Classification Methods for Airborne Disease Spores from Greenhouse Crops Based on Multifeature Fusion
Abstract
:1. Introduction
2. Materials and Methods
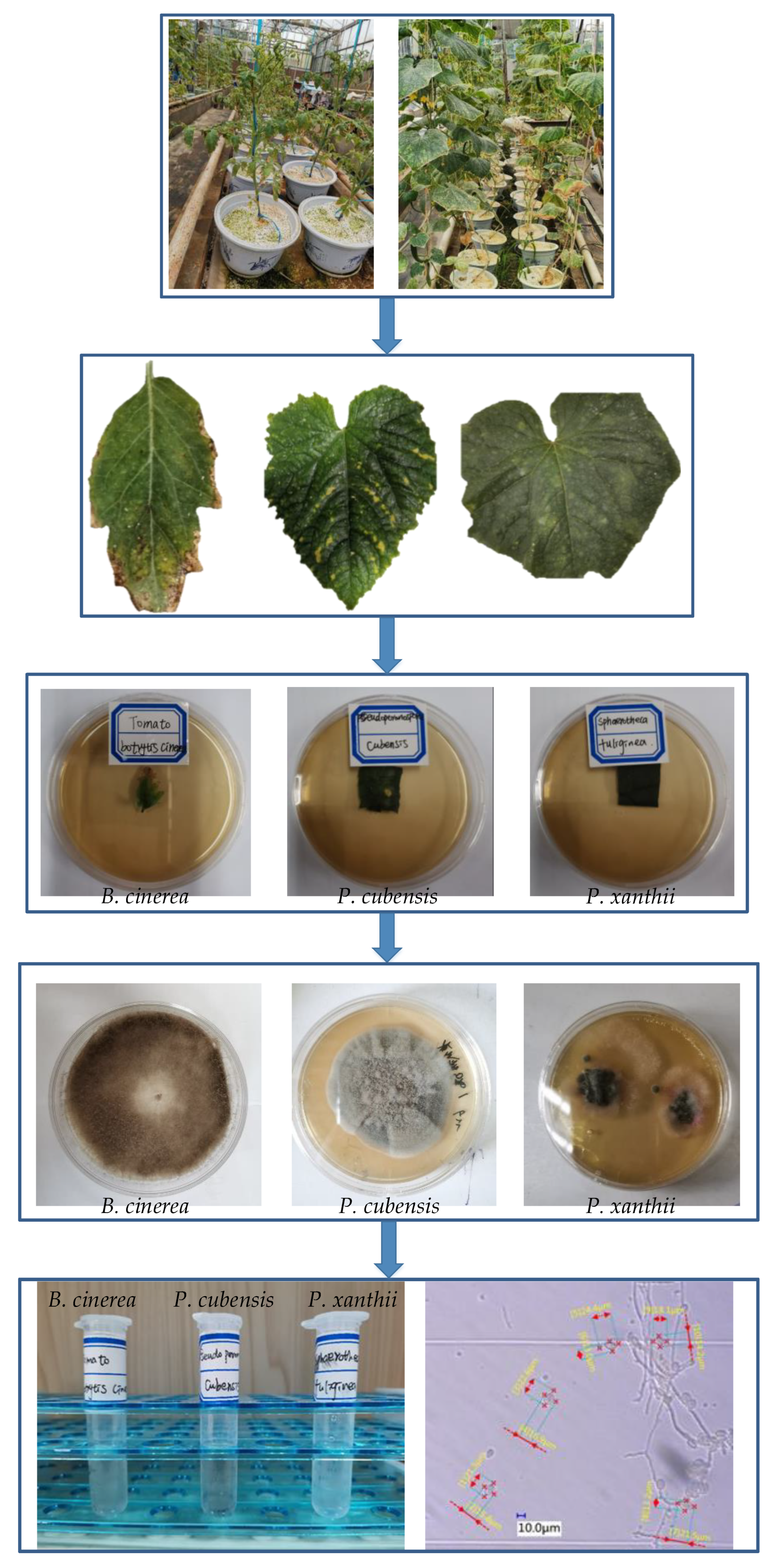
2.1. Spore Extraction and Identification
2.2. Spore Image Collection
2.3. Preprocessing for Spore Images
2.4. Feature Extraction of Spore Images
2.4.1. Color Features
2.4.2. Shape Features
- (1)
- Roundness ratio
- (2)
- Slenderness ratio
2.4.3. Texture Features
- (1)
- GLCM
- (2)
- LBP
3. Results
3.1. Test Platform and Parameter Settings
3.2. Analysis of Classification Results
3.2.1. Evaluation Index
3.2.2. Classification Results for Different Models
3.2.3. Recognition Rate of Different Feature Combinations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, T.Y.; Wu, G.X.; Chen, J.W.; Cui, P.; Chen, Z.X.; Yan, Y.Y.; Zhang, Y.; Li, M.C.; Niu, D.X.; Li, B.G. Integration of solar technology to modern greenhouse in China: Current status, challenges and prospect. Renew. Sustain. Energy Rev. 2017, 70, 1178–1188. [Google Scholar] [CrossRef]
- Jin, C.; Mao, H.P.; Chen, Y.; Shi, Q.; Wang, Q.R.; Ma, G.X.; Liu, Y. Engineering-Oriented dynamic optimal control of a greenhouse environment using an improved genetic algorithm with engineering constraint rules. Comput. Electron. Agric. 2020, 177, 105698. [Google Scholar] [CrossRef]
- Hafez, Y.M.; Attia, K.A.; Kamel, S.; Alamery, S.F.; El-Gendy, S.; Al-Doss, A.A.; Mehiar, F.; Ghazy, A.I.; Ibrahim, E.I.; Abdelaal, K.A.A. Bacillus subtilis as a bio-agent combined with nano molecules can control powdery mildew disease through histochemical and physiobiochemical changes in cucumber plants. Physiol. Mol. Plant Pathol. 2020, 111, 101489. [Google Scholar]
- Tanaka, K.; Fukuda, M.; Amaki, Y.; Sakaguchi, T.; Inai, K.; Ishihara, A.; Nakajima, H. Importance of prumycin produced by Bacillus amyloliquefaciens SD-32 in biocontrol against cucumber powdery mildew disease. Pest Manag. Sci. 2017, 73, 2419–2428. [Google Scholar] [CrossRef]
- Wallace, E.C.; D’Arcangelo, K.N.; Quesada-Ocampo, L.M. Population analyses reveal two host-adapted clades of Pseudoperonospora cubensis, the causal agent of cucurbit downy mildew, on commercial and wild cucurbits. Phytopathology 2020, 110, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.A.; Koike, S.T.; Fox, A.D.; Anchieta, A.; Subbarao, K.V.; Klosterman, S.J.; McRoberts, N. Season-Long dynamics of spinach downy mildew determined by spore trapping and disease incidence. Phytopathology 2016, 106, 1311–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, N.; Mamo, B.E.; Subbarao, K.V.; Koike, S.T.; Fox, A.; Anchieta, A.; Klosterman, S.J. Measurements of aerial spore load by qPCR facilitates lettuce downy mildew risk advisement. Plant Dis. 2020, 104, 82–93. [Google Scholar] [CrossRef]
- Torfs, S.; Van Poucke, K.; Van Campenhout, J.; Ceustermans, A.; Croes, S.; Bylemans, D.; Van Hemelrijck, W.; Keulemans, W.; Heungens, K. Venturia inaequalis trapped: Molecular quantification of airborne inoculum using volumetric and rotating arm samplers. Eur. J. Plant Pathol. 2019, 155, 1319–1332. [Google Scholar] [CrossRef]
- Chan, B.D.; Icoz, K.; Huang, W.F.; Chang, C.L.; Savran, C.A. On-Demand weighing of single dry biological particles over a 5-order-of-magnitude dynamic range. Lab Chip 2014, 14, 4188–4196. [Google Scholar] [CrossRef]
- Sireesha, Y.; Velazhahan, R. Rapid and specific detection of Peronosclerospora sorghi in maize seeds by conventional and real-time PCR. Eur. J. Plant Pathol. 2017, 150, 521–526. [Google Scholar] [CrossRef]
- Bandamaravuri, K.B.; Nayak, A.K.; Bandamaravuri, A.S.; Samad, A. Simultaneous detection of downy mildew and powdery mildew pathogens on Cucumis sativus and other cucurbits using duplex-qPCR and HRM analysis. AMB Express 2020, 10, 135. [Google Scholar] [CrossRef]
- Akhmadeev, A.A.; Salakhov, M.K. A new approach of recognition of ellipsoidal micro- and nanoparticles on AFM images and determination of their sizes. Meas. Sci. Technol. 2016, 27, 105402. [Google Scholar] [CrossRef]
- Xu, P.F.; Zhang, R.B.; Yang, N.; Oppong, P.K.; Sun, J.; Wang, P. High-Precision extraction and concentration detection of airborne disease microorganisms based on microfluidic chip. Biomicrofluidics 2019, 13, 024110. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Hu, J.Q.; Zhou, X.; Wang, A.Y.; Yu, J.J.; Tao, X.Y.; Tang, J. A rapid detection method of early spore viability based on AC impedance measurement. J. Food Process Eng. 2020, e13520. [Google Scholar] [CrossRef]
- Wang, P.; Yuan, S.Q.; Yang, N.; Wang, A.Y.; Fordjour, A.; Chen, S.B. The collection method for crop fungal spores based on an efficient microfluidic device. Aerosol Air Qual. Res. 2020, 20, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Quesada, T.; Hughes, J.; Smith, K.; Shin, K.; James, P.; Smith, J. A Low-Cost spore trap allows collection and real-time PCR quantification of airborne Fusarium circinatum spores. Forests 2018, 9, 586. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.D.; Zhang, J.Y.; Li, M.Y.; Zhao, L.J.; Ren, H.R.; Yan, L.G.; Zhao, G.M.; Zhu, C.Z. Rapid determination of spore germinability of Clostridium perfringens based on microscopic hyperspectral imaging technology and chemometrics. J. Food Eng. 2020, 280, 109896. [Google Scholar] [CrossRef]
- Setlow, P.; Wang, S.W.; Li, Y.Q. Germination of spores of the orders Bacillales and Clostridiales. Annu. Rev. Microbiol. 2017, 71, 459–477. [Google Scholar] [CrossRef]
- Lei, Y.; Yao, Z.F.; He, D.J. Automatic detection and counting of urediniospores of Puccinia striiformis f. sp. tritici using spore traps and image processing. Sci. Rep. 2018, 8, 13647. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.P.; Wang, B.T.; Yan, Y. The identification of powdery mildew spores image based on the integration of intelligent spore image sequence capture device. In Proceedings of the 2013 Ninth International Conference on Intelligent Information Hiding and Multimedia Signal Processing (Iih-Msp 2013), Beijing, China, 16–18 October 2013; pp. 177–180. [Google Scholar]
- Yang, N.; Yu, J.J.; Wang, A.Y.; Tang, J.; Zhang, R.B.; Xie, L.L.; Shu, F.Y.; Kwabena, O.P. A rapid rice blast detection and identification method based on crop disease spores’ diffraction fingerprint texture. J. Sci. Food Agric. 2020, 100, 3608–3621. [Google Scholar] [CrossRef]
- Bhunia, A.K.; Bhattacharyya, A.; Banerjee, P. A noval feature descriptor for image retrieval by combining modified color histogram and diagonally symmetric co-occurrence texture pattern. Pattern Anal. Appl. 2020, 2, 703–723. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Fieguth, P.; Guo, Y.; Wang, X.; Pietikainen, M. Local binary features for texture classification: Taxonomy and experimental study. Pattern Recognit. 2017, 62, 135–160. [Google Scholar] [CrossRef] [Green Version]
- Kou, Q.Q.; Cheng, D.Q.; Chen, L.L.; Zhao, K. A Multiresolution gray-scale and rotation invariant descriptor for texture classification. IEEE Access 2018, 6, 30691–30701. [Google Scholar] [CrossRef]
- Das, S.; Rudrapal, D. Analysis of color moment as a low level feature in improvement of content based image retrieval. In Proceedings of the Fourth International Conference on Signal and Image Processing 2012 (ICSIP); Springer: New Delhi, India, 2013; pp. 387–397. [Google Scholar]
- Yang, H.; Yin, J.; Jiang, M. Perceptual image hashing using latent low-rank representation and uniform LBP. Appl. Sci. 2018, 8, 317. [Google Scholar] [CrossRef] [Green Version]
- Vijayaragavan, P.; Ponnusaamy, R.; Aramudhan, M. An optimal support vector machine based classification model for sentimental analysis of online product reviews. Future Gener. Comput. Syst. Int. J. eSci. 2020, 111, 234–240. [Google Scholar] [CrossRef]
- Zeng, S.N.; Zhang, B.B.; Du, Y. Joint distances by sparse representation and locality-constrained dictionary learning for robust leaf recognition. Comput. Electron. Agric. 2017, 142, 563–571. [Google Scholar] [CrossRef]
- Chesmore, D.; Bernard, T.; Inman, A.J.; Bowyer, R.J. Image analysis for the identification of the quarantine pest Tilletia indica. EPPO Bull. 2003, 33, 495–499. [Google Scholar] [CrossRef]
- Marcos, J.V.; Nava, R.; Cristobal, G.; Redondo, R.; Escalante-Ramirez, B.; Bueno, G.; Deniz, O.; Gonzalez-Porto, A.; Pardo, C.; Chung, F.; et al. Automated pollen identification using microscopic imaging and texture analysis. Micron 2015, 68, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wang, W.; Huang, M.G.; Ni, X.Z.; Chu, X.; Li, C.Y. Evaluation and classification of five cereal fungi on culture medium using Visible/Near-Infrared (Vis/NIR) hyperspectral imaging. Infrared Phys. Technol. 2020, 105, 103206. [Google Scholar] [CrossRef]
- Yang, N.; Qian, Y.; EL-Mesery, H.S.; Zhang, R.B.; Wang, A.Y.; Tang, J. Rapid detection of rice disease using microscopy image identification based on the synergistic judgment of texture and shape features and decision tree-confusion matrix method. J. Sci. Food Agric. 2019, 99, 6589–6600. [Google Scholar] [CrossRef]




| Species | Spore Size/um |
|---|---|
| P. xanthii | 35.4(30.2~39.5) × 14.2(7.3~22.2) |
| P. cubensis | 30.6(21.1~39.8) × 20.5(13.8~23.6) |
| B. cinerea | 19.3(11.4~26.7) × 11.7(8.3~14.5) |
| Sample Class | Basic Indicators | |||
|---|---|---|---|---|
| TP | TN | FP | FN | |
| 1 | 55 | 110 | 8 | 5 |
| 2 | 58 | 107 | 1 | 2 |
| 3 | 52 | 113 | 6 | 8 |
| Class | Classification Results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Precision | Recall | F1-Score | ||||||||||
| LR | KNN | RF | SVM | LR | KNN | RF | SVM | LR | KNN | RF | SVM | |
| 1 | 89.26 | 82.40 | 88.57 | 87.30 | 91.35 | 89.13 | 88.16 | 91.67 | 86.57 | 85.63 | 88.36 | 89.43 |
| 2 | 98.32 | 95.16 | 97.08 | 98.31 | 92.75 | 92.83 | 89.34 | 96.67 | 95.45 | 93.98 | 93.05 | 97.48 |
| 3 | 87.94 | 85.35 | 83.28 | 89.66 | 85.97 | 83.98 | 80.26 | 86.67 | 86.94 | 86.94 | 81.74 | 88.14 |
| Indexes | Classification Model | |||
|---|---|---|---|---|
| LR | KNN | RF | SVM | |
| Accuracy (%) | 90.13 | 89.37 | 89.23 | 94.36 |
| Precision (%) | 89.51 | 87.64 | 89.64 | 91.76 |
| Recall (%) | 90.02 | 88.65 | 85.92 | 91.67 |
| F1-Score (%) | 89.65 | 88.09 | 87.72 | 91.68 |
| Recognition time (s) | 1.1241 | 1.6473 | 1.8597 | 1.5346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Du, X.; Ma, G.; Liu, Y.; Wang, B.; Mao, H. Classification Methods for Airborne Disease Spores from Greenhouse Crops Based on Multifeature Fusion. Appl. Sci. 2020, 10, 7850. https://doi.org/10.3390/app10217850
Wang Y, Du X, Ma G, Liu Y, Wang B, Mao H. Classification Methods for Airborne Disease Spores from Greenhouse Crops Based on Multifeature Fusion. Applied Sciences. 2020; 10(21):7850. https://doi.org/10.3390/app10217850
Chicago/Turabian StyleWang, Yafei, Xiaoxue Du, Guoxin Ma, Yong Liu, Bin Wang, and Hanping Mao. 2020. "Classification Methods for Airborne Disease Spores from Greenhouse Crops Based on Multifeature Fusion" Applied Sciences 10, no. 21: 7850. https://doi.org/10.3390/app10217850






