Laser Induced Aggregation of Light Absorbing Particles by Marangoni Convection
Abstract
:1. Introduction
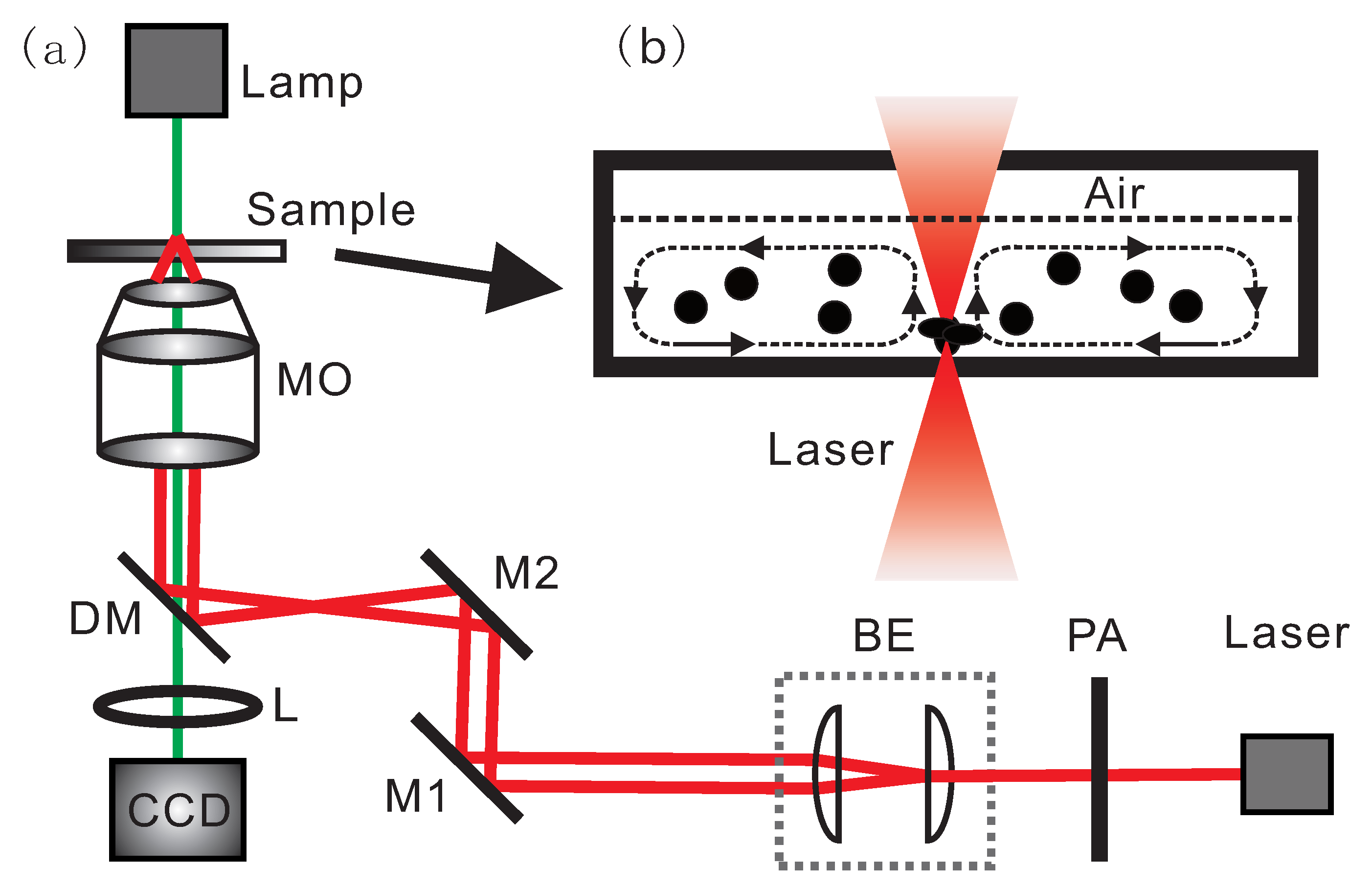
2. Experimental Methods
3. Results and Discussion
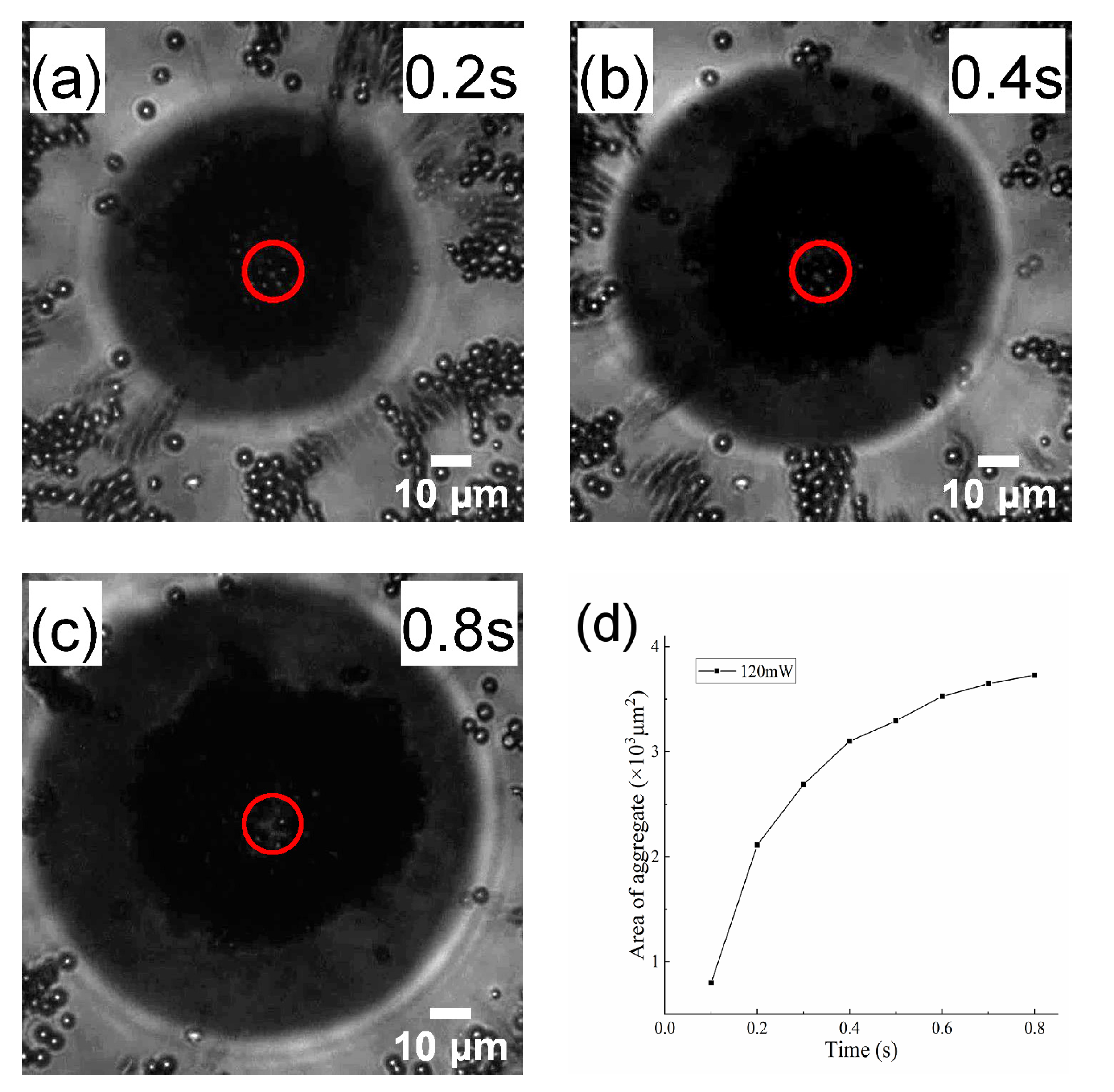
3.1. AP Aggregation
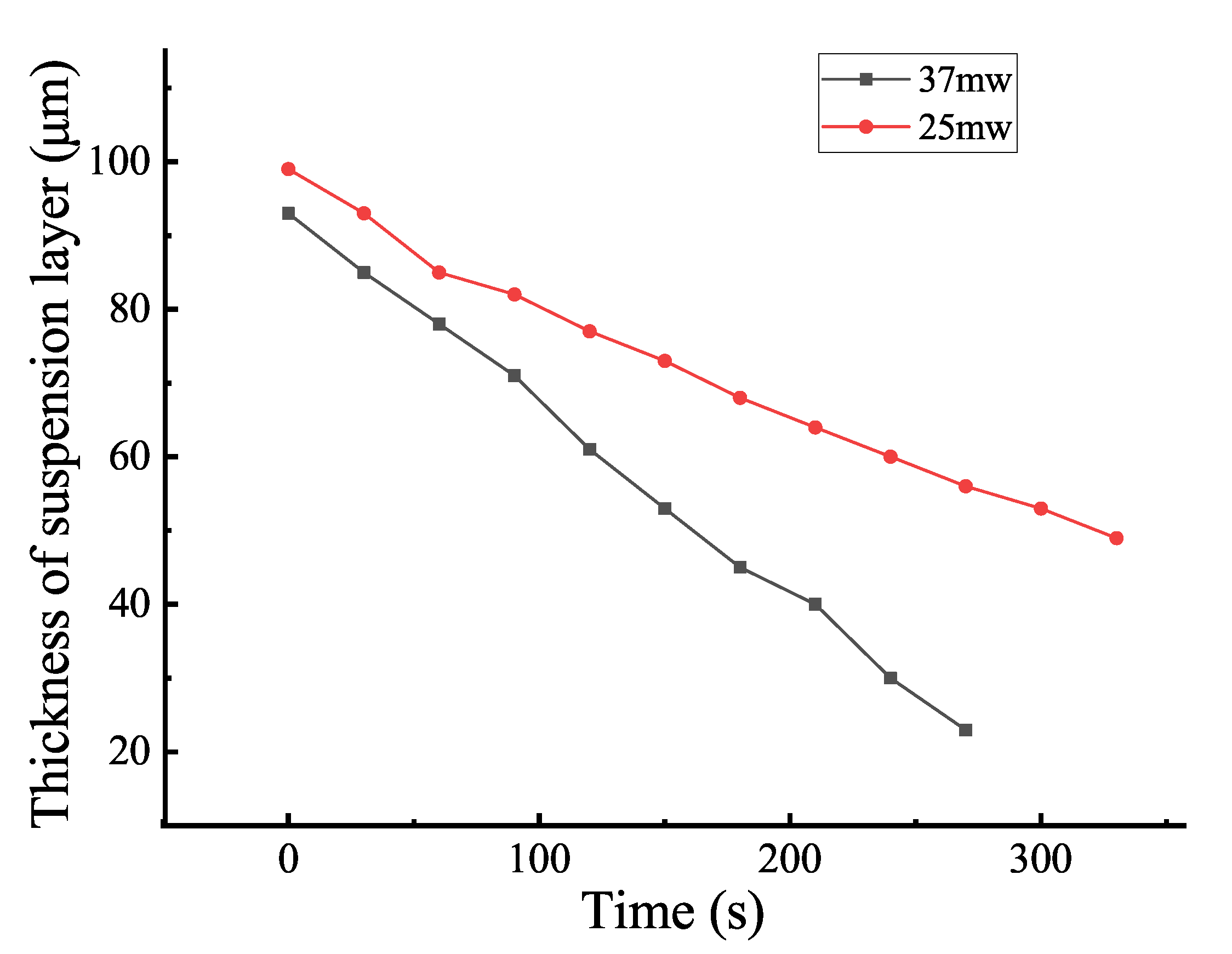
3.2. Effect of Thickness on AP Aggregation
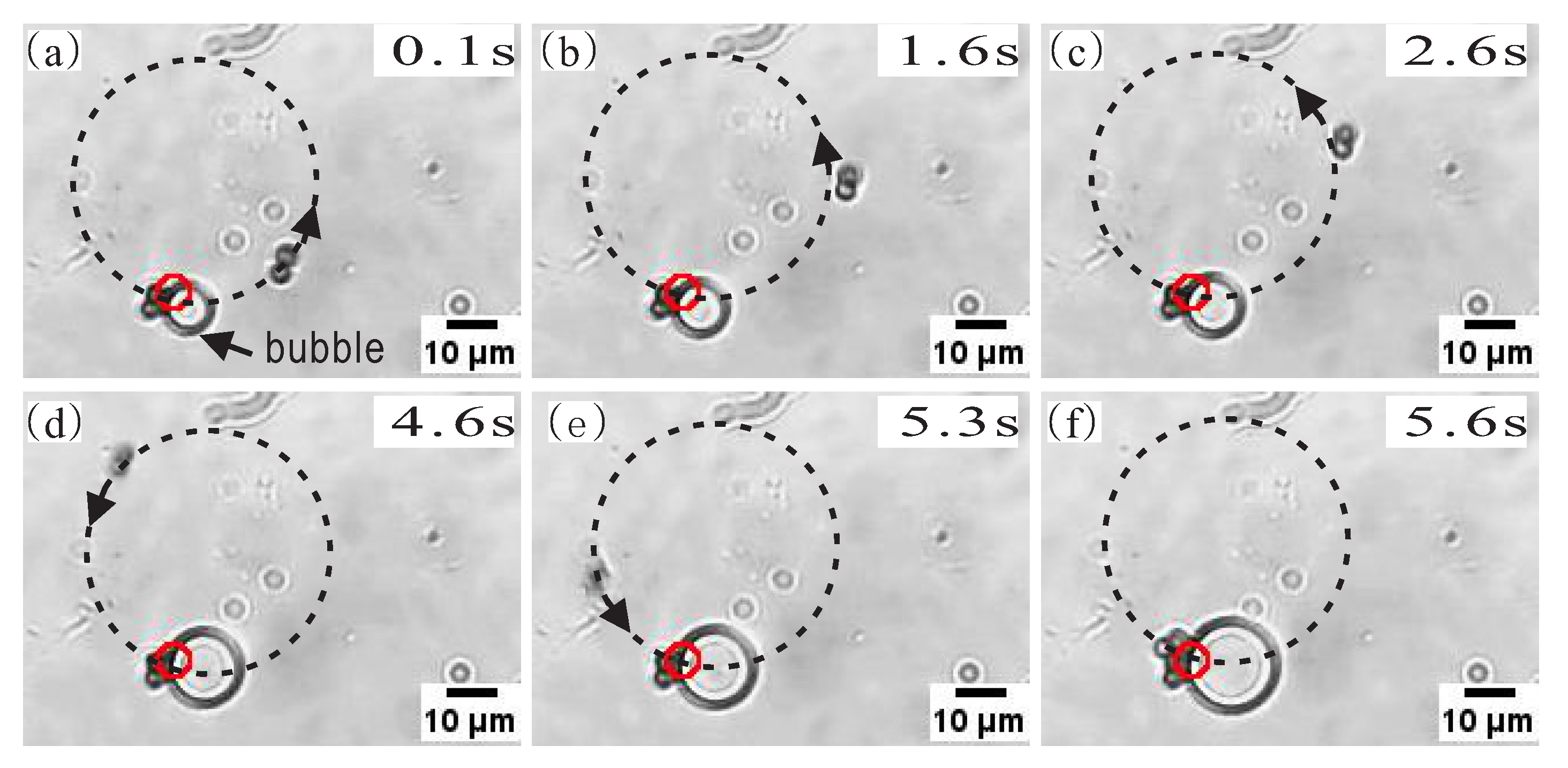
3.3. AP Accumulation Assisted by Bubbles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, P.; Ma, Y.; Zheng, Z.; Wu, C.; Wang, Y.; Liang, G. Facile syntheses of conjugated polymers for photothermal tumour therapy. Nat. Commun. 2019, 10, 1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Sun, E.; Ham, D.; Weissleder, R. Chip-NMR biosensor for detection and molecular analysis of cells. Nat. Med. 2008, 14, 869–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeili, E.; Khalili, M.; Alireza, N.S.; Hosseinzadeh, S.; Taheri, B.; Soleimani, M. Dendrimer functionalized magnetic nanoparticles as a promising platform for localized hyperthermia and magnetic resonance imaging diagnosis. J. Cell. Physiol. 2019, 234, 12615–12624. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986, 11, 288–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, J.E.; Grier, D.G. Structure of Optical Vortices. Phys. Rev. Lett. 2003, 90, 133901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, C.; Shen, Z.; Shen, J.; Zhang, Y.; Fang, H.; Yuan, G.; Du, L.; Zhu, S.; Lei, T.; Yuan, X. Focused plasmonic trapping of metallic particles. Nat. Commun. 2013, 4, 2891. [Google Scholar] [CrossRef]
- Zhong, M.C.; Wei, X.B.; Zhou, J.H.; Wang, Z.Q.; Li, Y.M. Trapping red blood cells in living animals using optical tweezers. Nat. Commun. 2013, 4, 1768. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.; Xiang, Y.; Lamstein, J.; Liang, Y.; Hansson, T.; Wetze, B.; Chen, Z. Optical force-induced nonlinearity and self-guiding of light in human red blood cell suspensions. Light. Sci. Appl. 2019, 8, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kinnunen, M.; Khokhlova, M.D.; Lyubin, E.V.; Priezzhev, A.V.; Meglinski, I.; Fedyanin, A.A. Optical tweezers study of red blood cell aggregation and disaggregation in plasma and protein solutions. J. Biomed. Opt. 2016, 21, 035001. [Google Scholar] [CrossRef]
- Barak, P.; Rai, A.; Rai, P.; Mallik, R. Quantitative optical trapping on single organelles in cell extract. Nat. Methods 2013, 10, 68–70. [Google Scholar] [CrossRef] [Green Version]
- Ashkin, A.; Dziedzic, J.M. Internal Cell Manipulation Using Infrared Laser Traps. Proc. Natl. Acad. Sci. USA 1989, 86, 7914–7918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparkes, I.A.; Ketelaar, T.; Ruijter, N.C.A.D.; Hawes, C. Grab a Golgi: Laser trapping of Golgi bodies reveals in vivo interactions with the endoplasmic reticulum. Traffic 2009, 10, 567–571. [Google Scholar] [CrossRef]
- Donato, M.G.; Brzobohaty, O.; Simpson, S.H.; Irrera, A.; Leonardi, A.A.; Faro, M.J.L.; Svak, V.; Marago, O.M.; Zemanek, P. Optical Trapping, Optical Binding, and Rotational Dynamics of Silicon Nanowires in Counter-Propagating Beams. Nano Lett. 2019, 19, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Maragò, O.M.; Jones, P.H.; Gucciardi, P.G.; Volpe, G.; Ferrari, A.C. Optical trapping and manipulation of nanostructures. Nat. Nanotechnol. 2013, 8, 807–819. [Google Scholar] [CrossRef] [Green Version]
- Jauffred, L.; Richardson, A.C.; Oddershede, L.B. Three-dimensional optical control of individual quantum dots. Nano Lett. 2008, 8, 3376–3380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, C.; Ye, A. Measurement of the binding force between RAS protein and a pathologic BRAF mutant using optical tweezers. Chin. Opt. Lett. 2013, 11, 80–83. [Google Scholar]
- Crocker, J.C.; Grier, D.G. Microscopic measurement of the pair interaction potential of charge-stabilized colloid. Phys. Rev. Lett. 1994, 73, 352–355. [Google Scholar] [CrossRef] [Green Version]
- Gutsche, C.; Keyser, U.F.; Kegler, K.; Kremer, F.; Linse, P. Forces between single pairs of charged colloids in aqueous salt solutions. Phys. Rev. E 2007, 76, 031403. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Li, S.W.; Jing, D.; Xu, J.H. Interactions between colliding oil drops coated with non-ionic surfactant determined using optical tweezers. Chem. Eng. Sci. 2019, 193, 276–281. [Google Scholar] [CrossRef]
- Chen, A.; Li, S.W.; Sang, F.; Zeng, H.B.; Xu, J.H. Interactions between Micro-Scale Oil Droplets in Aqueous Surfactant Solution Determined Using Optical Tweezers. J. Colloid Interface Sci. 2018, 532, 128–135. [Google Scholar] [CrossRef]
- Summers, M.D.; Burnham, D.R.; Mcgloin, D. Trapping solid aerosols with optical tweezers: A comparison between gas and liquid phase optical traps. Opt. Express 2008, 16, 7739–7747. [Google Scholar] [CrossRef]
- Tanaka, S.; Oki, Y.; Kimura, Y. Melting of a finite-sized two-dimensional colloidal crystal. Phys. Rev. E 2014, 89, 052305. [Google Scholar] [CrossRef]
- Zhong, M.C.; Wang, Z.Q.; Li, Y.M. Laser-accelerated self-assembly of colloidal particles at the water-air interface. Chin. Opt. Lett. 2017, 15, 051401. [Google Scholar] [CrossRef] [Green Version]
- Kudo, T.; Wang, S.F.; Yuyama, K.I.; Masuhara, H. Optical Trapping-Formed Colloidal Assembly with Horns Extended to the Outside of a Focus through Light Propagation. Nano Lett. 2016, 16, 3058–3062. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Z.Y. Light-driven crystallization of polystyrene micro-spheres. Photonics Res. 2017, 5, 201–206. [Google Scholar] [CrossRef]
- Zhong, M.C.; Liu, A.Y.; Zhu, R. Optical Assembling of Micro-Particles at a Glass-Water Interface with Diffraction Patterns Caused by the Limited Aperture of Objective. Appl. Sci. 2018, 8, 1522. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, Z.Y. Controlled Mechanical Motions of Microparticles in Optical Tweezers. Micromachines 2018, 9, 232. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Pan, Y.L.; Videen, G.; Wang, C. Optical trapping and manipulation of single particles in air: Principles, technical details, and applications. J. Quant. Spectrosc. Radiat. Transf. 2018, 214, 94–119. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, J.; Zhang, Y.; Zhang, Y.; Tang, X.; Yang, X.; Zhang, J.; Yang, J.; Yuan, L. Optical trapping and axial shifting for strongly absorbing particle with single focused TEM00 Gaussian beam. Appl. Phys. Lett. 2018, 113, 091101. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, Z.; Tang, X.; Yang, X.; Zhang, J.; Yang, J.; Yuan, L. Laser-Induced Microsphere Hammer-Hit Vibration in Liquid. Phys. Rev. Lett. 2018, 121, 133901. [Google Scholar] [CrossRef]
- Chen, J.; Cong, H.; Loo, F.C.; Kang, Z.; Tang, M.; Zhang, H.; Wu, S.Y.; Kong, S.K.; Ho, H.P. Thermal gradient induced tweezers for the manipulation of particles and cells. Sci. Rep. 2016, 6, 35814. [Google Scholar] [CrossRef] [Green Version]
- Piazza, R. Thermophoresis: Moving particles with thermal gradients. Soft Matter 2008, 4, 1740–1744. [Google Scholar] [CrossRef]
- Braun, D.; Libchaber, A. Trapping of DNA by Thermophoretic Depletion and Convection. Phys. Rev. Lett. 2002, 89, 188103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, T.; Sasai, Y.; Kawano, S. Thermophoretic Manipulation of Micro- and Nanoparticle Flow through a Sudden Contraction in a Microchannel with Near-Infrared Laser Irradiation. Phys. Rev. Appl. 2018, 10, 044005. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.R.; Wada, H.; Yoshinaga, N.; Sano, M. Manipulation of Colloids by a Nonequilibrium Depletion Force in a Temperature Gradient. Phys. Rev. Lett. 2009, 102, 208301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Z.; Chen, J.; Wu, S.Y.; Chen, K.; Kong, S.K.; Yong, K.T.; Ho, H.P. Trapping and assembling of particles and live cells on large-scale random gold nano-island substrates. Sci. Rep. 2015, 5, 9978. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Wang, M.; Peng, X.; Lissek, E.N.; Mao, Z.; Scarabelli, L.; Adkins, E.; Coskun, S.; Unalan, H.E.; Korgel, B.A. Opto-thermoelectric nanotweezers. Nat. Photonics 2018, 12, 195–201. [Google Scholar] [CrossRef]
- Li, J.; Lin, L.; Inoue, Y.; Zheng, Y. Opto-Thermophoretic Tweezers and Assembly. J. Micro Nano-Manuf. 2018, 6, 040801. [Google Scholar] [CrossRef]
- Zhong, M.C.; Liu, A.Y.; Ji, F. Opto-thermal oscillation and trapping of light absorbing particles. Opt. Express 2019, 27, 29730. [Google Scholar] [CrossRef]
- Lin, L.; Hill, E.H.; Peng, X.; Zheng, Y. Optothermal Manipulations of Colloidal Particles and Living Cells. Acc. Chem. Res. 2018, 51, 1465–1474. [Google Scholar] [CrossRef]
- Ilday, S.; Makey, G.; Akguc, G.B.; Tokel, O.; Pavlov, I.; Gülseren, O. Rich complex behaviour of self-assembled nanoparticles far from equilibrium. Nat. Commun. 2017, 8, 14942. [Google Scholar] [CrossRef] [Green Version]
- Louchev, O.A.; Juodkazis, S.; Murazawa, N.; Wada, S.; Misawa, H. Coupled laser molecular trapping, cluster assembly, and deposition fed by laser-induced Marangoni convection. Opt. Express 2008, 16, 5673–5680. [Google Scholar] [CrossRef]
- Flores-Flores, E.; Torres-Hurtado, S.; Páez, R.; Ruiz, U.; Beltrán-Pérez, G.; Neale, S.; Ramirez-San-Juan, J.; Ramos-García, R. Trapping and manipulation of microparticles using laser-induced convection currents and photophoresis. Biomed. Opt. Express 2015, 6, 4079–4087. [Google Scholar] [CrossRef] [Green Version]
- Chikazawa, J.I.; Uwada, T.; Furube, A.; Hashimoto, S. Flow-Induced Transport via Optical Heating of a Single Gold Nanoparticle. J. Phys. Chem. C 2019, 123, 4512–4522. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.-W.; Zhong, M.-C.; Ji, F. Laser Induced Aggregation of Light Absorbing Particles by Marangoni Convection. Appl. Sci. 2020, 10, 7795. https://doi.org/10.3390/app10217795
Li B-W, Zhong M-C, Ji F. Laser Induced Aggregation of Light Absorbing Particles by Marangoni Convection. Applied Sciences. 2020; 10(21):7795. https://doi.org/10.3390/app10217795
Chicago/Turabian StyleLi, Bo-Wei, Min-Cheng Zhong, and Feng Ji. 2020. "Laser Induced Aggregation of Light Absorbing Particles by Marangoni Convection" Applied Sciences 10, no. 21: 7795. https://doi.org/10.3390/app10217795




