Rapid Online Solid-State Battery Diagnostics with Optically Pumped Magnetometers
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
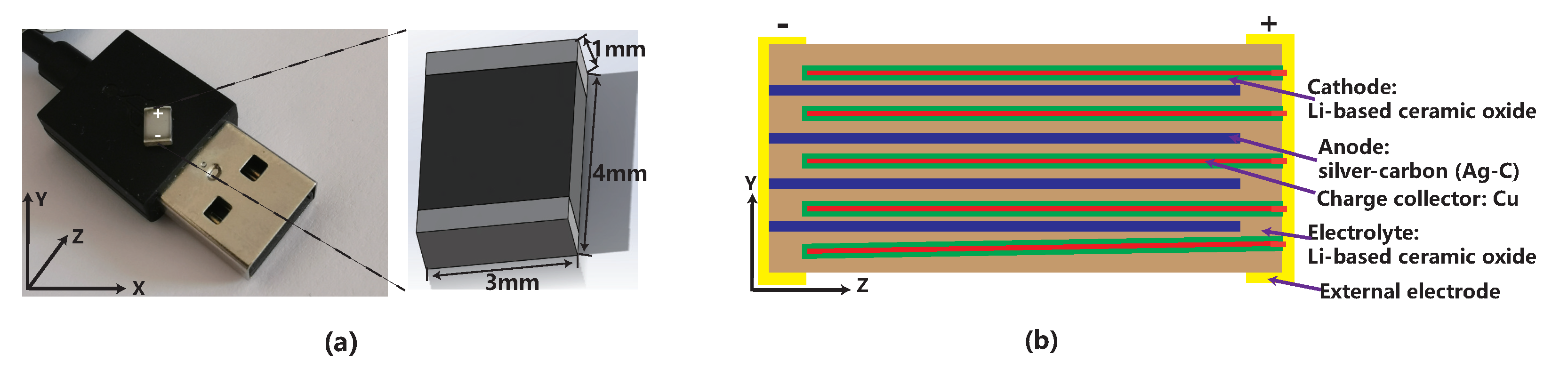
4.1. Solid-State Battery Cells
4.2. Measurement Apparatus
4.3. Cell Preparation and Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2008, 30, 1800561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A review of lithium and non-lithium based solid state batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar] [CrossRef]
- Robinson, J.B.; Maier, M.; Alster, G.; Compton, T.; Brett, D.J.; Shearing, P.R. Spatially resolved ultrasound diagnostics of Li-ion battery electrodes. Phys. Chem. Chem. Phys. 2019, 21, 6354–6361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauermann, L.P.; Mesquita, L.V.; Bischoff, C.; Drews, M.; Fitz, O.; Heuer, A.; Biro, D. Scanning acoustic microscopy as a non-destructive imaging tool to localize defects inside battery cells. J. Power Sources Adv. 2020, 6, 100035. [Google Scholar] [CrossRef]
- Lim, J.; Li, Y.; Alsem, D.H.; So, H.; Lee, S.C.; Bai, P.; Cogswell, D.A.; Liu, X.; Jin, N.; Yu, Y.S.; et al. Origin and hysteresis of lithium compositional spatiodynamics within battery primary particles. Science 2016, 353, 566–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Kunz, M.; Chen, K.; Tamura, N.; Richardson, T. Visualization of Charge Distribution in a Lithium-Ion Battery Electrode. Meet. Abstr. 2010, 2, 208. [Google Scholar]
- Panitz, J.C.; Novák, P.; Haas, O. Raman microscopy applied to rechargeable lithium-ion cells-Steps towards in situ Raman imaging with increased optical efficiency. Appl. Spectrosc. 2001, 9, 1131–1137. [Google Scholar] [CrossRef]
- Wood, V. X-ray tomography for battery research and development. Nat. Rev. Mater. 2018, 3, 293–295. [Google Scholar] [CrossRef]
- Väyrynen, A.; Salminen, J. Lithium ion battery production. J. Chem. Thermodyn. 2012, 46, 80–85. [Google Scholar] [CrossRef]
- Yufit, V.; Shearing, P.; Hamilton, R.W.; Lee, P.D.; Wu, M.; Brandon, N.P. Investigation of lithium-ion polymer battery cell failure using X-ray computed tomography. Electrochem. Commun. 2011, 13, 608–610. [Google Scholar] [CrossRef]
- Hsieh, A.G.; Bhadra, S.; Hertzberg, B.J.; Gjeltema, P.J.; Goy, A.; Fleischer, J.W.; Steingart, D.A. Electrochemical-acoustic time of flight: In operando correlation of physical dynamics with battery charge and health. Energy Environ. Sci. 2015, 8, 1569–1577. [Google Scholar] [CrossRef]
- Davies, G.; Knehr, K.W.; Van Tassell, B.; Hodson, T.; Biswas, S.; Hsieh, A.G.; Steingart, D.A. State of charge and state of health estimation using electrochemical acoustic time of flight analysis. J. Electrochem. Soc. 2017, 164, A2746–A2755. [Google Scholar] [CrossRef]
- Ilott, A.J.; Mohammadi, M.; Schauerman, C.M.; Ganter, M.J.; Jerschow, A. Rechargeable lithium-ion cell state of charge and defect detection by in-situ inside-out magnetic resonance imaging. Nat. Commun. 2018, 9, 1776. [Google Scholar] [CrossRef]
- Ilott, A.J.; Mohammadi, M.; Chang, H.J.; Grey, C.P.; Jerschow, A. Real-time 3D imaging of microstructure growth in battery cells using indirect MRI. Proc. Natl. Acad. Sci. USA 2016, 113, 10779–10784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, M.; Silletta, E.V.; Ilott, A.J.; Jerschow, A. Diagnosing current distributions in batteries with magnetic resonance imaging. J. Magn. Reson. 2019, 309, 106601. [Google Scholar] [CrossRef]
- Romanenko, K.; Jerschow, A. Distortion-free inside-out imaging for rapid diagnostics of rechargeable Li-ion cells. Proc. Natl. Acad. Sci. USA 2019, 116, 18783–18789. [Google Scholar] [CrossRef] [Green Version]
- Romanenko, K.; Kuchel, P.W.; Jerschow, A. Jerschow Alexej, Accurate visualization of operating commercial batteries using specialized magnetic resonance imaging with magnetic field sensing. Chem. Mater. 2020, 32, 2107–2113. [Google Scholar] [CrossRef]
- Benders, S.; Mohammadi, M.; Ganter, M.J.; Klug, C.A.; Jerschow, A. Mapping oscillating magnetic fields around rechargeable batteries. J. Magn. Reson. 2020, 319, 106811. [Google Scholar] [CrossRef]
- Pigliapochi, R.; Benders, S.; Silletta, E.; Glazier, S.; Lee, E.; Dahn, J.; Jerschow, A. Ultrafast inside-out NMR assessment of Rechargeable Cells. Batter. Supercaps 2020, 31, 1022. [Google Scholar] [CrossRef]
- Chernova, N.A.; Nolis, G.M.; Omenya, F.O.; Zhou, H.; Li, Z.; Whittingham, M.S. What can we learn about battery materials from their magnetic properties? J. Mater. Chem. 2011, 21, 9865–9875. [Google Scholar] [CrossRef]
- Klinser, G.; Topolovec, S.; Krenn, H.; Würschum, R. Process Monitoring of Charging/Discharging of Lithium Ion Battery Cathodes by Operando SQUID Magnetometry. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry: Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 849–855. [Google Scholar]
- Quantum Design, Inc. MPMS Application Note 1014-213; Quantum Design, Inc.: San Diego, CA, USA, 2002; Volume 21, pp. 10–20. [Google Scholar]
- Klinser, G.; Topolovec, S.; Kren, H.; Koller, S.; Krenn, H.; Wurschum, R. Charging of lithium cobalt oxide battery cathodes studied by means of magnetometry. Solid State Ion. 2016, 293, 64–71. [Google Scholar] [CrossRef]
- Hu, Y.; Iwata, G.Z.; Mohammadi, M.; Silletta, E.V.; Wickenbrock, A.; Blanchard, J.W.; Budker, D.; Jerschow, A. Sensitive magnetometry reveals inhomogeneities in charge storage and weak transient internal currents in Li-ion cells. Proc. Natl. Acad. Sci. USA 2020, 117, 10667–10672. [Google Scholar] [CrossRef] [PubMed]
- Kaboli, S.; Demers, H.; Paolella, A.; Darwiche, A.; Dontigny, M.; Clement, D.; Guerfi, A.; Trudeau, M.L.; Goodenough, J.B.; Zaghib, K. Behavior of Solid Electrolyte in Li-Polymer Battery with NMC Cathode via in-Situ Scanning Electron Microscopy. Nano Lett. 2020, 20, 1607–1613. [Google Scholar] [CrossRef]
- Danan, H.; Herr, A.; Meyer, A.J. New determinations of the saturation magnetization of nickel and iron. J. Appl. Phys. 1968, 39, 669–670. [Google Scholar] [CrossRef]
- Klinser, G.; Topolovec, S.; Kren, H.; Koller, S.; Gössler, W.; Krenn, H.; Würschum, R. Continuous monitoring of the bulk oxidation states in LixNi1/3Mn1/3Co1/3O2 during charging and discharging. Appl. Phys. Lett. 2016, 109, 213901. [Google Scholar] [CrossRef]
- Buchner, M.; Hofler, K.; Henne, B.; Ney, V.; Ney, A. Tutorial: Basic principles, limits of detection, and pitfalls of highly sensitive SQUID magnetometry for nanomagnetism and spintronics. J. Appl. Phys. 2018, 124, 161101. [Google Scholar] [CrossRef] [Green Version]
- Coey, J.M. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2010; Volume 124, pp. 128–194. [Google Scholar]
- Limes, M.E.; Foley, E.L.; Kornack, T.W.; Caliga, S.; McBride, S.; Braun, A.; Lee, W.; Lucivero, V.G.; Romalis, M.V. Portable magnetometry for detection of biomagnetism in ambient environments. Phys. Rev. Appl. 2020, 14, 011002. [Google Scholar] [CrossRef]
- Dang, H.B.; Maloof, A.C.; Romalis, M.V. Romalis Michael V, Ultrahigh sensitivity magnetic field and magnetization measurements with an atomic magnetometer. Appl. Phys. Lett. 2010, 979, 151110. [Google Scholar] [CrossRef] [Green Version]
- Rechargeable Solid-State SMD Battery for IoT Applications. 2018. Available online: www.tdk-electronics.tdk.com/en/2471330/tech-library/articles/applications—cases—video/rechargeable-solid-state-smd-battery-for-iot-applications/2431020 (accessed on 1 October 2020).


| Element | Unit | Value | ||
|---|---|---|---|---|
| Li | mg/kg | 12,685 ± 500 | ||
| Mg | mg/kg | 4 | ||
| Al | mg/kg | 417 ± 60 | ||
| K | mg/kg | 103 ± 9 | ||
| Ca | mg/kg | 24 | ||
| V | mg/kg | 27,889 ± 2000 | ||
| Cr | mg/kg | 42 | ||
| Mn | mg/kg | — | ||
| Fe | mg/kg | — | ||
| Co | mg/kg | — | ||
| Ni | mg/kg | 240 ± 22 | ||
| Cu | mg/kg | 188,628 ± 9000 | ||
| Zn | mg/kg | 271 | ||
| Pb | mg/kg | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Iwata, G.Z.; Bougas, L.; Blanchard, J.W.; Wickenbrock, A.; Jakob, G.; Schwarz, S.; Schwarzinger, C.; Jerschow, A.; Budker, D. Rapid Online Solid-State Battery Diagnostics with Optically Pumped Magnetometers. Appl. Sci. 2020, 10, 7864. https://doi.org/10.3390/app10217864
Hu Y, Iwata GZ, Bougas L, Blanchard JW, Wickenbrock A, Jakob G, Schwarz S, Schwarzinger C, Jerschow A, Budker D. Rapid Online Solid-State Battery Diagnostics with Optically Pumped Magnetometers. Applied Sciences. 2020; 10(21):7864. https://doi.org/10.3390/app10217864
Chicago/Turabian StyleHu, Yinan, Geoffrey Z. Iwata, Lykourgos Bougas, John W. Blanchard, Arne Wickenbrock, Gerhard Jakob, Stephan Schwarz, Clemens Schwarzinger, Alexej Jerschow, and Dmitry Budker. 2020. "Rapid Online Solid-State Battery Diagnostics with Optically Pumped Magnetometers" Applied Sciences 10, no. 21: 7864. https://doi.org/10.3390/app10217864
APA StyleHu, Y., Iwata, G. Z., Bougas, L., Blanchard, J. W., Wickenbrock, A., Jakob, G., Schwarz, S., Schwarzinger, C., Jerschow, A., & Budker, D. (2020). Rapid Online Solid-State Battery Diagnostics with Optically Pumped Magnetometers. Applied Sciences, 10(21), 7864. https://doi.org/10.3390/app10217864







