TD-DFT Prediction of the Intermolecular Charge-Transfer UV-Vis Spectra of Viologen Salts in Solution
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
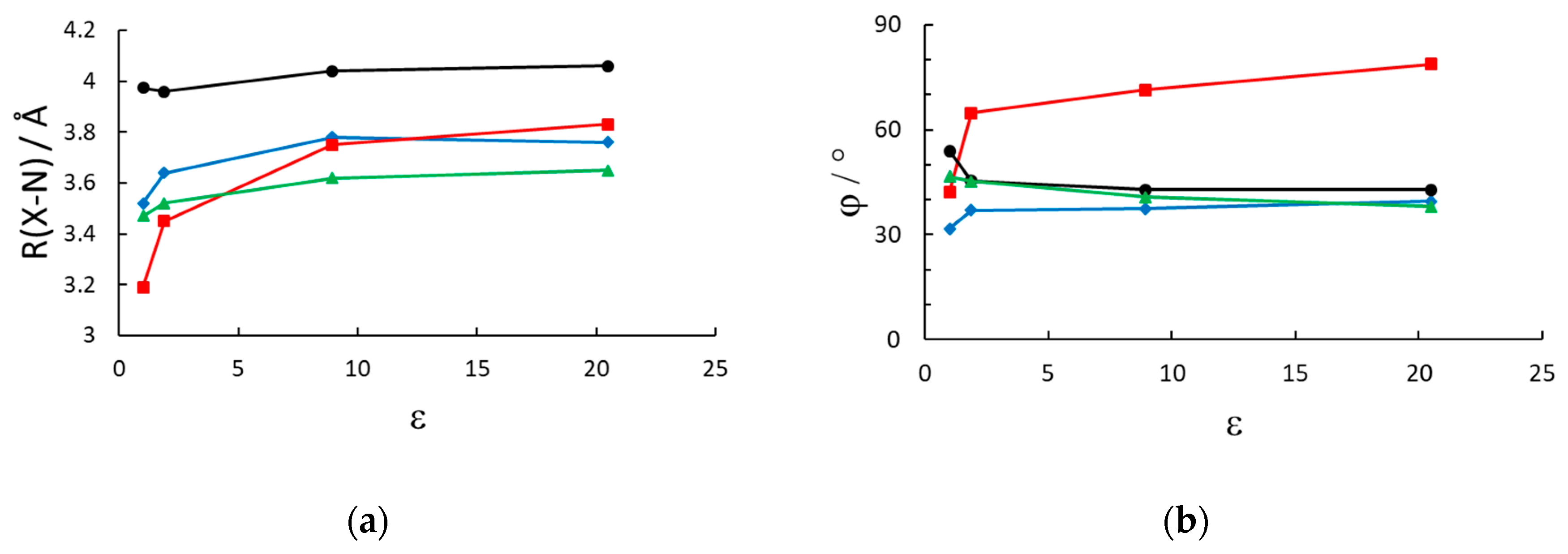
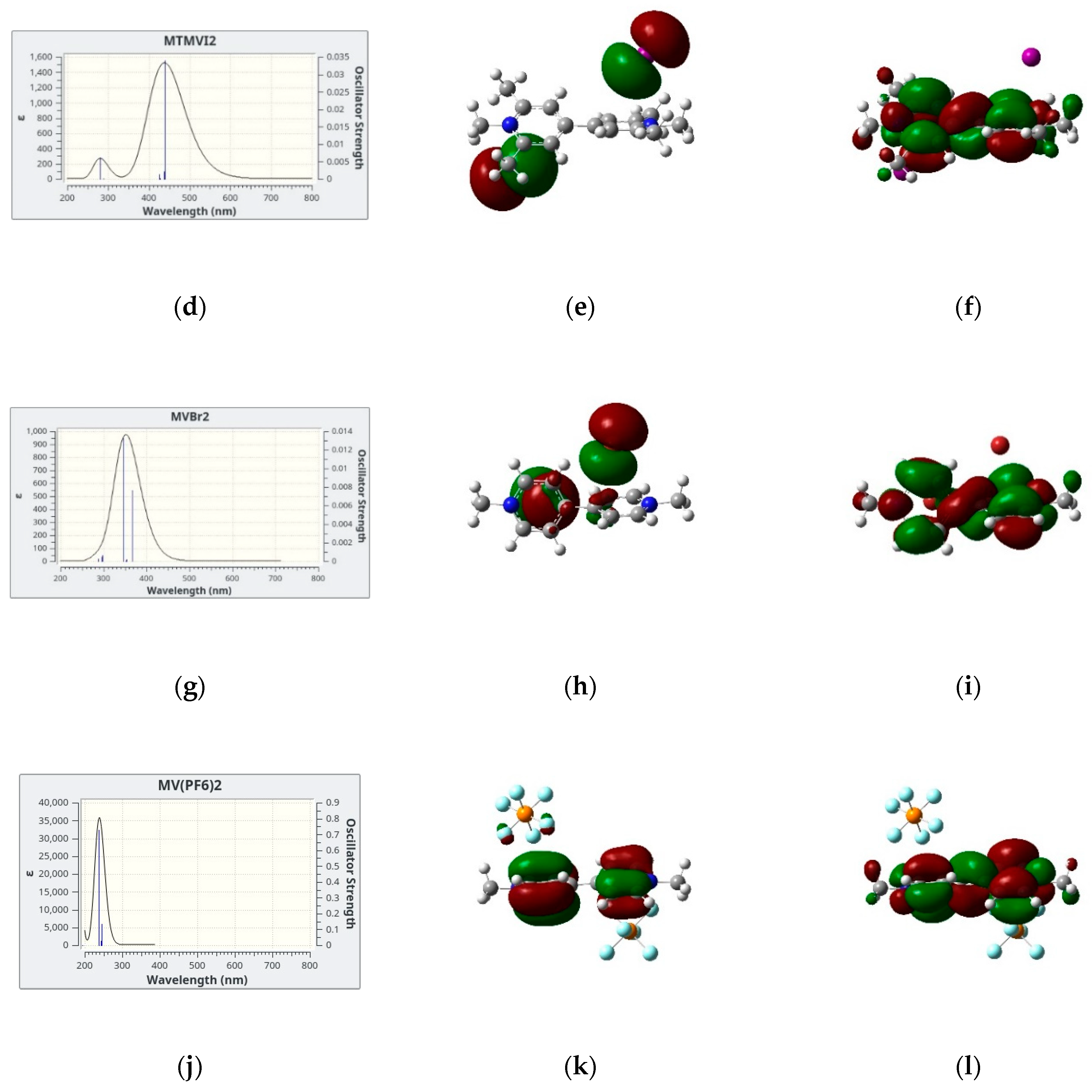
3.1. Geometry
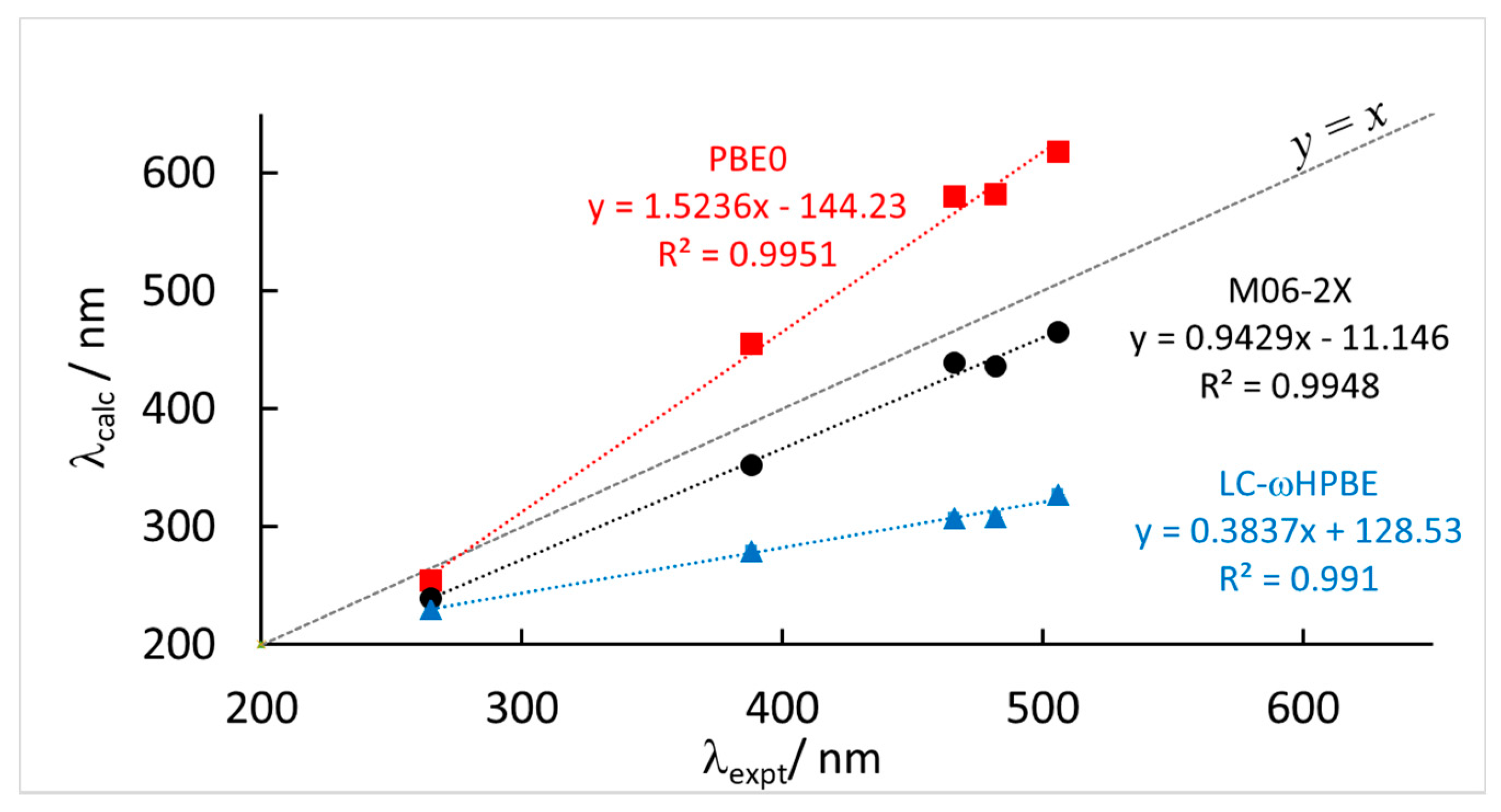
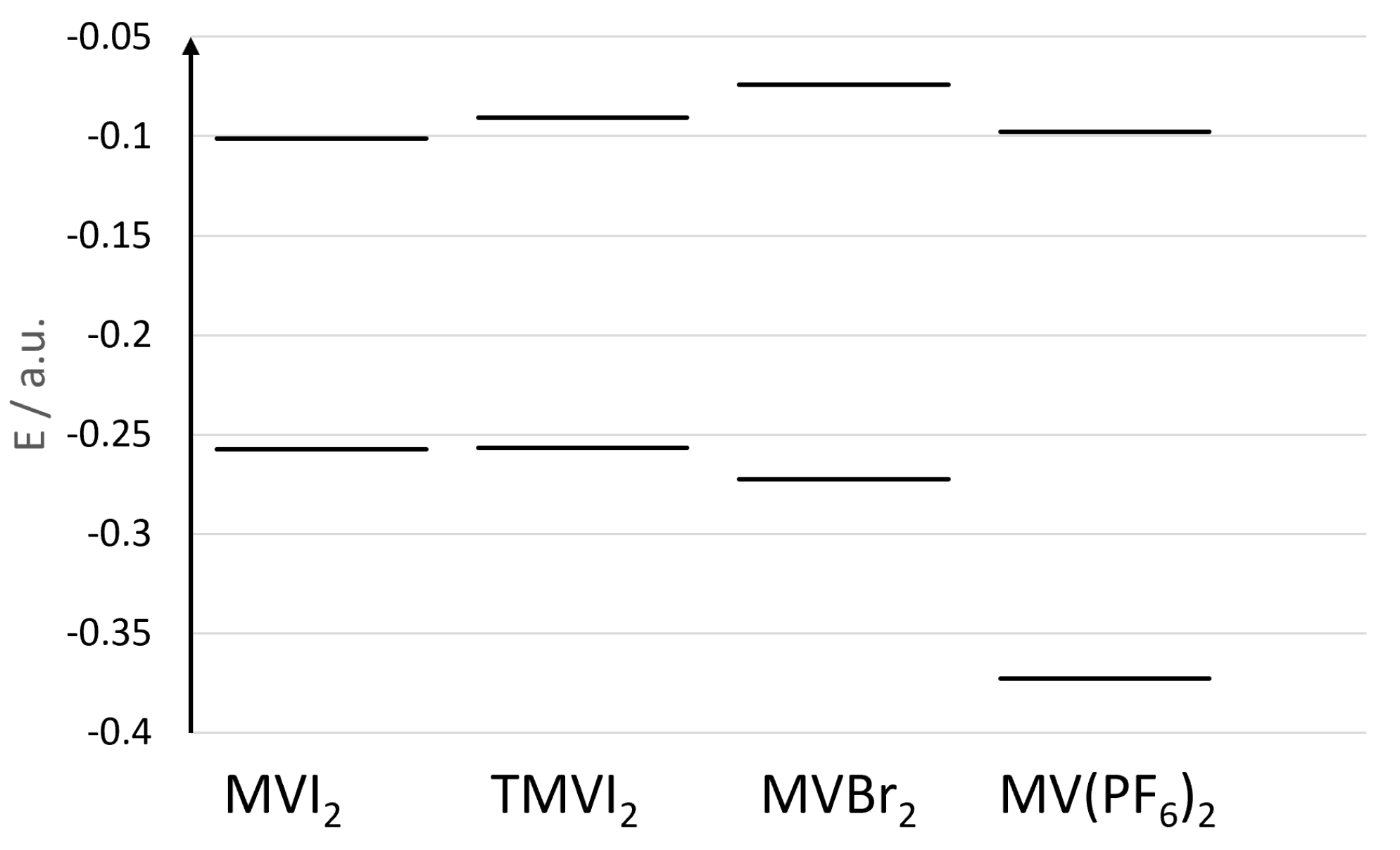
3.2. TDDFT Results
3.3. Direct and Indirect Solvent Effects
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Monk, P.M.S. The Viologens, Physicochemical Properties, Synthesis and Applications of the Salts of 4,4’-bipyridine; John Wiley & Sons, Ltd.: New York, NY, USA, 1998. [Google Scholar]
- Madasamy, K.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.-C. Viologen-based electrochromic materials and devices. J. Mater. Chem. C 2019, 7, 4622–4637. [Google Scholar] [CrossRef]
- Pibiri, I.; Beneduci, A.; Carraro, M.; Causin, V.; Casella, G.; Corrente, G.A.; Chidichimo, G.; Pace, A.; Riccobono, A.; Saielli, G. Mesomorphic and electrooptical properties of viologens based on non-symmetric alkyl/polyfluoroalkyl functionalization and on an oxadiazolyl-extended bent core. J. Mater. Chem. C 2019, 7, 7974–7983. [Google Scholar] [CrossRef]
- Hu, M.; Xing, F.; Zhao, Y.; Bai, Y.-L.; Li, M.-X.; Zhu, S. Phenolacetyl Viologen as Multifunctional Chromic Material for Fast and Reversible Sensor of Solvents, Base, Temperature, Metal Ions, NH3 Vapor, and Grind in Solution and Solid State. ACS Omega 2017, 2, 1128–1133. [Google Scholar] [CrossRef]
- Kinuta, T.; Sato, T.; Tajima, N.; Kuroda, R.; Matsubara, Y.; Imai, Y. Solid-state thermochromism observed in charge-transfer complex composed of binaphthol and viologen. J. Mol. Struct. 2010, 982, 45–49. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Zhao, G. Photochromism of supramolecular assemblies based on benzenecarboxylate donors and viologen acceptors. New J. Chem. 2019, 43, 6607–6614. [Google Scholar] [CrossRef]
- Kan, W.-Q.; Wen, S.-Z.; He, Y.-C.; Xu, C.-Y. Viologen-Based Photochromic Coordination Polymers for Inkless and Erasable Prints. Inorg. Chem. 2017, 56, 14926–14935. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-N.; Tu, Z.-M.; Li, L.; Wang, Z.-H.; Zhang, H. A novel viologen-based coordination polymer with multi-stimuli responsive chromic properties: Photochromism{,} thermochromism{,} chemochromism and electrochromism. Dalt. Trans. 2020, 49, 3228–3233. [Google Scholar] [CrossRef] [PubMed]
- Saielli, G. Ion-pairing of octyl viologen diiodide in low-polar solvents: An experimental and computational study. J. Phys. Chem. A 2008, 112, 7987–7995. [Google Scholar] [CrossRef] [PubMed]
- Causin, V.; Saielli, G. Effect of a structural modification of the bipyridinium core on the phase behaviour of viologen-based bistriflimide salts. J. Mol. Liq. 2009, 145, 41–47. [Google Scholar] [CrossRef]
- Causin, V.; Saielli, G. Effect of asymmetric substitution on the mesomorphic behaviour of low-melting viologen salts of bis(trifluoromethanesulfonyl)amide. J. Mater. Chem. 2009, 19, 9153–9162. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.S.; Nedeltchev, I.K.; Cebe, J.J. Room-temperature thermotropic ionic liquid crystals: Viologenbis(triflimide) salts. Mol. Cryst. Liq. Cryst. 2004, 419, 27–46. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.S.; Cebe, J.J.; Burchett, R.A.; Acharya, B.; Kumar, S. Ambient temperature thermotropic liquid crystalline viologen bis(triflimide) salts. Liq. Cryst. 2003, 30, 1433–1440. [Google Scholar] [CrossRef]
- Casella, G.; Causin, V.; Rastrelli, F.; Saielli, G. Ionic liquid crystals based on viologen dimers: Tuning the mesomorphism by varying the conformational freedom of the ionic layer. Liq. Cryst. 2016, 43, 1161–1173. [Google Scholar] [CrossRef]
- Martynov, A.G.; Mack, J.; May, A.K.; Nyokong, T.; Gorbunova, Y.G.; Tsivadze, A.Y. Methodological Survey of Simplified TD-DFT Methods for Fast and Accurate Interpretation of UV–Vis–NIR Spectra of Phthalocyanines. ACS Omega 2019, 4, 7265–7284. [Google Scholar] [CrossRef]
- Shao, Y.; Mei, Y.; Sundholm, D.; Kaila, V.R.I. Benchmarking the Performance of Time-Dependent Density Functional Theory Methods on Biochromophores. J. Chem. Theory Comput. 2020, 16, 587–600. [Google Scholar] [CrossRef]
- Charaf-Eddin, A.; Planchat, A.; Mennucci, B.; Adamo, C.; Jacquemin, D. Choosing a Functional for Computing Absorption and Fluorescence Band Shapes with TD-DFT. J. Chem. Theory Comput. 2013, 9, 2749–2760. [Google Scholar] [CrossRef]
- Saielli, G. Computational Spectroscopy of Ionic Liquids for Bulk Structure Elucidation. Adv. Theory Simulations 2018, 1, 1800084. [Google Scholar] [CrossRef]
- Arduini, A.; Calzavacca, F.; Pochini, A.; Secchi, A. Unidirectional Threading of Triphenylureidocalix[6]arene-Based Wheels: Oriented Pseudorotaxane Synthesis. Chem. Eur. J. 2003, 9, 793–799. [Google Scholar] [CrossRef]
- Credi, A.; Dumas, S.; Silvi, S.; Venturi, M.; Arduini, A.; Pochini, A.; Secchi, A. Viologen-Calix[6]arene Pseudorotaxanes. Ion-Pair Recognition and Threading/Dethreading Molecular Motions. J. Org. Chem. 2004, 69, 5881–5887. [Google Scholar] [CrossRef]
- Li, S.; Saielli, G.; Wang, Y. Aggregation behavior of dihexadecylviologen bistriflimide ionic liquid crystal in different solvents: Influence of polarity and concentration. Phys. Chem. Chem. Phys. 2018, 20, 22730–22738. [Google Scholar] [CrossRef]
- Marotta, E.; Rastrelli, F.; Saielli, G. Aggregation behavior of octyl viologen di[bis(trifluoromethanesulfonyl) amide] in nonpolar solvents. J. Phys. Chem. B 2008, 112, 16566–16574. [Google Scholar] [CrossRef] [PubMed]
- Schaftenaar, G.; Noordik, J.H. Molden: A pre- and post-processing program for molecular and electronic structures. J. Comput. Aided. Mol. Des. 2000, 14, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model RID C-7344-2008. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Henderson, T.M.; Izmaylov, A.F.; Scalmani, G.; Scuseria, G.E. Can short-range hybrids describe long-range-dependent properties? J. Chem. Phys. 2009, 131, 44108. [Google Scholar] [CrossRef]
- Vydrov, O.A.; Scuseria, G.E.; Perdew, J.P. Tests of functionals for systems with fractional electron number. J. Chem. Phys. 2007, 126, 154109. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian16 2016; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView 2019; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- di Matteo, A. Structural, electronic and magnetic properties of methylviologen in its reduced forms. Chem. Phys. Lett. 2007, 439, 190–198. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Russell, J.H.; Wallwork, S.C. The crystal structures of the dichloride and isomorphous dibromide and diiodide of the N,N’-dimethyl-4,4’-bipyridylium ion. Acta Crystallogr. Sect. B 1972, 28, 1527–1533. [Google Scholar] [CrossRef]
- Onsager, L. Electric Moments of Molecules in Liquids. J. Am. Chem. Soc. 1936, 58, 1486–1493. [Google Scholar] [CrossRef]
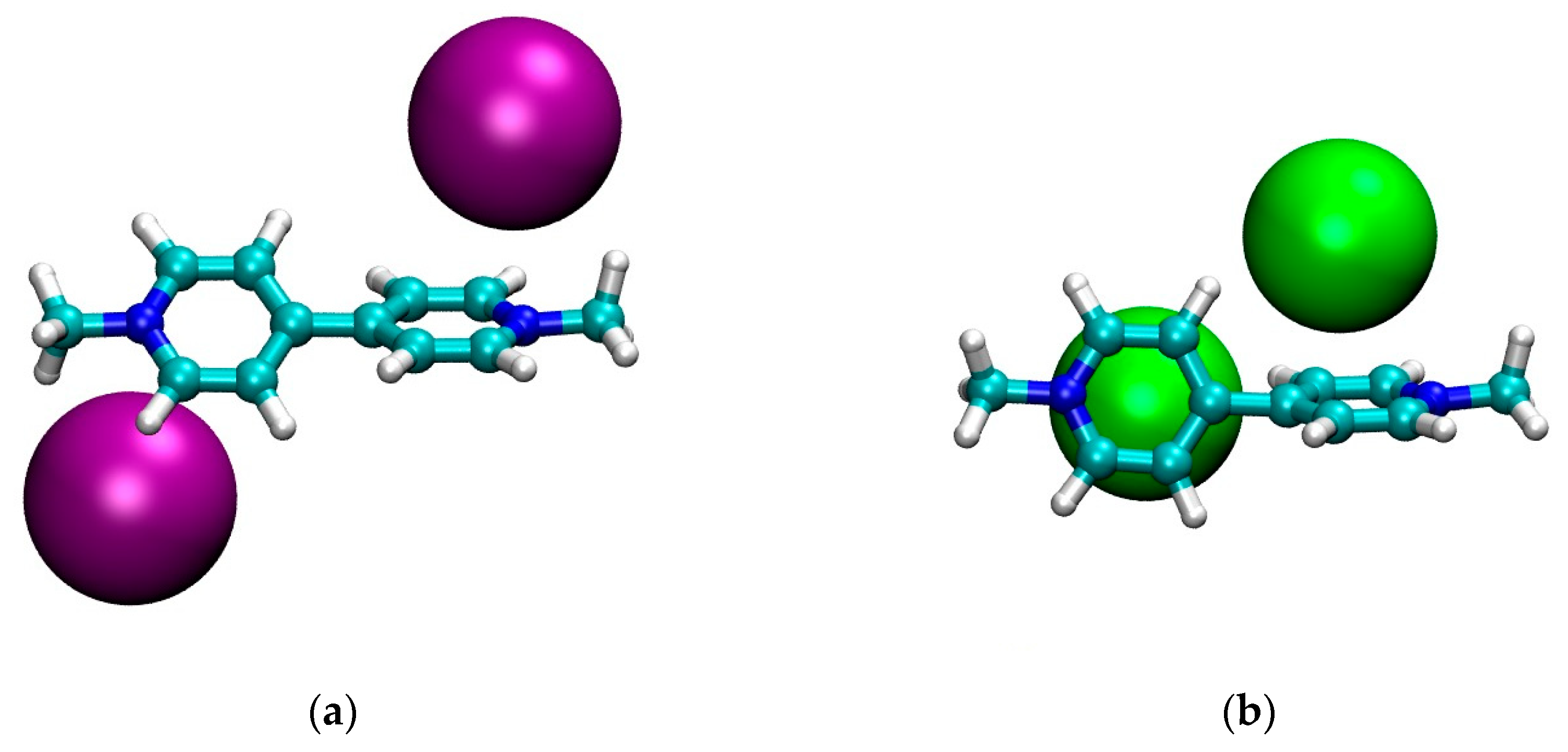
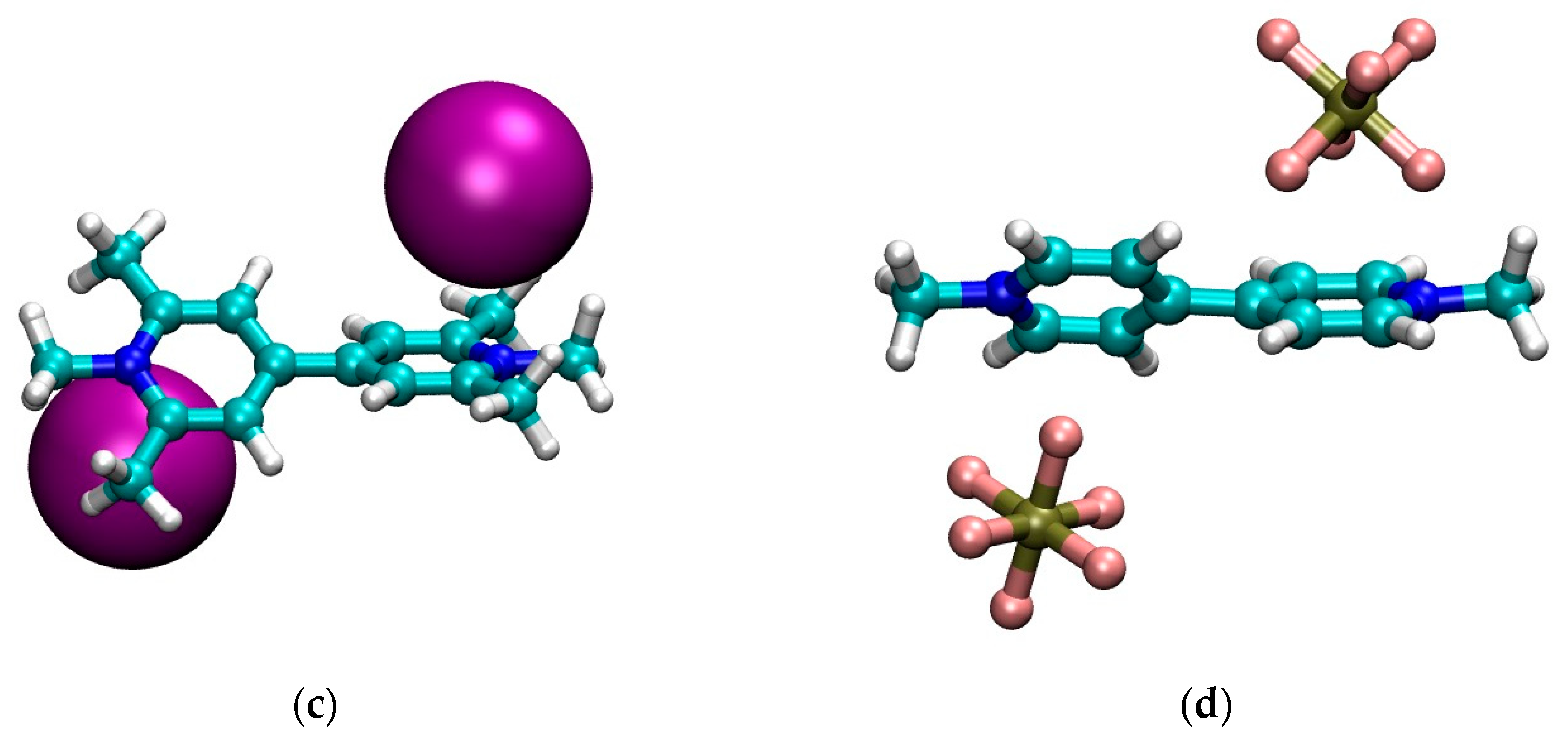

| Compound | λmax (nm) Lowest Energy Band | Ref. |
|---|---|---|
| [OV][PF6] in CH2Cl2 | 265 | [20] |
| [OV]Br2 in CH2Cl2 | 388 | [9] |
| [DTMV]I2 in CH2Cl2 | 466 | [10] |
| [OV]I2 in acetone | 482 | [9] |
| [OV]I2 in CH2Cl2 | 506 | [9] |
| R(X-N)/Å | φ (C-C-C-C)/° | |||||||
|---|---|---|---|---|---|---|---|---|
| Vacuum | n-Hexane | CH2Cl2 | Acetone | Vacuum | n-Hexane | CH2Cl2 | Acetone | |
| MVI2 | 3.52 | 3.64 | 3.78 | 3.76 | 31.7 | 37.0 | 37.5 | 39.5 |
| MVBr2 | 3.19 | 3.45 | 3.75 | 3.83 | 42.3 | 64.8 | 71.4 | 78.7 |
| MV(PF6)2 | 3.97 | 3.96 | 4.04 | 4.06 | 53.9 | 45.4 | 42.8 | 42.9 |
| MTMVI2 | 3.47 | 3.52 | 3.62 | 3.65 | 46.6 | 45.3 | 40.8 | 38.0 |
| 6-31+G(d,p) | aug-cc-pVTZ | 6-31+G(d,p) | ||||||
|---|---|---|---|---|---|---|---|---|
| Expt | PBE0 | M06-2X | ωB97-XD | CAM-B3LYP | LC-ωHPBE | CAM-B3LYP | Expt (eV) | M06-2X (eV) |
| 265 | 254 | 239 | 240 | 242 | 230 | 244 | 4.68 | 5.19 |
| 388 | 455 | 352 | 342 | 349 | 279 | 349 | 3.20 | 3.52 |
| 466 | 580 | 439 | 384 | 407 | 307 | 414 | 2.66 | 2.82 |
| 482 | 582 | 436 | 379 | 406 | 308 | 412 | 2.57 | 2.84 |
| 506 | 618 | 465 | 404 | 432 | 327 | 440 | 2.45 | 2.67 |
| a | 1.5236 | 0.9429 | 0.6593 | 0.7761 | 0.3837 | 0.8032 | 1.136 | |
| b | −144.23 | −11.146 | 71.977 | 40.161 | 128.53 | 33.349 | −0.126 | |
| R2 | 0.9951 | 0.9948 | 0.9773 | 0.9927 | 0.9910 | 0.9946 | 0.9982 | |
| MAE | 80.8 | 35.2 | 71.6 | 54.2 | 131.2 | 49.6 | 0.30 | |
| Solvent | Direct Effect 1 (λmax) | Indirect Effect 2 (λmax) |
|---|---|---|
| vacuum | 890 | 455 |
| n-hexane | 683 | 463 |
| CH2Cl2 | 465 | 465 |
| Acetone | 436 | 466 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saielli, G. TD-DFT Prediction of the Intermolecular Charge-Transfer UV-Vis Spectra of Viologen Salts in Solution. Appl. Sci. 2020, 10, 8108. https://doi.org/10.3390/app10228108
Saielli G. TD-DFT Prediction of the Intermolecular Charge-Transfer UV-Vis Spectra of Viologen Salts in Solution. Applied Sciences. 2020; 10(22):8108. https://doi.org/10.3390/app10228108
Chicago/Turabian StyleSaielli, Giacomo. 2020. "TD-DFT Prediction of the Intermolecular Charge-Transfer UV-Vis Spectra of Viologen Salts in Solution" Applied Sciences 10, no. 22: 8108. https://doi.org/10.3390/app10228108
APA StyleSaielli, G. (2020). TD-DFT Prediction of the Intermolecular Charge-Transfer UV-Vis Spectra of Viologen Salts in Solution. Applied Sciences, 10(22), 8108. https://doi.org/10.3390/app10228108





