A Comprehensive Review of the Application Characteristics of Biodiesel Blends in Diesel Engines
Abstract
:1. Introduction
2. Physicochemical Properties of Biodiesel
3. Engine Performance
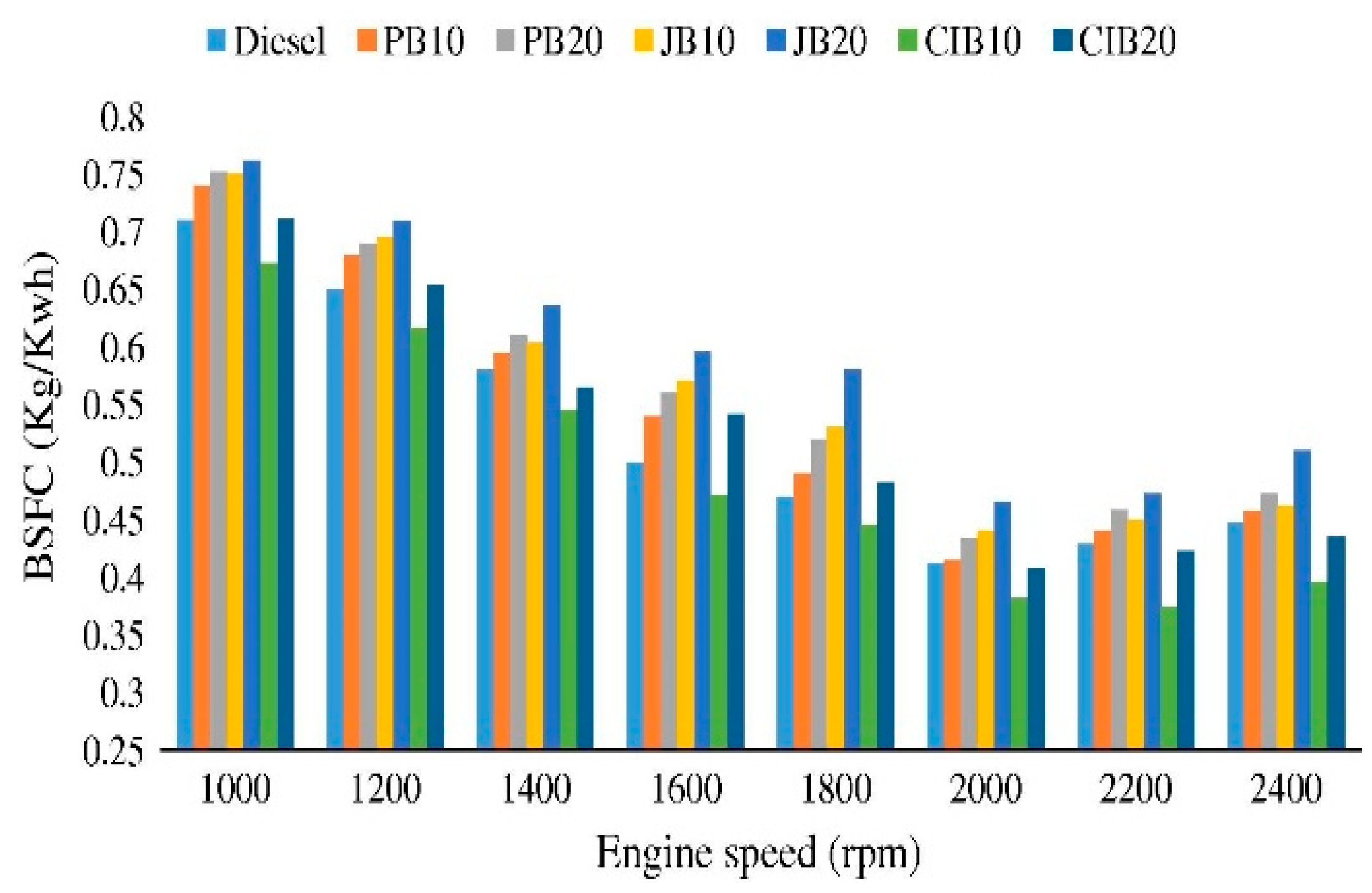
3.1. Brake Specific Fuel Consumption
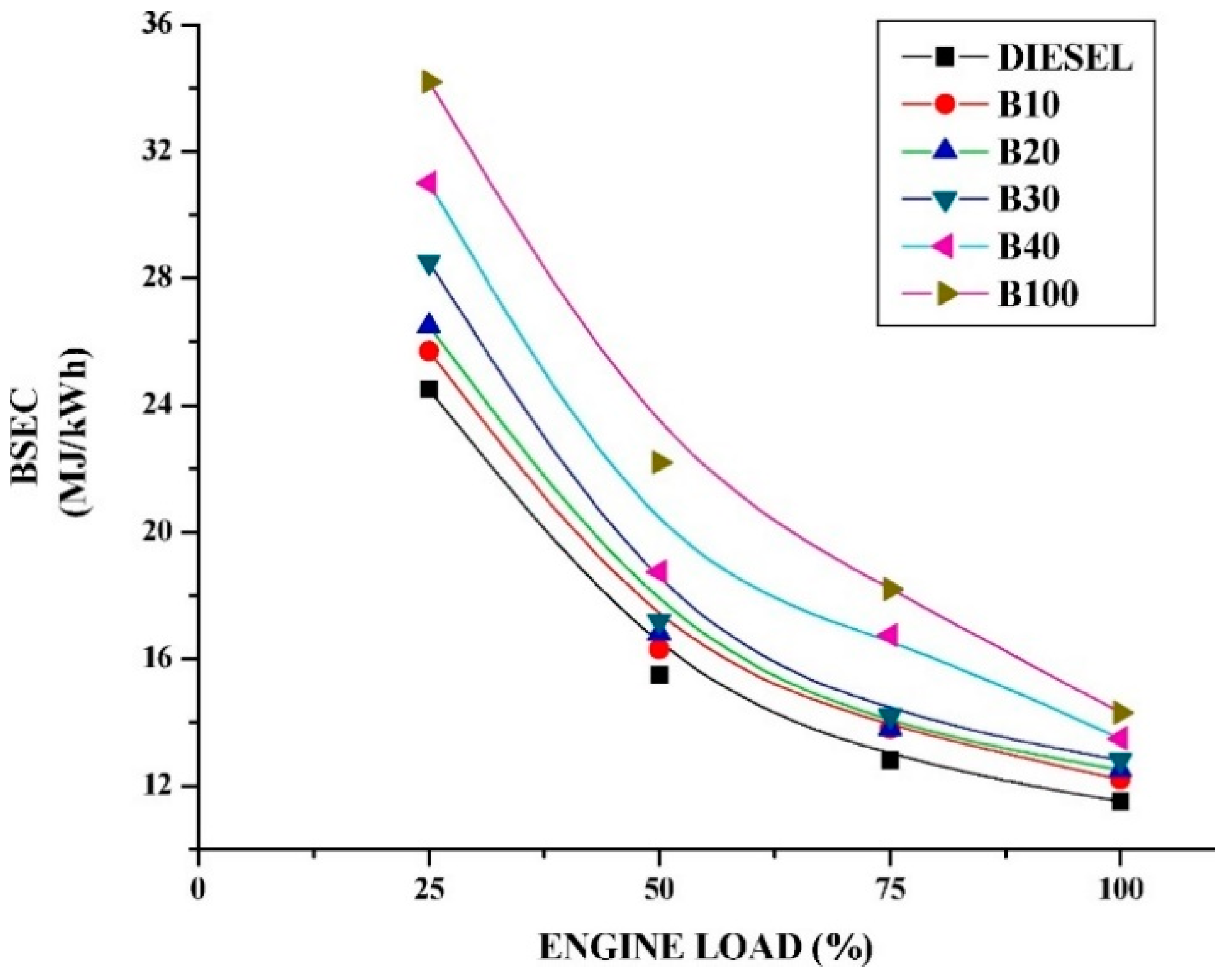
3.2. Brake Specific Energy Consumption
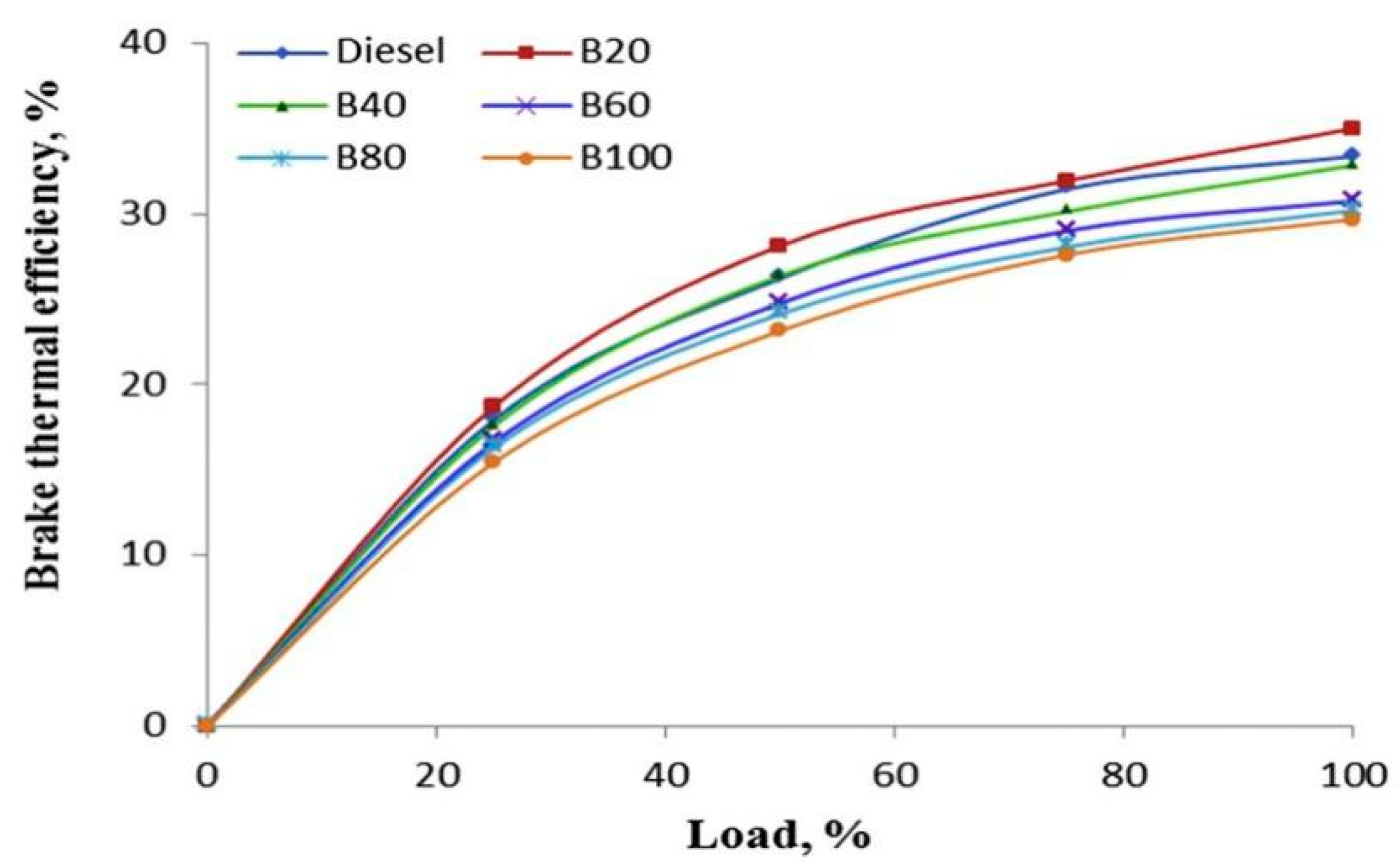
3.3. Brake Thermal Efficiency
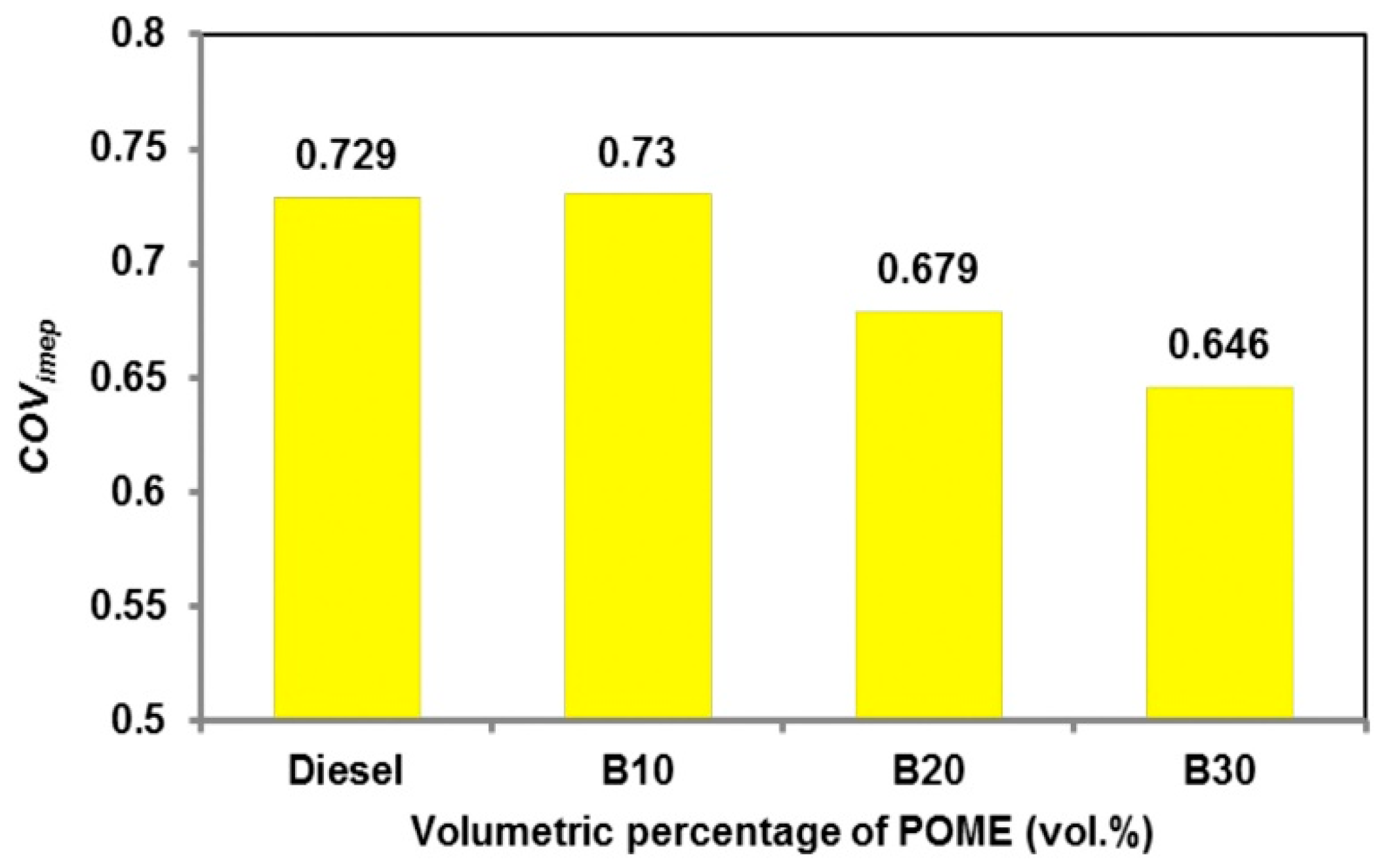
3.4. Coefficient of Variation in Indicated Mean Effective Pressure
3.5. Exhaust Gas Temperature
4. Combustion Characteristics
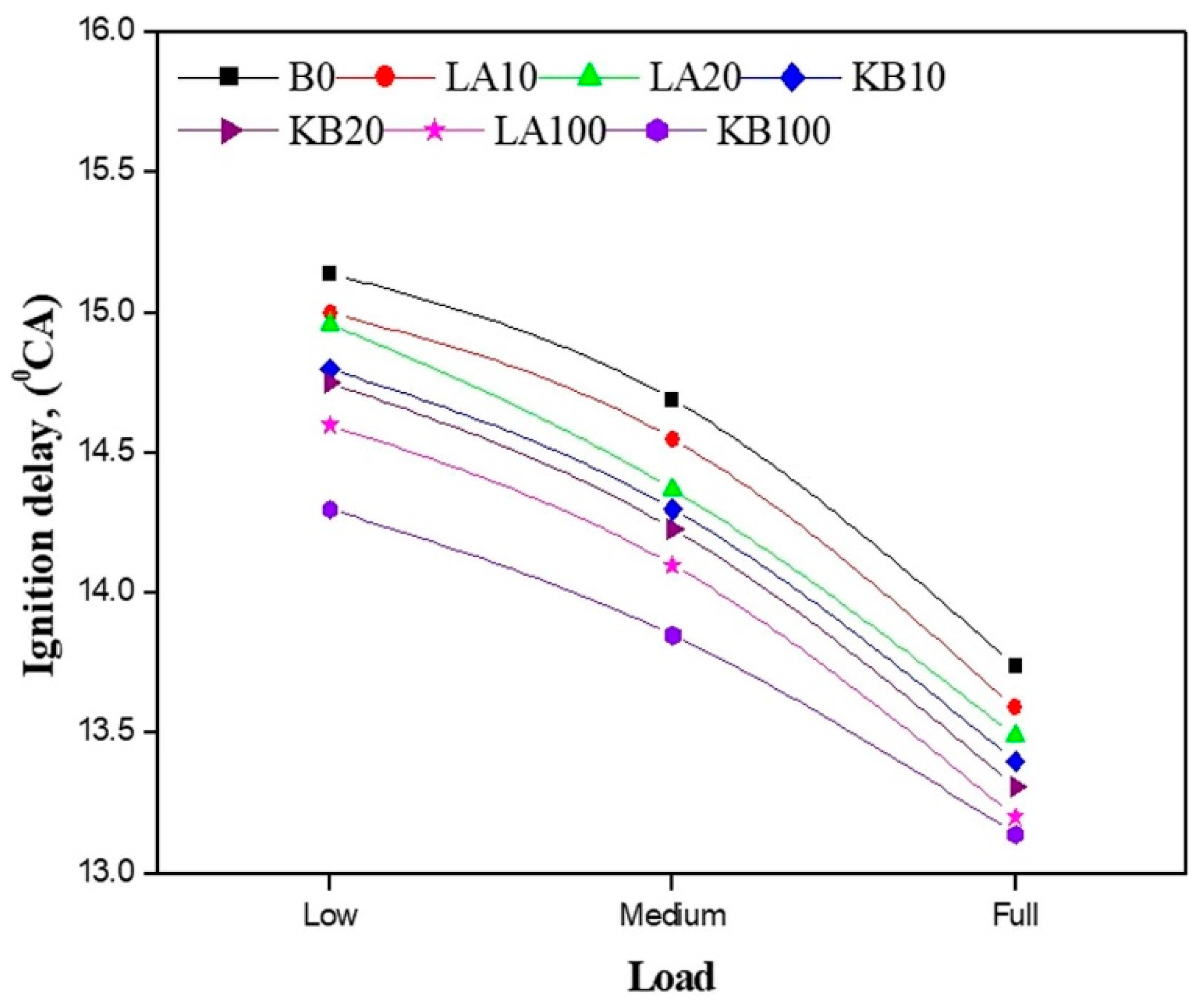
4.1. Ignition Delay
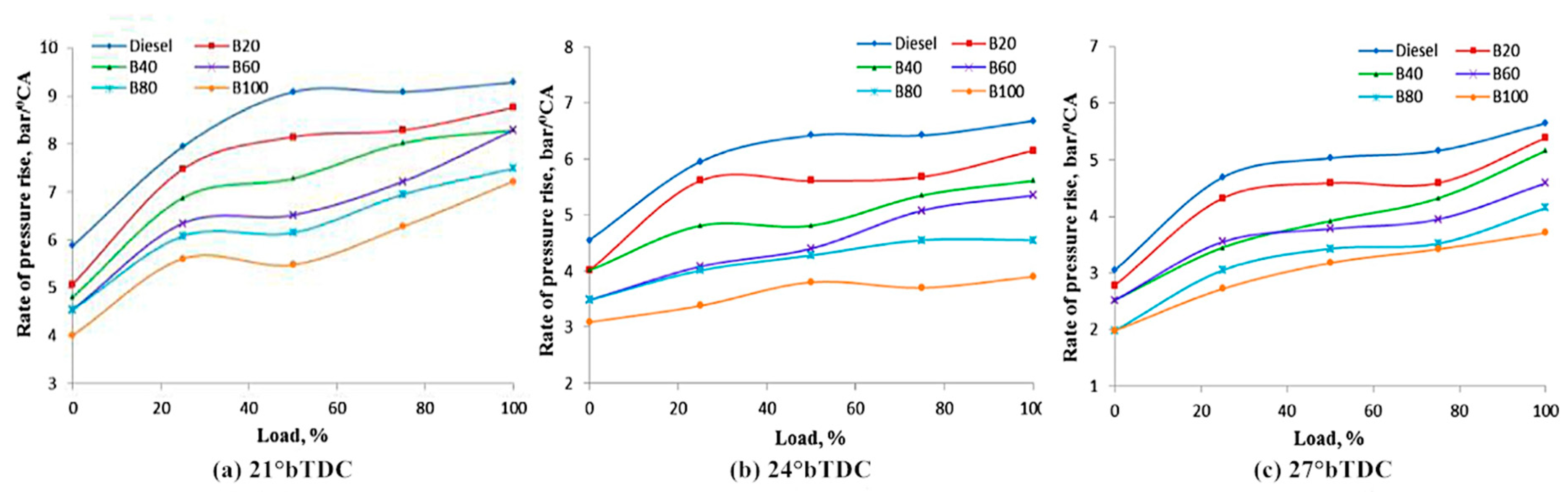
4.2. Cylinder Pressure
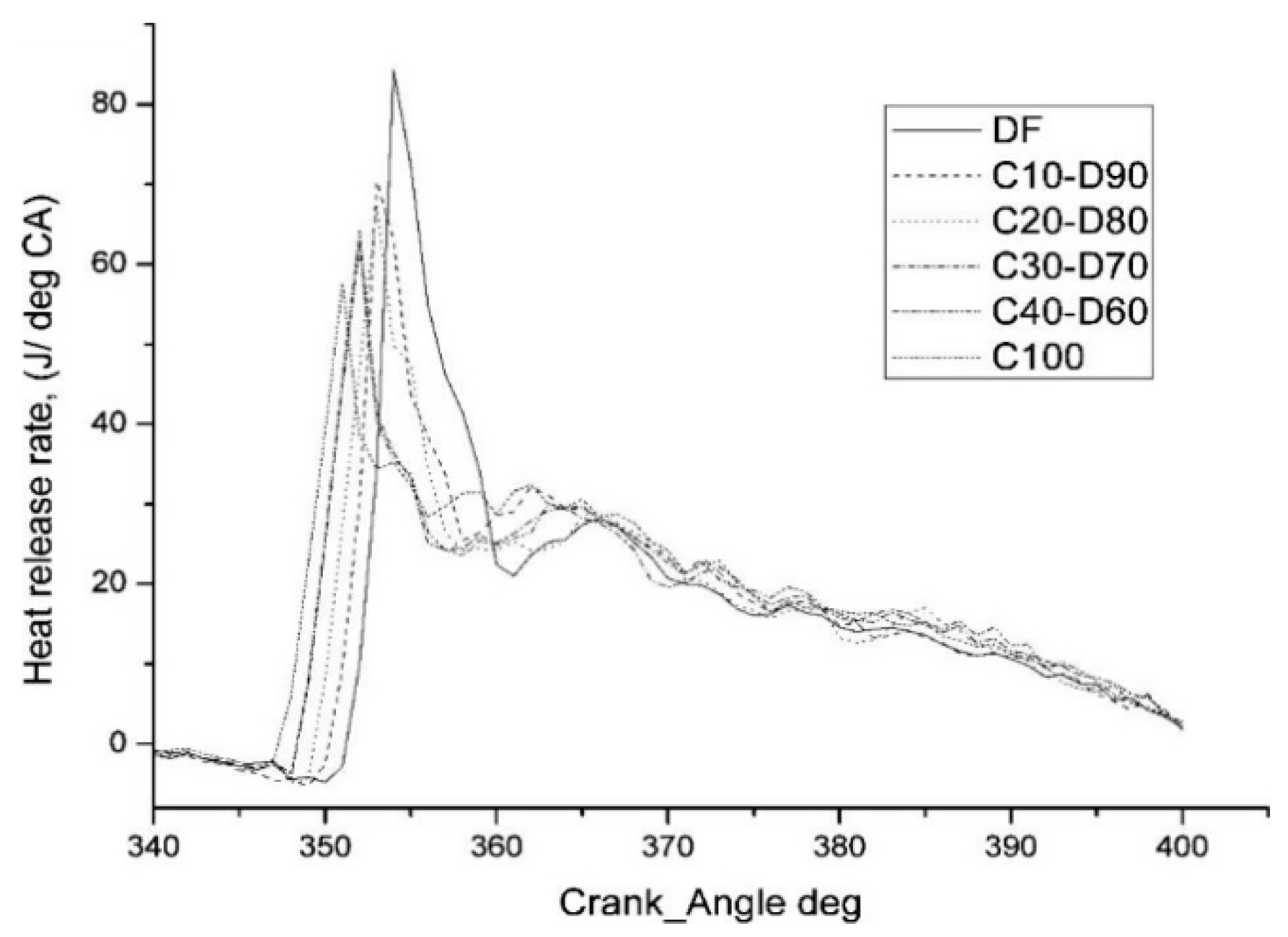
4.3. Heat Release Rate
5. Emission Characteristics
5.1. Regulated Emissions
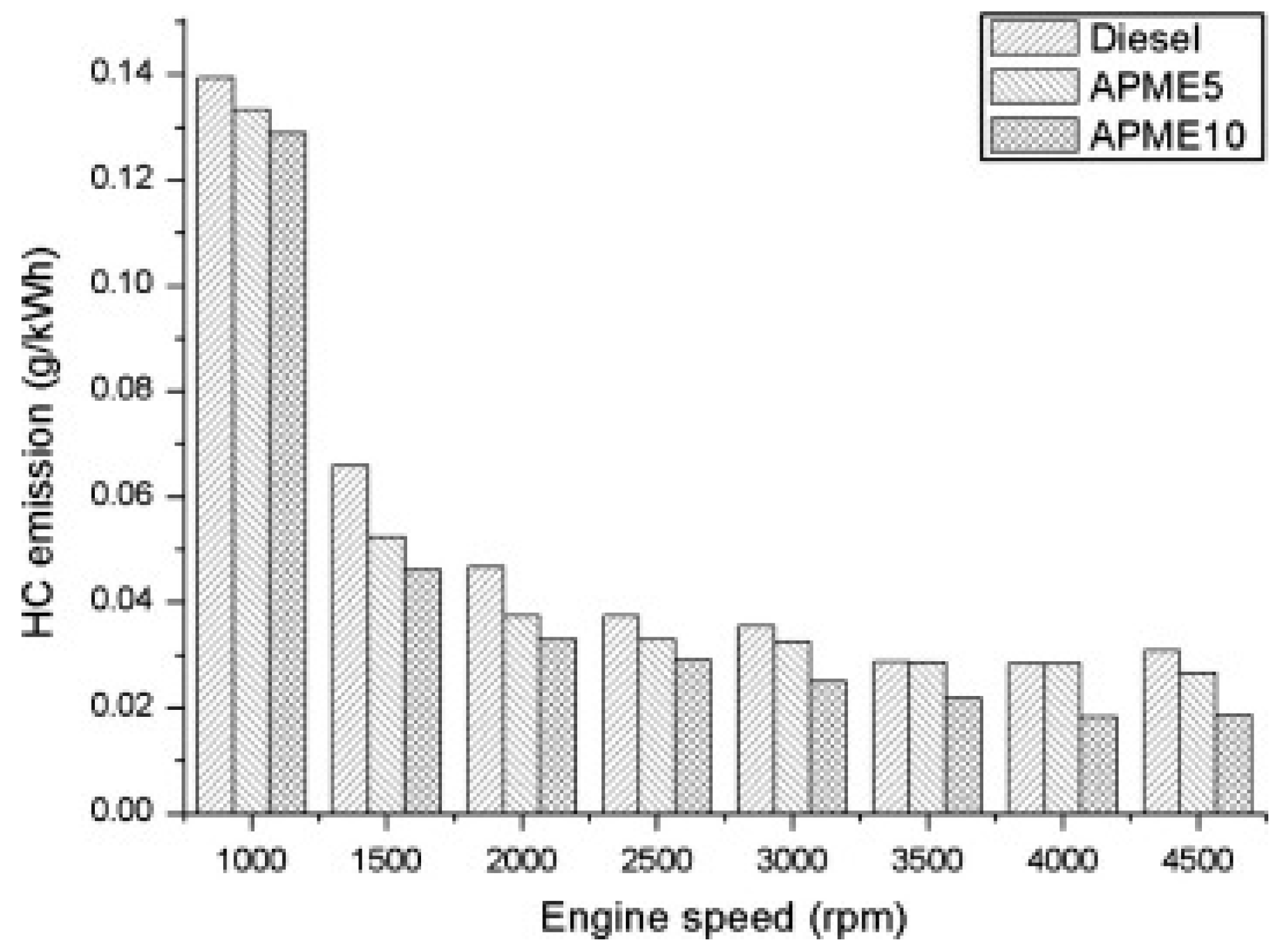
5.1.1. Hydrocarbon
5.1.2. Carbon Monoxide
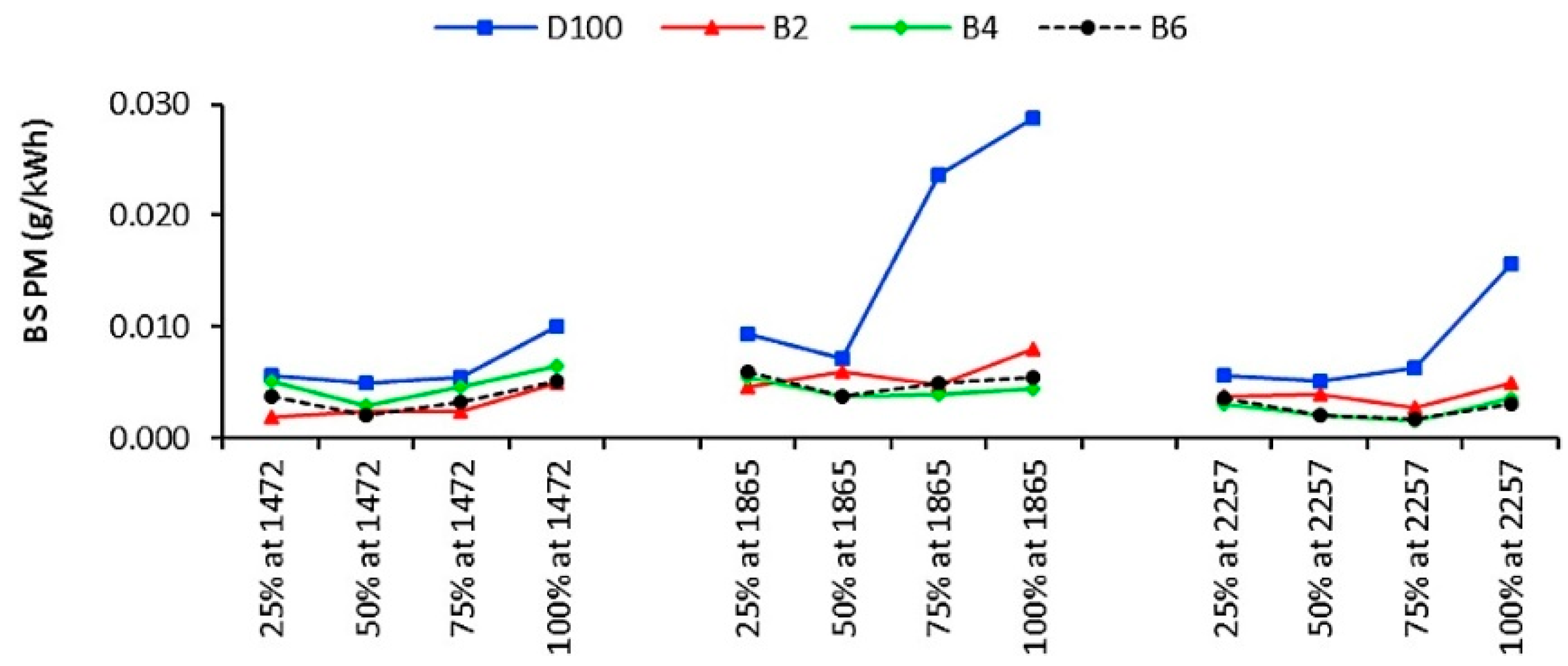
5.1.3. Particulate Matter
5.1.4. Nitrogen Oxides
5.2. Unregulated Emissions
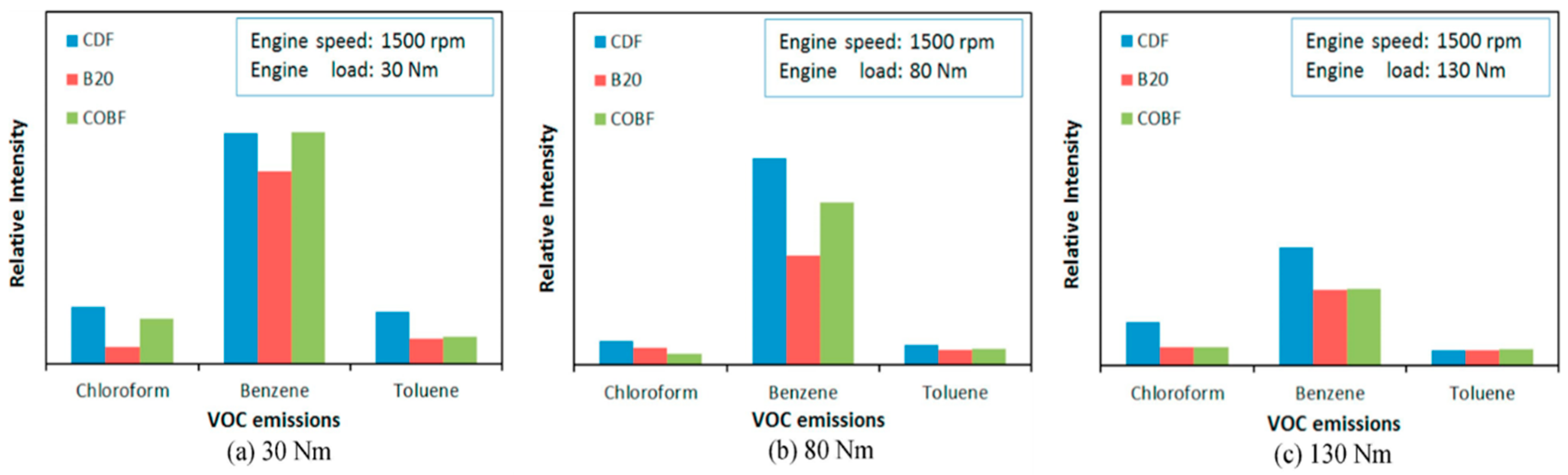
5.2.1. Volatile Organic Compounds
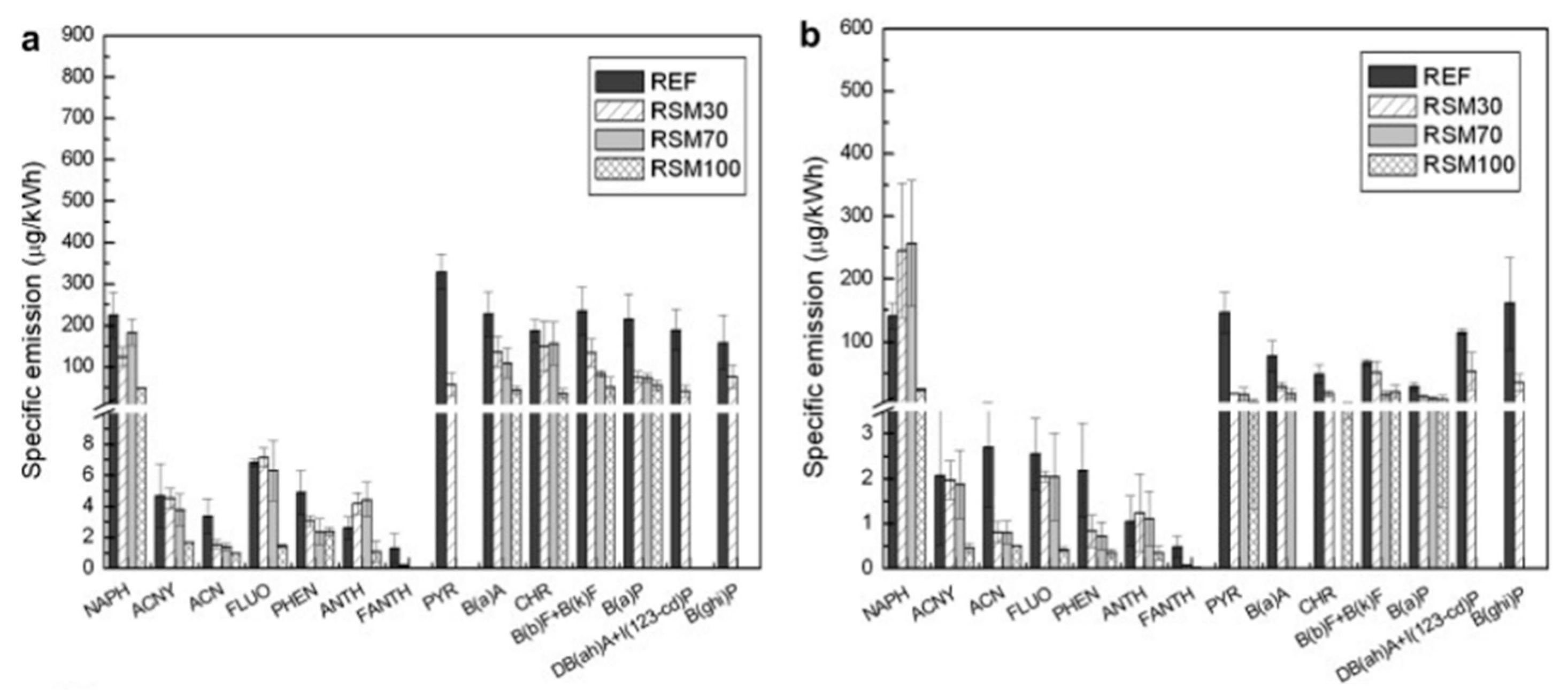
5.2.2. Polycyclic Aromatic Hydrocarbons
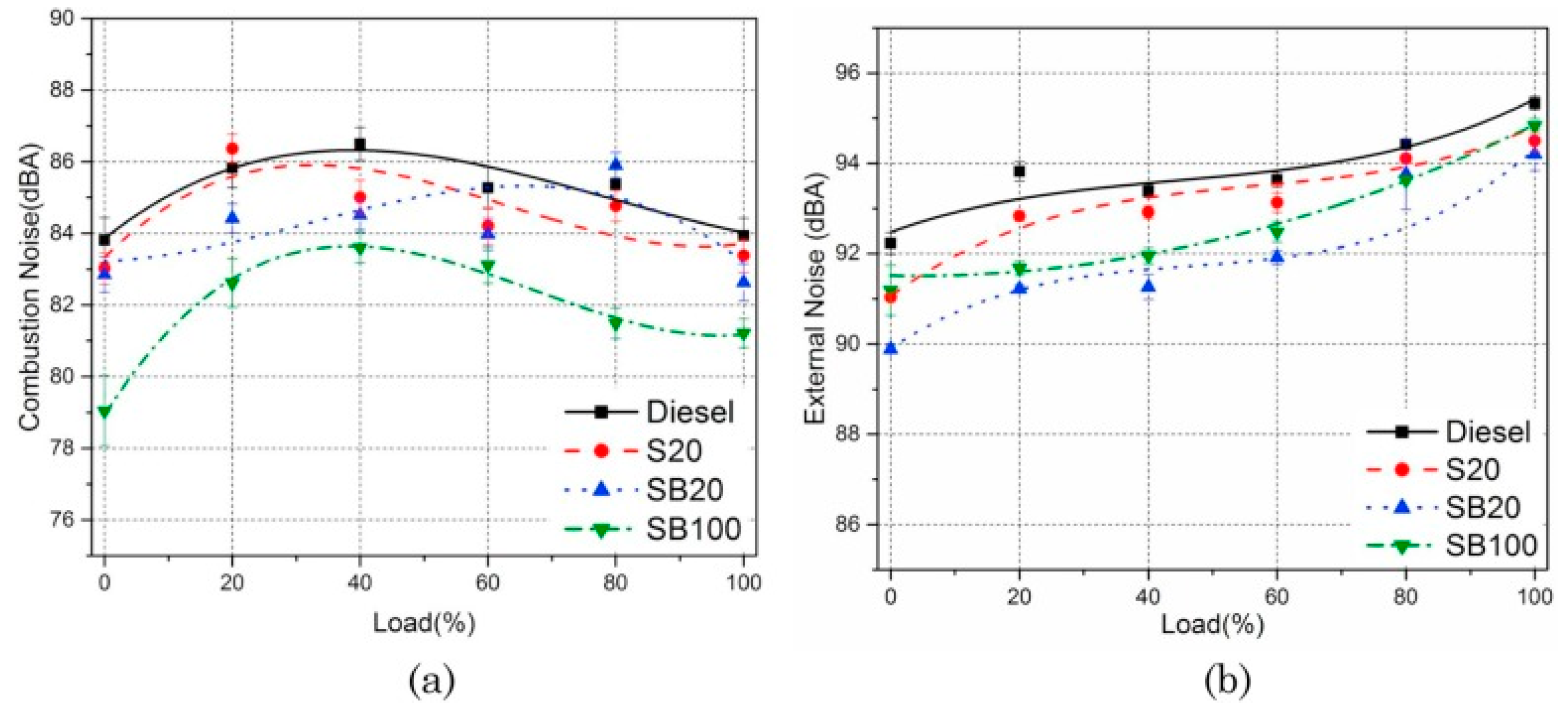
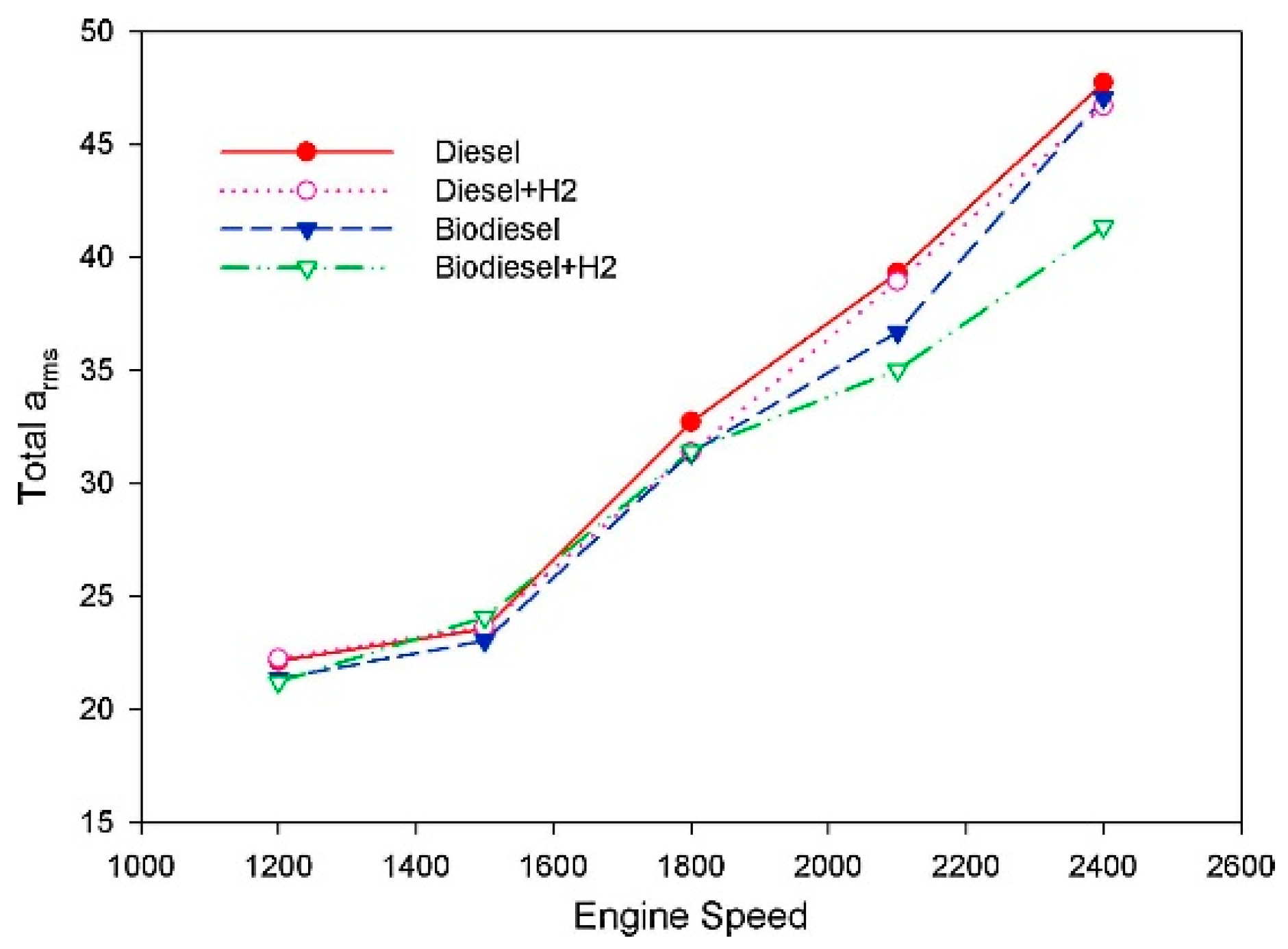
6. Noise and Vibration
7. Compatibility
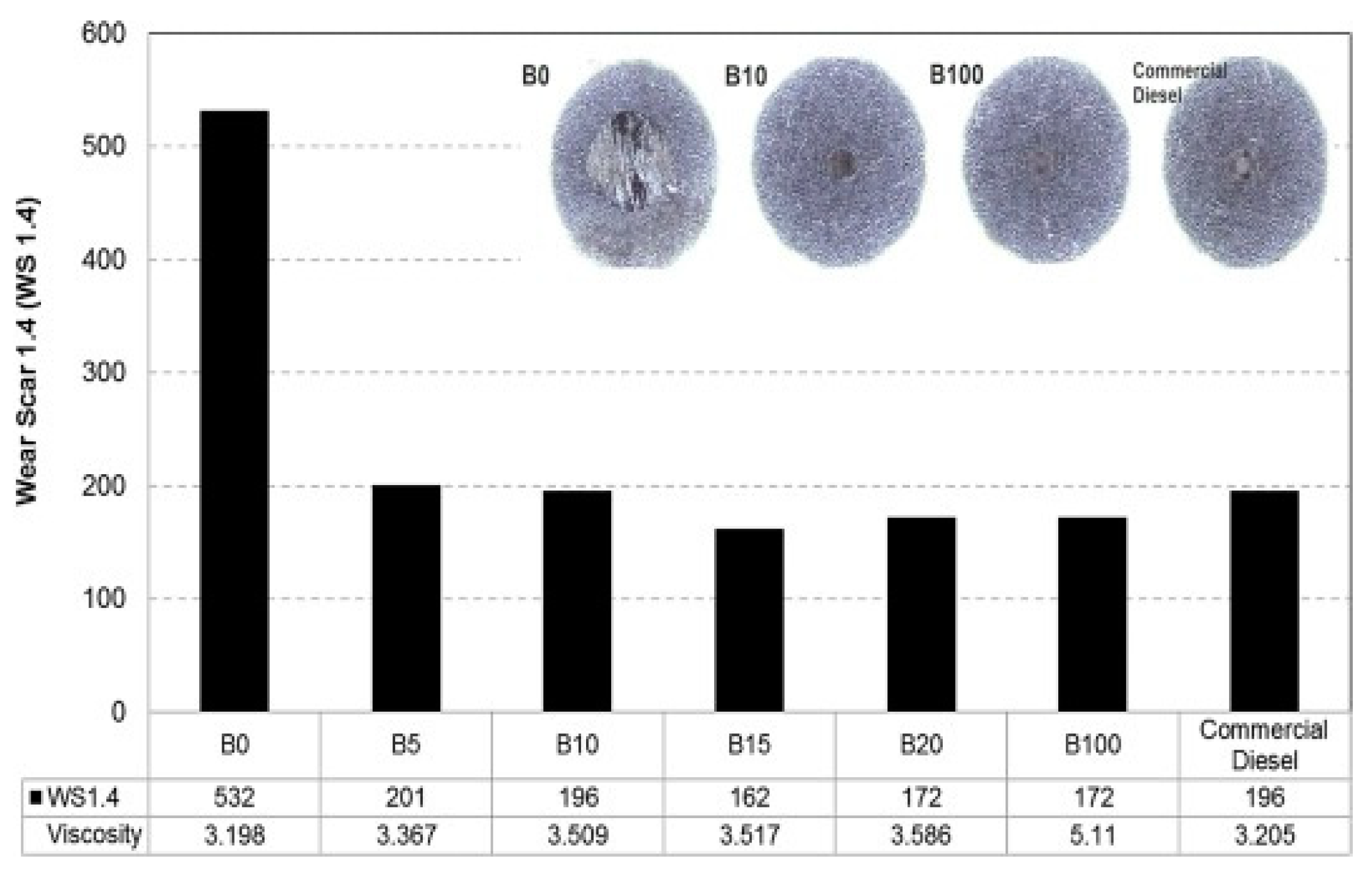
7.1. Lubricity
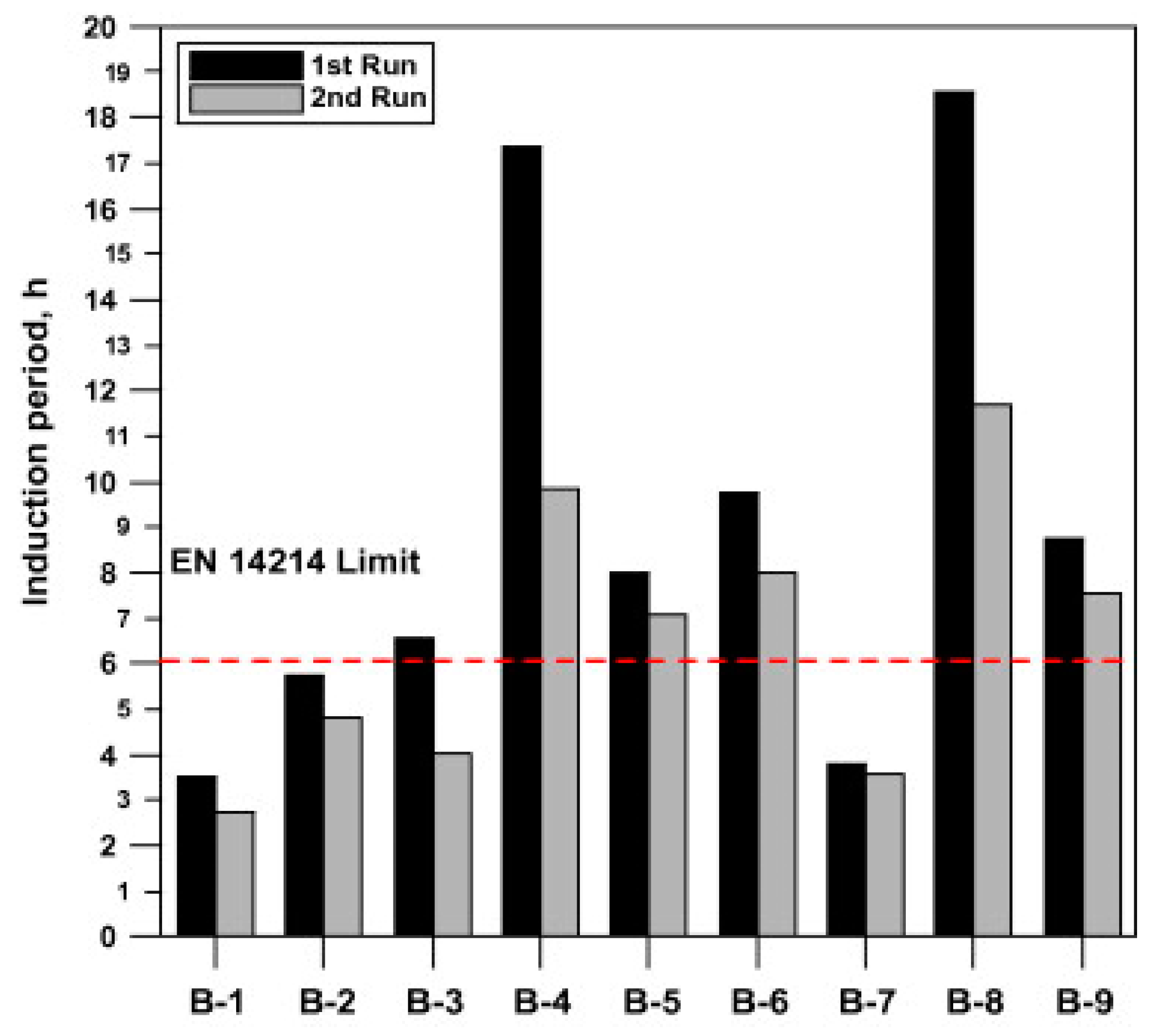
7.2. Oxidation Stability
7.3. Corrosion
8. Conclusions
- ◆
- In most cases, the BSFC and BSEC of biodiesel are slightly higher than that of diesel, and BTE may be slightly lower. COVIMEP is generally low, which will help the engine run smoothly. The increase and decrease of EGT both occur, depending on the specific operating conditions.
- ◆
- The effect of biodiesel on emissions is more obvious. The emissions of HC, CO and PM are significantly reduced due to the addition of biodiesel, but NOx increases. Biodiesel also has a very good reduction effect on VOCs and PAHs emissions.
- ◆
- The ignition delay of biodiesel is generally shorter than that of diesel, and the maximum cylinder pressure would be reduced accordingly. As for the heat release rate, a downward trend in the peak value is usually observed, but the corresponding crank angle seems earlier.
- ◆
- The addition of biodiesel helps reduce engine noise and improve noise quality. At the same time, it would significantly reduce the vibration of the engine in the vertical direction. These changes may be related to the exothermic characteristics of biodiesel.
- ◆
- Biodiesel is generally highly corrosive, which would cause corrosion and deterioration on some metals and elastomers. However, the impact of biodiesel on the fuel system is still poorly understood, and more scientific and detailed investigations are needed.
- ◆
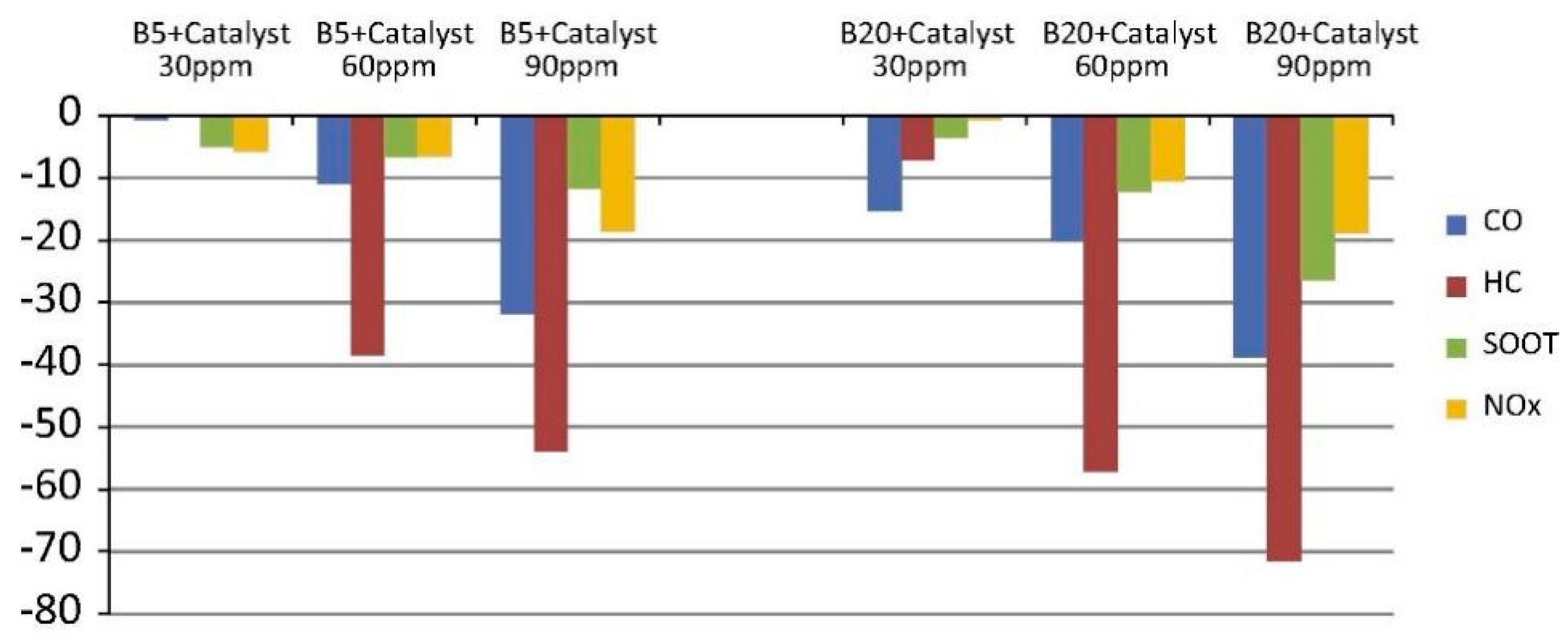
- As for the increased NOx emissions caused by biodiesel, weak oxidation stability, and strong corrosiveness, these problems can be solved by adding special additives. In the future, a multifunctional additive can also be explored to optimize the multiple properties of biodiesel simultaneously.
Author Contributions
Funding
Conflicts of Interest
References
- Rajaeifar, M.A.; Akram, A.; Ghobadian, B.; Rafiee, S.; Heijungs, R.; Tabatabaei, M. Environmental impact assessment of olive pomace oil biodiesel production and consumption: A comparative lifecycle assessment. Energy 2016, 106, 87–102. [Google Scholar] [CrossRef]
- Kodjak, D. Policies to Reduce Fuel Consumption, Air Pollution, and Carbon Emissions from Vehicles in G20 Nations; International Council on Clean Transportation: Washington, DC, USA, 2015. [Google Scholar]
- Man, X.J.; Cheung, C.S.; Ning, Z.; Wei, L.; Huang, Z.H. Influence of engine load and speed on regulated and unregulated emissions of a diesel engine fueled with diesel fuel blended with waste cooking oil biodiesel. Fuel 2016, 180, 41–49. [Google Scholar] [CrossRef]
- Özener, O.; Yüksek, L.; Ergenç, A.T.; Özkan, M. Effects of soybean biodiesel on a DI diesel engine performance, emission and combustion characteristics. Fuel 2014, 115, 875–883. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Demirbas, A. Biodiesel: Hopes and dreads. Biofuel Res. J. 2016, 3, 379. [Google Scholar] [CrossRef] [Green Version]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- How, H.G.; Masjuki, H.H.; Kalam, M.A.; Teoh, Y.H. An investigation of the engine performance, emissions and combustion characteristics of coconut biodiesel in a high-pressure common-rail diesel engine. Energy 2014, 69, 749–759. [Google Scholar] [CrossRef]
- Das, M.; Sarkar, M.; Datta, A.; Santra, A.K. An experimental study on the combustion, performance and emission characteristics of a diesel engine fuelled with diesel-castor oil biodiesel blends. Renew. Energy 2018, 119, 174–184. [Google Scholar] [CrossRef]
- DeCicco, J.M.; Liu, D.Y.; Heo, J.; Krishnan, R.; Kurthen, A.; Wang, L. Carbon balance effects of US biofuel production and use. Clim. Chang. 2016, 138, 667–680. [Google Scholar] [CrossRef] [Green Version]
- Milazzo, M.F.; Spina, F. The use of the risk assessment in the life cycle assessment framework. Manag. Environ. Q. Int. J. 2015, 26, 389–406. [Google Scholar] [CrossRef]
- Curran, M.A. Assessing environmental impacts of biofuels using lifecycle-based approaches. Manag. Environ. Q. Int. J. 2013, 24, 34–52. [Google Scholar] [CrossRef]
- Hu, N.; Tan, J.; Wang, X.; Zhang, X.; Yu, P. Volatile organic compound emissions from an engine fueled with an ethanol-biodiesel-diesel blend. J. Energy Inst. 2017, 90, 101–109. [Google Scholar] [CrossRef]
- Ballesteros, R.; Hernández, J.J.; Lyons, L.L. An experimental study of the influence of biofuel origin on particle-associated PAH emissions. Atmos. Environ. 2010, 44, 930–938. [Google Scholar] [CrossRef]
- Uludamar, E.; Tosun, E.; Aydın, K. Experimental and regression analysis of noise and vibration of a compression ignition engine fuelled with various biodiesels. Fuel 2016, 177, 326–333. [Google Scholar] [CrossRef]
- World Health Organization. Burden of Disease from Environmental Noise: Quantification of Healthy Life Years Lost in Europe; World Health Organization, Regional Office for Europe: Geneva, Switzerland, 2011. [Google Scholar]
- Fattah, I.R.; Masjuki, H.; Liaquat, A.; Ramli, R.; Kalam, M.; Riazuddin, V. Impact of various biodiesel fuels obtained from edible and non-edible oils on engine exhaust gas and noise emissions. Renew. Sustain. Energy Rev. 2013, 18, 552–567. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Investigation of friction and wear characteristics of palm biodiesel. Energy Convers. Manag. 2013, 67, 251–256. [Google Scholar] [CrossRef]
- Habibullah, M.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Masum, B.M.; Arslan, A.; Gulzar, M. Friction and wear characteristics of Calophyllum inophyllum biodiesel. Ind. Crops Prod. 2015, 76, 188–197. [Google Scholar] [CrossRef]
- Muñoz, M.; Moreno, F.; Monné, C.; Morea, J.; Terradillos, J. Biodiesel improves lubricity of new low sulphur diesel fuels. Renew. Energy 2011, 36, 2918–2924. [Google Scholar] [CrossRef]
- Wadumesthrige, K.; Ara, M.; Salley, S.O.; Ng, K.S. Investigation of lubricity characteristics of biodiesel in petroleum and synthetic fuel. Energy Fuels 2009, 23, 2229–2234. [Google Scholar] [CrossRef]
- Tamilselvan, P.; Nallusamy, N.; Rajkumar, S. A comprehensive review on performance, combustion and emission characteristics of biodiesel fuelled diesel engines. Renew. Sustain. Energy Rev. 2017, 79, 1134–1159. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Giakoumis, E.G. A statistical investigation of biodiesel physical and chemical properties, and their correlation with the degree of unsaturation. Renew. Energy 2013, 50, 858–878. [Google Scholar] [CrossRef]
- Graboski, M.S.; McCormick, R.L. Combustion of fat and vegetable oil derived fuels in diesel engines. Prog. Energy Combust. Sci. 1998, 24, 125–164. [Google Scholar] [CrossRef]
- Singh, M.; Sandhu, S.S. Performance, emission and combustion characteristics of multi-cylinder CRDI engine fueled with argemone biodiesel/diesel blends. Fuel 2020, 265, 117024. [Google Scholar] [CrossRef]
- Teoh, Y.H.; How, H.G.; Masjuki, H.H.; Nguyen, H.T.; Kalam, M.A.; Alabdulkarem, A. Investigation on particulate emissions and combustion characteristics of a common-rail diesel engine fueled with Moringa oleifera biodiesel-diesel blends. Renew. Energy 2019, 136, 521–534. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Dhar, A.; Gupta, J.G.; Kim, W.I.; Choi, K.; Lee, C.S.; Park, S. Effect of fuel injection pressure and injection timing of Karanja biodiesel blends on fuel spray, engine performance, emissions and combustion characteristics. Energy Convers. Manag. 2015, 91, 302–314. [Google Scholar] [CrossRef]
- El-Kasaby, M.; Nemit-allah, M.A. Experimental investigations of ignition delay period and performance of a diesel engine operated with Jatropha oil biodiesel. Alex. Eng. J. 2013, 52, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Monirul, I.M.; Masjuki, H.H.; Kalam, M.A.; Mosarof, M.H.; Zulkifli, N.W.M.; Teoh, Y.H.; How, H.G. Assessment of performance, emission and combustion characteristics of palm, jatropha and Calophyllum inophyllum biodiesel blends. Fuel 2016, 181, 985–995. [Google Scholar] [CrossRef]
- Alagu, K.; Venu, H.; Jayaraman, J.; Raju, V.D.; Subramani, L.; Appavu, P.; Dhanasekar, S. Novel water hyacinth biodiesel as a potential alternative fuel for existing unmodified diesel engine: Performance, combustion and emission characteristics. Energy 2019, 179, 295–305. [Google Scholar] [CrossRef]
- Nantha Gopal, K.; Thundil Karupparaj, R. Effect of pongamia biodiesel on emission and combustion characteristics of DI compression ignition engine. Ain Shams Eng. J. 2015, 6, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Alloune, R.; Balistrou, M.; Awad, S.; Loubar, K.; Tazerout, M. Performance, combustion and exhaust emissions characteristics investigation using Citrullus colocynthis L. biodiesel in DI diesel engine. J. Energy Inst. 2018, 91, 434–444. [Google Scholar] [CrossRef]
- Gnanasekaran, S.; Saravanan, N.; Ilangkumaran, M. Influence of injection timing on performance, emission and combustion characteristics of a DI diesel engine running on fish oil biodiesel. Energy 2016, 116, 1218–1229. [Google Scholar] [CrossRef]
- Dhamodaran, G.; Krishnan, R.; Pochareddy, Y.K.; Pyarelal, H.M.; Sivasubramanian, H.; Ganeshram, A.K. A comparative study of combustion, emission, and performance characteristics of rice-bran-, neem-, and cottonseed-oil biodiesels with varying degree of unsaturation. Fuel 2017, 187, 296–305. [Google Scholar] [CrossRef]
- Uyumaz, A. Experimental evaluation of linseed oil biodiesel/diesel fuel blends on combustion, performance and emission characteristics in a DI diesel engine. Fuel 2020, 267, 117150. [Google Scholar] [CrossRef]
- Turkcan, A. The effects of different types of biodiesels and biodiesel-bioethanol-diesel blends on the cyclic variations and correlation coefficient. Fuel 2020, 261, 116453. [Google Scholar] [CrossRef]
- Alptekin, E. Emission, injection and combustion characteristics of biodiesel and oxygenated fuel blends in a common rail diesel engine. Energy 2017, 119, 44–52. [Google Scholar] [CrossRef]
- Raman, L.A.; Deepanraj, B.; Rajakumar, S.; Sivasubramanian, V. Experimental investigation on performance, combustion and emission analysis of a direct injection diesel engine fuelled with rapeseed oil biodiesel. Fuel 2019, 246, 69–74. [Google Scholar] [CrossRef]
- Qi, D.H.; Chen, H.; Geng, L.M.; Bian, Y.Z. Experimental studies on the combustion characteristics and performance of a direct injection engine fueled with biodiesel/diesel blends. Energy Convers. Manag. 2010, 51, 2985–2992. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Using canola oil biodiesel as an alternative fuel in diesel engines: A review. Appl. Sci. 2017, 7, 881. [Google Scholar] [CrossRef]
- Can, Ö. Combustion characteristics, performance and exhaust emissions of a diesel engine fueled with a waste cooking oil biodiesel mixture. Energy Convers. Manag 2014, 87, 676–686. [Google Scholar] [CrossRef]
- EL_Kassaby, M.; Nemit_allah, M.A. Studying the effect of compression ratio on an engine fueled with waste oil produced biodiesel/diesel fuel. Alex. Eng. J. 2013, 52, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Goga, G.; Chauhan, B.S.; Mahla, S.K.; Cho, H.M.; Dhir, A.; Lim, H.C. Properties and characteristics of various materials used as biofuels: A review. Mater. Today Proc. 2018, 5, 28438–28445. [Google Scholar] [CrossRef]
- Ali, O.M.; Mamat, R.; Abdullah, N.R.; Abdullah, A.A. Analysis of blended fuel properties and engine performance with palm biodiesel–diesel blended fuel. Renew. Energy 2016, 86, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Habibullah, M.; Masjuki, H.H.; Kalam, M.; Fattah, I.R.; Ashraful, A.; Mobarak, H. Biodiesel production and performance evaluation of coconut, palm and their combined blend with diesel in a single-cylinder diesel engine. Energy Convers. Manag. 2014, 87, 250–257. [Google Scholar] [CrossRef]
- Buyukkaya, E. Effects of biodiesel on a DI diesel engine performance, emission and combustion characteristics. Fuel 2010, 89, 3099–3105. [Google Scholar] [CrossRef]
- Rahman, S.A.; Masjuki, H.; Kalam, M.; Abedin, M.; Sanjid, A.; Rahman, M.M. Assessing idling effects on a compression ignition engine fueled with Jatropha and Palm biodiesel blends. Renew. Energy 2014, 68, 644–650. [Google Scholar] [CrossRef]
- Gumus, M.; Kasifoglu, S. Performance and emission evaluation of a compression ignition engine using a biodiesel (apricot seed kernel oil methyl ester) and its blends with diesel fuel. Biomass Bioenergy 2010, 34, 134–139. [Google Scholar] [CrossRef]
- Teoh, Y.; Yu, K.H.; How, H.; Nguyen, H.-T. Experimental investigation of performance, emission and combustion characteristics of a common-rail diesel engine fuelled with bioethanol as a fuel additive in coconut oil biodiesel blends. Energies 2019, 12, 1954. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.C.; Yoon, S.K.; Kim, M.S.; Choi, N.J. Application of canola oil biodiesel/diesel blends in a common rail diesel engine. Appl. Sci. 2017, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Ge, J.C.; Choi, N.J. Effects of fuel injection pressure on combustion and emission characteristics under low speed conditions in a diesel engine fueled with palm oil biodiesel. Energies 2019, 12, 3264. [Google Scholar] [CrossRef] [Green Version]
- Asokan, M.A.; Senthur Prabu, S.; Bade, P.K.K.; Nekkanti, V.M.; Gutta, S.S.G. Performance, combustion and emission characteristics of juliflora biodiesel fuelled DI diesel engine. Energy 2019, 173, 883–892. [Google Scholar] [CrossRef]
- Raheman, H.; Kumari, S. Combustion characteristics and emissions of a compression ignition engine using emulsified jatropha biodiesel blend. Biosyst. Eng. 2014, 123, 29–39. [Google Scholar] [CrossRef]
- Dhinesh, B.; Isaac JoshuaRamesh Lalvani, J.; Parthasarathy, M.; Annamalai, K. An assessment on performance, emission and combustion characteristics of single cylinder diesel engine powered by Cymbopogon flexuosus biofuel. Energy Convers. Manag. 2016, 117, 466–474. [Google Scholar] [CrossRef]
- Rajak, U.; Verma, T.N. Spirulina microalgae biodiese—A novel renewable alternative energy source for compression ignition engine. J. Clean. Prod. 2018, 201, 343–357. [Google Scholar] [CrossRef]
- Uyumaz, A. Combustion, performance and emission characteristics of a DI diesel engine fueled with mustard oil biodiesel fuel blends at different engine loads. Fuel 2018, 212, 256–267. [Google Scholar] [CrossRef]
- Ceviz, M.A.; Yüksel, F. Effects of ethanol–unleaded gasoline blends on cyclic variability and emissions in an SI engine. Appl. Therm. Eng. 2005, 25, 917–925. [Google Scholar] [CrossRef]
- Gürgen, S.; Ünver, B.; Altın, İ. Prediction of cyclic variability in a diesel engine fueled with n-butanol and diesel fuel blends using artificial neural network. Renew. Energy 2018, 117, 538–544. [Google Scholar] [CrossRef]
- Gharehghani, A.; Mirsalim, M.; Hosseini, R. Effects of waste fish oil biodiesel on diesel engine combustion characteristics and emission. Renew. Energy 2017, 101, 930–936. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Masjuki, H.H.; Kalam, M.A.; Abedin, M.J.; Sanjid, A.; Sajjad, H. Production of palm and Calophyllum inophyllum based biodiesel and investigation of blend performance and exhaust emission in an unmodified diesel engine at high idling conditions. Energy Convers. Manag. 2013, 76, 362–367. [Google Scholar] [CrossRef]
- Sanjid, A.; Masjuki, H.H.; Kalam, M.A.; Rahman, S.M.A.; Abedin, M.J.; Palash, S.M. Impact of palm, mustard, waste cooking oil and Calophyllum inophyllum biofuels on performance and emission of CI engine. Renew. Sustain. Energy Rev. 2013, 27, 664–682. [Google Scholar] [CrossRef]
- Anwar, M.; Rasul, M.G.; Ashwath, N. A pragmatic and critical analysis of engine emissions for biodiesel blended fuels. Fuel 2020, 270, 117513. [Google Scholar] [CrossRef]
- Azad, A.K.; Rasul, M.; Khan, M.M.; Sharma, S. Macadamia Biodiesel as a Sustainable and Alternative Transport Fuel in Australia. Energy Procedia 2017, 110, 543–548. [Google Scholar] [CrossRef]
- Altaie, M.A.H.; Janius, R.B.; Rashid, U.; Taufiq-Yap, Y.H.; Yunus, R.; Zakaria, R.; Adam, N.M. Performance and exhaust emission characteristics of direct-injection diesel engine fueled with enriched biodiesel. Energy Convers. Manag. 2015, 106, 365–372. [Google Scholar] [CrossRef]
- Elkelawy, M.; Alm-Eldin Bastawissi, H.; Esmaeil, K.K.; Radwan, A.M.; Panchal, H.; Sadasivuni, K.K.; Ponnamma, D.; Walvekar, R. Experimental studies on the biodiesel production parameters optimization of sunflower and soybean oil mixture and DI engine combustion, performance, and emission analysis fueled with diesel/biodiesel blends. Fuel 2019, 255, 115791. [Google Scholar] [CrossRef]
- Shrivastava, P.; Nath Verma, T. An experimental investigation into engine characteristics fueled with Lal ambari biodiesel and its blends. Therm. Sci. Eng. Prog. 2020, 17, 100356. [Google Scholar] [CrossRef]
- Sakthivel, G. Prediction of CI engine performance, emission and combustion characteristics using fish oil as a biodiesel at different injection timing using fuzzy logic. Fuel 2016, 183, 214–229. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Nguyen, D.D.; Shobana, S.; Saratale, G.D.; Arvindnarayan, S.; Atabani, A.E.; Chang, S.W.; Kumar, G. Engine performance, emission and bio characteristics of rice bran oil derived biodiesel blends. Fuel 2019, 239, 153–161. [Google Scholar] [CrossRef]
- Shameer, P.M.; Ramesh, K. Assessment on the consequences of injection timing and injection pressure on combustion characteristics of sustainable biodiesel fuelled engine. Renew. Sustain. Energy Rev. 2018, 81, 45–61. [Google Scholar] [CrossRef]
- Aldhaidhawi, M.; Chiriac, R.; Badescu, V. Ignition delay, combustion and emission characteristics of Diesel engine fueled with rapeseed biodiesel—A literature review. Renew. Sustain. Energy Rev. 2017, 73, 178–186. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Liaquat, A.M.; Masjuki, H.H.; Kalam, M.A.; Mofijur, M. Ignition delay, combustion and emission characteristics of diesel engine fueled with biodiesel. Renew. Sustain. Energy Rev. 2013, 21, 623–632. [Google Scholar] [CrossRef]
- Lakshminarayanan, P.; Aghav, Y.V. Ignition delay in a diesel engine. In Modelling Diesel Combustion; Springer: Berlin/Heidelberg, Germany, 2010; pp. 59–78. [Google Scholar]
- Shrivastava, P.; Verma, T.N.; Pugazhendhi, A. An experimental evaluation of engine performance and emisssion characteristics of CI engine operated with Roselle and Karanja biodiesel. Fuel 2019, 254, 115652. [Google Scholar] [CrossRef]
- Hoang, V.N.; Thi, L.D. Experimental study of the ignition delay of diesel/biodiesel blends using a shock tube. Biosyst. Eng. 2015, 134, 1–7. [Google Scholar] [CrossRef]
- Guardiola, C.; Triantopoulos, V.; Bares, P.; Bohac, S.; Stefanopoulou, A. Simultaneous estimation of intake and residual mass using in-cylinder pressure in an engine with negative valve overlap. IFAC PapersOnLine 2016, 49, 461–468. [Google Scholar] [CrossRef]
- Shahlari, A.J.; Kurtz, E.; Hocking, C.; Antonov, S. Correlation of cylinder pressure–based engine noise metrics to measured microphone data. Int. J. Eng. Res. 2015, 16, 829–850. [Google Scholar] [CrossRef]
- Jaliliantabar, F.; Ghobadian, B.; Carlucci, A.P.; Najafi, G.; Ficarella, A.; Strafella, L.; Santino, A.; De Domenico, S. Comparative evaluation of physical and chemical properties, emission and combustion characteristics of brassica, cardoon and coffee based biodiesels as fuel in a compression-ignition engine. Fuel 2018, 222, 156–174. [Google Scholar] [CrossRef]
- Shehata, M.S.; Attia, A.M.A.; Abdel Razek, S.M. Corn and soybean biodiesel blends as alternative fuels for diesel engine at different injection pressures. Fuel 2015, 161, 49–58. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Das, L.M. Combustion analysis of Jatropha, Karanja and Polanga based biodiesel as fuel in a diesel engine. Fuel 2009, 88, 994–999. [Google Scholar] [CrossRef]
- Wei, L.; Cheung, C.S.; Ning, Z. Influence of waste cooking oil biodiesel on combustion, unregulated gaseous emissions and particulate emissions of a direct-injection diesel engine. Energy 2017, 127, 175–185. [Google Scholar] [CrossRef]
- Indudhar, M.; Banapurmath, N.; Rajulu, K.G.; Khandal, S. Effect of injection timing and injection pressure on the performance of biodiesel ester of hongeoil fuelled common rail direct injection (CRDI) engine. Int. J. Eng. Sci. Technol. 2015, 7, 37–48. [Google Scholar] [CrossRef]
- Kathirvelu, B.; Subramanian, S.; Kathirvelu, B.; Subramanian, S. Performance and emission characteristics of biodiesel blends in a premixed compression ignition engine with exhaust gas recirculation. Environ. Eng. Res. 2017, 22, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Imtenan, S.; Varman, M.; Masjuki, H.H.; Kalam, M.A.; Sajjad, H.; Arbab, M.I.; Rizwanul Fattah, I.M. Impact of low temperature combustion attaining strategies on diesel engine emissions for diesel and biodiesels: A review. Energy Convers. Manag. 2014, 80, 329–356. [Google Scholar] [CrossRef]
- Mwangi, J.K.; Lee, W.-J.; Chang, Y.-C.; Chen, C.-Y.; Wang, L.-C. An overview: Energy saving and pollution reduction by using green fuel blends in diesel engines. Appl. Energy 2015, 159, 214–236. [Google Scholar] [CrossRef]
- Han, D.; Ickes, A.M.; Bohac, S.V.; Huang, Z.; Assanis, D.N. HC and CO emissions of premixed low-temperature combustion fueled by blends of diesel and gasoline. Fuel 2012, 99, 13–19. [Google Scholar] [CrossRef]
- Perumal, V.; Ilangkumaran, M. Experimental analysis of engine performance, combustion and emission using pongamia biodiesel as fuel in CI engine. Energy 2017, 129, 228–236. [Google Scholar] [CrossRef]
- Palash, S.M.; Masjuki, H.H.; Kalam, M.A.; Atabani, A.E.; Rizwanul Fattah, I.M.; Sanjid, A. Biodiesel production, characterization, diesel engine performance, and emission characteristics of methyl esters from Aphanamixis polystachya oil of Bangladesh. Energy Convers. Manag. 2015, 91, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Sanjid, A.; Kalam, M.A.; Masjuki, H.H.; Varman, M.; Zulkifli, N.W.B.M.; Abedin, M.J. Performance and emission of multi-cylinder diesel engine using biodiesel blends obtained from mixed inedible feedstocks. J. Clean. Prod. 2016, 112, 4114–4122. [Google Scholar] [CrossRef]
- Arunkumar, M.; Kannan, M.; Murali, G. Experimental studies on engine performance and emission characteristics using castor biodiesel as fuel in CI engine. Renew. Energy 2019, 131, 737–744. [Google Scholar] [CrossRef]
- Rajak, U.; Nashine, P.; Verma, T.N. Assessment of diesel engine performance using spirulina microalgae biodiesel. Energy 2019, 166, 1025–1036. [Google Scholar] [CrossRef]
- Can, Ö.; Öztürk, E.; Yücesu, H.S. Combustion and exhaust emissions of canola biodiesel blends in a single cylinder DI diesel engine. Renew. Energy 2017, 109, 73–82. [Google Scholar] [CrossRef]
- Nabi, M.N.; Rahman, M.M.; Islam, M.A.; Hossain, F.M.; Brooks, P.; Rowlands, W.N.; Tulloch, J.; Ristovski, Z.D.; Brown, R.J. Fuel characterisation, engine performance, combustion and exhaust emissions with a new renewable Licella biofuel. Energy Convers. Manag. 2015, 96, 588–598. [Google Scholar] [CrossRef]
- Özçelik, A.E.; Aydoğan, H.; Acaroğlu, M. Determining the performance, emission and combustion properties of camelina biodiesel blends. Energy Convers. Manag. 2015, 96, 47–57. [Google Scholar] [CrossRef]
- Gad, M.S.; El-Araby, R.; Abed, K.A.; El-Ibiari, N.N.; El Morsi, A.K.; El-Diwani, G.I. Performance and emissions characteristics of C.I. engine fueled with palm oil/palm oil methyl ester blended with diesel fuel. Egypt. J. Pet. 2018, 27, 215–219. [Google Scholar] [CrossRef]
- Tüccar, G.; Tosun, E.; Özgür, T.; Aydın, K. Diesel engine emissions and performance from blends of citrus sinensis biodiesel and diesel fuel. Fuel 2014, 132, 7–11. [Google Scholar] [CrossRef]
- Al-lwayzy, S.H.; Yusaf, T. Diesel engine performance and exhaust gas emissions using Microalgae Chlorella protothecoides biodiesel. Renew. Energy 2017, 101, 690–701. [Google Scholar] [CrossRef]
- An, H.; Yang, W.; Chou, S.; Chua, K. Combustion and emissions characteristics of diesel engine fueled by biodiesel at partial load conditions. Appl. Energy 2012, 99, 363–371. [Google Scholar] [CrossRef]
- Mohankumar, S.; Senthilkumar, P. Particulate matter formation and its control methodologies for diesel engine: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 1227–1238. [Google Scholar] [CrossRef]
- Yusop, A.F.; Mamat, R.; Yusaf, T.; Najafi, G.; Yasin, M.H.M.; Khathri, A.M. Analysis of particulate matter (pm) emissions in diesel engines using palm oil biodiesel blended with diesel fuel. Energies 2018, 11, 1039. [Google Scholar] [CrossRef] [Green Version]
- Glassman, I.; Yetter, R.A.; Glumac, N.G. Combustion; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Nabi, M.N.; Zare, A.; Hossain, F.M.; Ristovski, Z.D.; Brown, R.J. Reductions in diesel emissions including PM and PN emissions with diesel-biodiesel blends. J. Clean. Prod. 2017, 166, 860–868. [Google Scholar] [CrossRef] [Green Version]
- Abed, K.A.; El Morsi, A.K.; Sayed, M.M.; Shaib, A.A.E.; Gad, M.S. Effect of waste cooking-oil biodiesel on performance and exhaust emissions of a diesel engine. Egypt. J. Pet. 2018, 27, 985–989. [Google Scholar] [CrossRef]
- Pinzi, S.; Rounce, P.; Herreros, J.M.; Tsolakis, A.; Pilar Dorado, M. The effect of biodiesel fatty acid composition on combustion and diesel engine exhaust emissions. Fuel 2013, 104, 170–182. [Google Scholar] [CrossRef]
- Gumus, M.; Sayin, C.; Canakci, M. The impact of fuel injection pressure on the exhaust emissions of a direct injection diesel engine fueled with biodiesel–diesel fuel blends. Fuel 2012, 95, 486–494. [Google Scholar] [CrossRef]
- Mohd Noor, C.W.; Noor, M.M.; Mamat, R. Biodiesel as alternative fuel for marine diesel engine applications: A review. Renew. Sustain. Energy Rev. 2018, 94, 127–142. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Varatharajan, K.; Cheralathan, M. Influence of fuel properties and composition on NOx emissions from biodiesel powered diesel engines: A review. Renew. Sustain. Energy Rev. 2012, 16, 3702–3710. [Google Scholar] [CrossRef]
- Chen, H.; Xie, B.; Ma, J.; Chen, Y. NOx emission of biodiesel compared to diesel: Higher or lower? Appl. Therm. Eng. 2018, 137, 584–593. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Kumar, N.; Cho, H.M. A study on the performance and emission of a diesel engine fueled with Jatropha biodiesel oil and its blends. Energy 2012, 37, 616–622. [Google Scholar] [CrossRef]
- Nabi, M.N.; Rasul, M.G.; Anwar, M.; Mullins, B.J. Energy, exergy, performance, emission and combustion characteristics of diesel engine using new series of non-edible biodiesels. Renew. Energy 2019, 140, 647–657. [Google Scholar] [CrossRef]
- Pimenidou, P.; Shanmugapriya, N.; Shah, N. Performance and emissions study of diesel and waste biodiesel blends with nanosized CZA2 of high oxygen storage capacity. Fuel 2019, 239, 1072–1082. [Google Scholar] [CrossRef]
- Mirzajanzadeh, M.; Tabatabaei, M.; Ardjmand, M.; Rashidi, A.; Ghobadian, B.; Barkhi, M.; Pazouki, M. A novel soluble nano-catalysts in diesel–biodiesel fuel blends to improve diesel engines performance and reduce exhaust emissions. Fuel 2015, 139, 374–382. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.-N.; Abul Kalam, M.; Badruddin, I.A.; Banapurmath, N.R.; Akram, N. The effect of nano-additives in diesel-biodiesel fuel blends: A comprehensive review on stability, engine performance and emission characteristics. Energy Convers. Manag. 2018, 178, 146–177. [Google Scholar] [CrossRef]
- Sarigiannis, D.A.; Karakitsios, S.P.; Gotti, A.; Liakos, I.L.; Katsoyiannis, A. Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ. Int. 2011, 37, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Jing, S.-A.; Lou, S.-R.; Hu, Q.-Y.; Li, L.; Tao, S.-K.; Huang, C.Q.; Li, P.; Chen, C.-H. Volatile organic compounds (VOCs) source profiles of on-road vehicle emissions in China. Sci. Total Environ. 2017, 607–608, 253–261. [Google Scholar] [CrossRef]
- Shuai, J.; Kim, S.; Ryu, H.; Park, J.; Lee, C.K.; Kim, G.-B.; Ultra, V.U.; Yang, W. Health risk assessment of volatile organic compounds exposure near Daegu dyeing industrial complex in South Korea. BMC Public Health 2018, 18, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.-Y.; Lan, C.-H.; Yang, C.-Y. Effects of biodiesel blend fuel on volatile organic compound (VOC) emissions from diesel engine exhaust. Biomass Bioenergy 2012, 36, 96–106. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Reducing volatile organic compound emissions from diesel engines using canola oil biodiesel fuel and blends. Fuel 2018, 218, 266–274. [Google Scholar] [CrossRef]
- Lopes, M.; Serrano, L.; Ribeiro, I.; Cascão, P.; Pires, N.; Rafael, S.; Tarelho, L.; Monteiro, A.; Nunes, T.; Evtyugina, M.; et al. Emissions characterization from EURO 5 diesel/biodiesel passenger car operating under the new European driving cycle. Atmos. Environ. 2014, 84, 339–348. [Google Scholar] [CrossRef]
- Shahab, A.N.; Yun-shan, G.E.; Tan, J.-W.; Liu, Z.-H. Experimental investigation of VOCs emitted from a DI-CI engine fuelled with biodiesel, diesel and biodiesel-diesel blend. Biol. Sci. PJSIR 2009, 52, 158–166. [Google Scholar]
- Guarieiro, L.L.N.; de Paula Pereira, P.A.; Torres, E.A.; da Rocha, G.O.; de Andrade, J.B. Carbonyl compounds emitted by a diesel engine fuelled with diesel and biodiesel–diesel blends: Sampling optimization and emissions profile. Atmos. Environ. 2008, 42, 8211–8218. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Akyüz, M.; Çabuk, H. Gas–particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak, Turkey. Sci. Total Environ. 2010, 408, 5550–5558. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hsu, K.-H.; Chen, C.-B. Experimental investigation of the performance and emissions of a heavy-duty diesel engine fueled with waste cooking oil biodiesel/ultra-low sulfur diesel blends. Energy 2011, 36, 241–248. [Google Scholar] [CrossRef]
- Borillo, G.C.; Tadano, Y.S.; Godoi, A.F.L.; Pauliquevis, T.; Sarmiento, H.; Rempel, D.; Yamamoto, C.I.; Marchi, M.R.R.; Potgieter-Vermaak, S.; Godoi, R.H.M. Polycyclic Aromatic Hydrocarbons (PAHs) and nitrated analogs associated to particulate matter emission from a Euro V-SCR engine fuelled with diesel/biodiesel blends. Sci. Total Environ. 2018, 644, 675–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vojtisek-Lom, M.; Pechout, M.; Dittrich, L.; Beránek, V.; Kotek, M.; Schwarz, J.; Vodička, P.; Milcová, A.; Rossnerová, A.; Ambrož, A.; et al. Polycyclic aromatic hydrocarbons (PAH) and their genotoxicity in exhaust emissions from a diesel engine during extended low-load operation on diesel and biodiesel fuels. Atmos. Environ. 2015, 109, 9–18. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y.; Guan, C.; Cheung, C.S.; Huang, Z. Effect of biodiesel on PAH, OPAH, and NPAH emissions from a direct injection diesel engine. Environ. Sci. Pollut. Res. 2018, 25, 34131–34138. [Google Scholar] [CrossRef]
- He, C.; Ge, Y.; Tan, J.; You, K.; Han, X.; Wang, J. Characteristics of polycyclic aromatic hydrocarbons emissions of diesel engine fueled with biodiesel and diesel. Fuel 2010, 89, 2040–2046. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Lee, C.-F.F. Particulate matter emission characteristics of diesel engines with biodiesel or biodiesel blending: A review. Renew. Sustain. Energy Rev. 2016, 64, 569–581. [Google Scholar] [CrossRef]
- Mills, C.; Aspinall, D. Some aspects of commercial vehicle noise. Appl. Acoust. 1968, 1, 47–66. [Google Scholar] [CrossRef]
- Patel, C.; Tiwari, N.; Agarwal, A.K. Experimental investigations of Soyabean and Rapeseed SVO and biodiesels on engine noise, vibrations, and engine characteristics. Fuel 2019, 238, 86–97. [Google Scholar] [CrossRef]
- Uludamar, E.; Yıldızhan, Ş.; Aydın, K.; Özcanlı, M. Vibration, noise and exhaust emissions analyses of an unmodified compression ignition engine fuelled with low sulphur diesel and biodiesel blends with hydrogen addition. Int. J. Hydrogen Energy 2016, 41, 11481–11490. [Google Scholar] [CrossRef]
- Patel, C.; Agarwal, A.K.; Tiwari, N.; Lee, S.; Lee, C.S.; Park, S. Combustion, noise, vibrations and spray characterization for Karanja biodiesel fuelled engine. Appl. Therm. Eng. 2016, 106, 506–517. [Google Scholar] [CrossRef]
- Redel-Macías, M.; Pinzi, S.; Leiva, D.; Cubero-Atienza, A.; Dorado, M. Air and noise pollution of a diesel engine fueled with olive pomace oil methyl ester and petrodiesel blends. Fuel 2012, 95, 615–621. [Google Scholar] [CrossRef]
- Çalık, A. Determination of vibration characteristics of a compression ignition engine operated by hydrogen enriched diesel and biodiesel fuels. Fuel 2018, 230, 355–358. [Google Scholar] [CrossRef]
- Jaikumar, S.; Bhatti, S.; Srinivas, V. Emission and vibration characteristics of Niger seed oil biodiesel fueled diesel engine. J. Mech. Eng. Sci. 2019, 13, 5862–5874. [Google Scholar] [CrossRef] [Green Version]
- Taghizadeh-Alisaraei, A.; Ghobadian, B.; Tavakoli-Hashjin, T.; Mohtasebi, S.S. Vibration analysis of a diesel engine using biodiesel and petrodiesel fuel blends. Fuel 2012, 102, 414–422. [Google Scholar] [CrossRef]
- Patel, C.; Lee, S.; Tiwari, N.; Agarwal, A.K.; Lee, C.S.; Park, S. Spray characterization, combustion, noise and vibrations investigations of Jatropha biodiesel fuelled genset engine. Fuel 2016, 185, 410–420. [Google Scholar] [CrossRef]
- Tongroon, M.; Suebwong, A.; Kananont, M.; Aunchaisri, J.; Chollacoop, N. High quality jatropha biodiesel (H-FAME) and its application in a common rail diesel engine. Renew. Energy 2017, 113, 660–668. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Lubricity of components of biodiesel and petrodiesel. The origin of biodiesel lubricity. Energy Fuels 2005, 19, 1192–1200. [Google Scholar] [CrossRef]
- Karavalakis, G.; Stournas, S.; Karonis, D. Evaluation of the oxidation stability of diesel/biodiesel blends. Fuel 2010, 89, 2483–2489. [Google Scholar] [CrossRef]
- Yang, Z.; Hollebone, B.P.; Wang, Z.; Yang, C.; Landriault, M. Factors affecting oxidation stability of commercially available biodiesel products. Fuel Process. Technol. 2013, 106, 366–375. [Google Scholar] [CrossRef]
- Yaakob, Z.; Narayanan, B.N.; Padikkaparambil, S.; Unni, K.S.; Akbar, P.M. A review on the oxidation stability of biodiesel. Renew. Sustain. Energy Rev. 2014, 35, 136–153. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Almeida, E.S.; Portela, F.M.; Sousa, R.M.F.; Daniel, D.; Terrones, M.G.H.; Richter, E.M.; Muñoz, R.A.A. Behaviour of the antioxidant tert-butylhydroquinone on the storage stability and corrosive character of biodiesel. Fuel 2011, 90, 3480–3484. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Sharma, M.P. Stability of biodiesel and its blends: A review. Renew. Sustain. Energy Rev. 2010, 14, 667–678. [Google Scholar] [CrossRef]
- Chandran, D. Compatibility of diesel engine materials with biodiesel fuel. Renew. Energy 2020, 147, 89–99. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Degradation of automotive materials in palm biodiesel. Energy 2012, 40, 76–83. [Google Scholar] [CrossRef]
- Ahmmad, M.S.; Haji Hassan, M.B.; Kalam, M.A. Comparative corrosion characteristics of automotive materials in Jatropha biodiesel. Int. J. Green Energy 2018, 15, 393–399. [Google Scholar] [CrossRef]
- Fazal, M.; Haseeb, A.; Masjuki, H.H. Comparative corrosive characteristics of petroleum diesel and palm biodiesel for automotive materials. Fuel Process. Technol. 2010, 91, 1308–1315. [Google Scholar] [CrossRef]
- Fazal, M.A.; Suhaila, N.R.; Haseeb, A.S.M.A.; Rubaiee, S.; Al-Zahrani, A. Influence of copper on the instability and corrosiveness of palm biodiesel and its blends: An assessment on biodiesel sustainability. J. Clean. Prod. 2018, 171, 1407–1414. [Google Scholar] [CrossRef]
- Fazal, M.A.; Suhaila, N.R.; Haseeb, A.S.M.A.; Rubaiee, S. Sustainability of additive-doped biodiesel: Analysis of its aggressiveness toward metal corrosion. J. Clean. Prod. 2018, 181, 508–516. [Google Scholar] [CrossRef]
- Kass, M.; Janke, C.; Connatser, R.; West, B.; Szybist, J.; Sluder, S. Influence of biodiesel decomposition chemistry on elastomer compatibility. Fuel 2018, 233, 714–723. [Google Scholar] [CrossRef]
- Zhu, L.; Cheung, C.S.; Zhang, W.G.; Huang, Z. Compatibility of different biodiesel composition with acrylonitrile butadiene rubber (NBR). Fuel 2015, 158, 288–292. [Google Scholar] [CrossRef]
- Chandran, D.; Ng, H.; Harrison, L.; Gan, S.; Choo, Y.; Jahis, S. Compatibility of biodiesel fuel with metals and elastomers in fuel delivery system of a diesel engine. J. Oil Palm Res. 2016, 28, 64–73. [Google Scholar] [CrossRef]



| Biodiesel Types | Trends Compared to Conventional Diesel | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|
| Density (kg/m3) | Kinematic Viscosity at 40 °C (mm2/s) | Calorific Value (MJ/kg) | Flash Point (°C) | Cetane Number | Oxidation Stability (h) or (%) | Acid Value (mg KOH/g) | ||
| Argemone mexicana biodiesel | 870/830 (15 °C) | 4.38/2.8 | 37.5/44.5 | 193/65.5 | [25] | |||
| Moringa oleifera biodiesel | 866.1/834.3 (40 °C) | 4.03/3.63 | 39.9/45.21 | 189.0/71.5 | 54.3/52.4 | 10.8/0.1 h | 0.24/- | [26] |
| Karanja biodiesel | 881/831 (40 °C) | 4.42/2.78 | 37.98/43.79 | 50.8/51.2 | [27] | |||
| Jatropha oil biodiesel | 865/841 | 5.2/4.5 | 34.5/42 | 175/50 | 51/49 | [28] | ||
| Calophyllum inophyllum biodiesel | 871.8/834.7 (40 °C) | 4.9762/3.4926 | 39.17/45.6 | 92.6/68.5 | 56/48 | 2.53/35 h | 0.41/0.072 | [29] |
| Water hyacinth biodiesel | 887/838 (15 °C) | 3.96/2.76 | 36.9/42.7 | 212/68 | 52.5/48 | 0.42/- | [30] | |
| Pongamia biodiesel | 912/824 (15 °C) | 10.29/2.3 | 912/824 | 175/53 | 2.3~11.6/- h | >1.53 | [31] | |
| Citrullus colocynthis L. biodiesel | 886/830 (15 °C) | 3.45/2.43 | 37.64/42.82 | 134/69 | 66.89/60.82 | 0.27/- | [32] | |
| Fish oil biodiesel | 885/850 | 4.741/3.05 | 40.057/42.8 | 114/56 | 52.6/52 | [33] | ||
| Rice bran oil biodiesel | 887/843 | 4.98/3.58 | 38.725/43.2 | 55.7/48 | 11.25%/0 | [34] | ||
| Neem oil biodiesel | 871/843 | 4.63/3.58 | 41/43.2 | 53.5/48 | 11%/0 | [34] | ||
| Cottonseed oil biodiesel | 864/843 | 4.14/3.58 | 36.8/43.2 | 52/48 | ~10%/0 | [34] | ||
| Linseed oil biodiesel | 924/842 (15 °C) | 16.23/2.44 | 39.750/45.343 | 108/47 | 35/>50 | [35] | ||
| Fleshing oil biodiesel | 876.7/829 (15 °C) | 4.7/3 | 37.3/43.2 | 168/63 | 58.8/56.8 | [36] | ||
| Chicken fat biodiesel | 889.7/829 (15 °C) | 5.3/3 | 37.1/43.2 | 169/63 | 52.3/56.8 | [36] | ||
| Canola-safflower biodiesel | 884.3/831.7 (15 °C) | 4.35/2.58 | 40.10/45.98 | 168/63 | [37] | |||
| Rapeseed oil biodiesel | 874/850 | 4.8/2.6 | 37.6/42 | >140/68 | 54/51 | 10.2/- h | [38] | |
| Engine | Test Condition | Test Fuels | Blend Ratio | Increase Rate of BSFC (%) | Ref. |
|---|---|---|---|---|---|
| Single cylinder, direct injection diesel engine, 582 cc | An average value for the three compression ratios (14, 16, 18), 2000 rpm | Diesel blend with wasted cooking oil | B10 | 2.72 | [42] |
| B20 | 2.3 | ||||
| B30 | 4.08 | ||||
| B50 | 7.12 | ||||
| Single cylinder diesel engine-generating set of 5 kVA rating | Full load conditions | Diesel blend with eucalyptus biodiesel | B10 | 2.94 | [43] |
| B30 | 7.21 | ||||
| B50 | 12.04 | ||||
| B100 | 22.83 | ||||
| Single cylinder, direct injection diesel engine, 454 cc | Over the entire speed range under full load | Diesel blend with soybean biodiesel | B10 | 2 | [4] |
| B20 | 4 | ||||
| B50 | 7 | ||||
| B100 | 9 | ||||
| Four-cylinder direct injection diesel engine, 1998 cc | A constant engine speed of 2500 rpm with 50% open throttle | Diesel blend with palm biodiesel | B10 | 1 | [44] |
| B20 | 2.1 | ||||
| B30 | 3 | ||||
| Single cylinder, direct injection diesel engine, 638 cc | Over the entire speed range | Diesel blend with palm and coconut biodiesel | PB30 | 8.58 | [45] |
| CB30 | 9.03 | ||||
| PB15CB15 | 8.55 | ||||
| Six-cylinder direct injection diesel engine, 12,822 cc | 1400 rpm engine speed at full-load condition | Diesel blend with rapeseed oil biodiesel | B5 | 2.5 | [46] |
| B20 | 3 | ||||
| B70 | 5.5 | ||||
| B100 | 7.5 | ||||
| Four-cylinder direct injection diesel engine, 2476 cc | Two idling conditions (1000 RPM 10% load and 1200 RPM 12% load) | Diesel blend with jatropha biodiesel | JB5 | 2.28–2.69 | [47] |
| JB10 | 3.98–5.39 | ||||
| JB20 | 8.83–9.29 |
| Raw Materials | Blends | Trends Compared to Conventional Diesel | Reference | |||
|---|---|---|---|---|---|---|
| HC | CO | NOx | PM | |||
| Waste cooking-oil biodiesel | D100, B10, B20, B30 | ↓ | ↓ | ↑ | ↓ | [103] |
| Soybean biodiesel | D2, B10, B20, B50, B100 | ↓ | ↓ | ↑ | ↑ | [4] |
| Palm oil | D100, B20, B100, PO20 | ↓ | ↓ | ↑ | [94] | |
| Waste fish oil | D, B25, B50, B75, B100 | ↓ | ↓ | ↑ | [59] | |
| Mixed inedible feedstocks | B0, KB5MB5, KB10, MB10, KB10MB10, KB20, MB20 | ↓ | ↓ | ↑ | [88] | |
| Pongamia biodiesel | D, B20, B40, B60, B80, B100 | ↓ | ↓ | ↑ | [86] | |
| Castor biodiesel | D, B20, B40, B60, B80, B100 | ↓ | ↓ | ↑ | ↑↓ | [89] |
| Mustard oil biodiesel | D100, M10, M20, M30 | ↓ | ↑ | ↓ | [56] | |
| Spirulina microalgae biodiesel | B0, B20, B40, B60, B80, B100 | ↓ | ↓ | ↓ | ↓ | [90] |
| Citrus sinensis biodiesel | D, B5, B10, B20 | ↓ | ↑ | [95] | ||
| Camelina biodiesel | D, B7, B100 | ↓ | ↑ | [93] | ||
| Canola biodiesel | D, B5, B10, B15, B20 | ↓ | ↓ | ↑ | ↑ | [91] |
| Cymbopogon flexuosus biofuel | DF, C10, C20, C30, C40, C100 | ↓ | ↓ | ↑ | ↓ | [54] |
| Microalgae Chlorella protothecoides biodiesel | PD, B20, B50, B100 | ↓ | ↓ | ↓ | [96] | |
| Licella biofuel | R0, R5, R10, R20 | ↑ | ↑ | ↓ | [92] | |
| Aphanamixis polystachya oil | Diesel, APME5, APME10 | ↓ | ↓ | [87] | ||
| Jatropha biodiesel | D100, JME5, JME10, JME20, JME30, JME100 | ↓ | ↓ | ↑ | ↓ | [110] |
| Argemone biodiesel | D, AB10, AB20, AB30, AB50 | ↓ | ↓ | ↓↑ | ↓ | [25] |
| New series of non-edible biodiesel | D, MD, WD, MWD | ↓ | ↓ | ↑ | ↓ | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Ge, J.C.; Choi, N.J. A Comprehensive Review of the Application Characteristics of Biodiesel Blends in Diesel Engines. Appl. Sci. 2020, 10, 8015. https://doi.org/10.3390/app10228015
Wu G, Ge JC, Choi NJ. A Comprehensive Review of the Application Characteristics of Biodiesel Blends in Diesel Engines. Applied Sciences. 2020; 10(22):8015. https://doi.org/10.3390/app10228015
Chicago/Turabian StyleWu, Guirong, Jun Cong Ge, and Nag Jung Choi. 2020. "A Comprehensive Review of the Application Characteristics of Biodiesel Blends in Diesel Engines" Applied Sciences 10, no. 22: 8015. https://doi.org/10.3390/app10228015





