 2017, 82, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Mano, J. Reactive carbonyl species: Their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol. Biochem. 2012, 59, 90–97. [Google Scholar] [CrossRef]
- Thornalley, P.J. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems--role in ageing and disease. Drug Metabol. Drug Interact. 2008, 23, 125–150. [Google Scholar] [CrossRef]
- Peppa, M.; Uribarri, J.; Vlassara, H. Aging and glycoxidant stress. Hormones 2008, 7, 123–132. [Google Scholar] [CrossRef]
- Shipanova, I.N.; Glomb, M.A.; Nagaraj, R.H. Protein modification by methylglyoxal: Chemical nature and synthetic mechanism of a major fluorescent adduct. Arch. Biochem. Biophys. 1997, 344, 29–36. [Google Scholar] [CrossRef]
- Thornalley, P.J. Glyoxalase I—Structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef]
- Kuhla, B.; Boeck, K.; Lüth, H.-J.; Schmidt, A.; Weigle, B.; Schmitz, M.; Ogunlade, V.; Münch, G.; Arendt, T. Age-dependent changes of glyoxalase I expression in human brain. Neurobiol. Aging 2006, 27, 815–822. [Google Scholar] [CrossRef]
- Shinohara, M.; Thornalley, P.J.; Giardino, I.; Beisswenger, P.; Thorpe, S.R.; Onorato, J.; Brownlee, M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J. Clin. Investig. 1998, 101, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. The glyoxalase system in health and disease. Mol. Asp. Med. 1993, 14, 287–371. [Google Scholar] [CrossRef]
- Tatone, C.; Carbone, M.C.; Campanella, G.; Festuccia, C.; Artini, P.G.; Talesa, V.; Focarelli, R.; Amicarelli, F. Female reproductive dysfunction during ageing: Role of methylglyoxal in the formation of advanced glycation endproducts in ovaries of reproductively-aged mice. J. Biol. Regul. Homeost. Agents 2010, 24, 63–72. [Google Scholar] [PubMed]
- Merhi, Z. Advanced glycation end products and their relevance in female reproduction. Hum. Reprod. 2014, 29, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Sauer, M.V. Reproduction at an advanced maternal age and maternal health. Fertil. Steril. 2015, 103, 1136–1143. [Google Scholar] [CrossRef]
- Haucke, E.; Navarrete Santos, A.; Simm, A.; Henning, C.; Glomb, M.A.; Gürke, J.; Schindler, M.; Fischer, B.; Navarrete Santos, A. Accumulation of advanced glycation end products in the rabbit blastocyst under maternal diabetes. Reproduction 2014, 148, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Seeling, T.; Haucke, E.; Navarrete Santos, A.; Grybel, K.J.; Gürke, J.; Pendzialek, S.M.; Schindler, M.; Simm, A.; Navarrete Santos, A. Glyoxalase 1 expression is downregulated in preimplantation blastocysts of diabetic rabbits. Reprod. Domest. Anim. 2019, 54 (Suppl. S3), 4–11. [Google Scholar] [CrossRef]
- Fischer, B.; Chavatte-Palmer, P.; Viebahn, C.; Navarrete Santos, A.; Duranthon, V. Rabbit as a reproductive model for human health. Reproduction 2012, 144, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Navarrete Santos, A.; Augustin, R.; Lazzari, G.; Galli, C.; Sreenan, J.M.; Fischer, B. The insulin-dependent glucose transporter isoform 4 is expressed in bovine blastocysts. Biochem. Biophys. Res. Commun. 2000, 271, 753–760. [Google Scholar] [CrossRef]
- Tonack, S.; Fischer, B.; Navarrete Santos, A. Expression of the insulin-responsive glucose transporter isoform 4 in blastocysts of C57/BL6 mice. Anatomy Embryol. 2004, 208, 225–230. [Google Scholar] [CrossRef]
- Pendzialek, S.M.; Knelangen, J.M.; Schindler, M.; Gürke, J.; Grybel, K.J.; Gocza, E.; Fischer, B.; Navarrete Santos, A. Trophoblastic microRNAs are downregulated in a diabetic pregnancy through an inhibition of Drosha. Mol. Cell. Endocrinol. 2019, 480, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Pendzialek, S.M.; Schindler, M.; Plösch, T.; Gürke, J.; Haucke, E.; Hecht, S.; Fischer, B.; Santos, A.N. Cholesterol metabolism in rabbit blastocysts under maternal diabetes. Reprod. Fertil. Dev. 2017, 29, 1921–1931. [Google Scholar] [CrossRef]
- Bélanger, M.; Yang, J.; Petit, J.-M.; Laroche, T.; Magistretti, P.J.; Allaman, I. Role of the glyoxalase system in astrocyte-mediated neuroprotection. J. Neurosci. 2011, 31, 18338–18352. [Google Scholar] [CrossRef]
- Arai, M.; Nihonmatsu-Kikuchi, N.; Itokawa, M.; Rabbani, N.; Thornalley, P.J. Measurement of glyoxalase activities. Biochem. Soc. Trans. 2014, 42, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Kandaraki, E.; Chatzigeorgiou, A.; Piperi, C.; Palioura, E.; Palimeri, S.; Korkolopoulou, P.; Koutsilieris, M.; Papavassiliou, A.G. Reduced ovarian glyoxalase-I activity by dietary glycotoxins and androgen excess: A causative link to polycystic ovarian syndrome. Mol. Med. 2012, 18, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, B.; Goldstein, B.; Thornalley, P.J.; Rabbani, N.; Tschoepe, D. Intracellular Accumulation of Methylglyoxal by Glyoxalase 1 Knock Down Alters Collagen Homoeostasis in L6 Myoblasts. Int. J. Mol. Sci. 2017, 18, 480. [Google Scholar] [CrossRef] [PubMed]
- Nigro, C.; Leone, A.; Longo, M.; Prevenzano, I.; Fleming, T.H.; Nicolò, A.; Parrillo, L.; Spinelli, R.; Formisano, P.; Nawroth, P.P.; et al. Methylglyoxal accumulation de-regulates HoxA5 expression, thereby impairing angiogenesis in glyoxalase 1 knock-down mouse aortic endothelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 73–85. [Google Scholar] [CrossRef]
- Vannuccini, S.; Clifton, V.L.; Fraser, I.S.; Taylor, H.S.; Critchley, H.; Giudice, L.C.; Petraglia, F. Infertility and reproductive disorders: Impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum. Reprod. Update 2016, 22, 104–115. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Piperi, C.; Patsouris, E.; Korkolopoulou, P.; Panidis, D.; Pawelczyk, L.; Papavassiliou, A.G.; Duleba, A.J. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem. Cell Biol. 2007, 127, 581–589. [Google Scholar] [CrossRef]
- Papachroni, K.K.; Piperi, C.; Levidou, G.; Korkolopoulou, P.; Pawelczyk, L.; Diamanti-Kandarakis, E.; Papavassiliou, A.G. Lysyl oxidase interacts with AGE signalling to modulate collagen synthesis in polycystic ovarian tissue. J. Cell. Mol. Med. 2010, 14, 2460–2469. [Google Scholar] [CrossRef] [Green Version]
- Tatone, C.; Amicarelli, F. The aging ovary—The poor granulosa cells. Fertil. Steril. 2013, 99, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Matsumine, M.; Shibata, N.; Ishitani, K.; Kobayashi, M.; Ohta, H. Pentosidine accumulation in human oocytes and their correlation to age-related apoptosis. Acta Histochem. Cytochem. 2008, 41, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Emidio, G.; Santini, S.J.; D’Alessandro, A.M.; Vetuschi, A.; Sferra, R.; Artini, P.G.; Carta, G.; Falone, S.; Amicarelli, F.; Tatone, C. SIRT1 participates in the response to methylglyoxal-dependent glycative stress in mouse oocytes and ovary. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, G.; Chen, M.; Zuo, T.; Xu, W.; Liu, X. The Role of Antioxidant Enzymes in the Ovaries. Oxid. Med. Cell. Longev. 2017, 2017, 4371714. [Google Scholar] [CrossRef] [Green Version]
- Al-Gubory, K.H.; Bolifraud, P.; Garrel, C. Regulation of key antioxidant enzymatic systems in the sheep endometrium by ovarian steroids. Endocrinology 2008, 149, 4428–4434. [Google Scholar] [CrossRef] [Green Version]
- ProteinSimple. Simple Western Assays: Immunoassay. Available online: https://www.proteinsimple.com/simple_western_assays.html (accessed on 18 September 2020).

| Antigen | Name of Antibody | Manufacturer/Company Catalogue NO. | Type | Dilution |
|---|---|---|---|---|
| GLO1 | GLO1 (D-5) | santa cruz, sc-133214 | MM | 1:50 |
| Vinculin | Vinculin (7F9) | santa cruz, sc-73614 | MM | 1:300 |
| Mouse IgG | Peroxidase-conjugated AffiniPure Donkey anti-mouse IgG (H + L) | Jackson ImmunoResearch, 715-035-150 | DP | 1:300 |
| Mouse IgG | Secondary HRP Conjugate Goat anti-mouse IgG (H + L) | ProteinSimple, 042-205 | MP | undiluted |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Nivelle, J.; Thoma, J.; Toto Nienguesso, A.; Seeling, T.; Jung, J.-S.; Navarrete Santos, A.; Schindler, M. Rabbit as an Aging Model in Reproduction: Advanced Maternal Age Alters GLO1 Expression in the Endometrium at the Time of Implantation. Appl. Sci. 2020, 10, 7732. https://doi.org/10.3390/app10217732
de Nivelle J, Thoma J, Toto Nienguesso A, Seeling T, Jung J-S, Navarrete Santos A, Schindler M. Rabbit as an Aging Model in Reproduction: Advanced Maternal Age Alters GLO1 Expression in the Endometrium at the Time of Implantation. Applied Sciences. 2020; 10(21):7732. https://doi.org/10.3390/app10217732
Chicago/Turabian Stylede Nivelle, Johanna, Juliane Thoma, Alicia Toto Nienguesso, Tom Seeling, Juliane-Susanne Jung, Anne Navarrete Santos, and Maria Schindler. 2020. "Rabbit as an Aging Model in Reproduction: Advanced Maternal Age Alters GLO1 Expression in the Endometrium at the Time of Implantation" Applied Sciences 10, no. 21: 7732. https://doi.org/10.3390/app10217732





