Preparation and Evaluation of Microcapsules Encapsulating Royal Jelly Sieve Residue: Flavor and Release Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Pretreatment of RJSR
2.3. Preparation of Microcapsules
2.4. Efficiency of Microencapsulation
2.5. Characterization of Microcapsules
2.5.1. Optical Microscope
2.5.2. Scanning Electron Microscope (SEM)
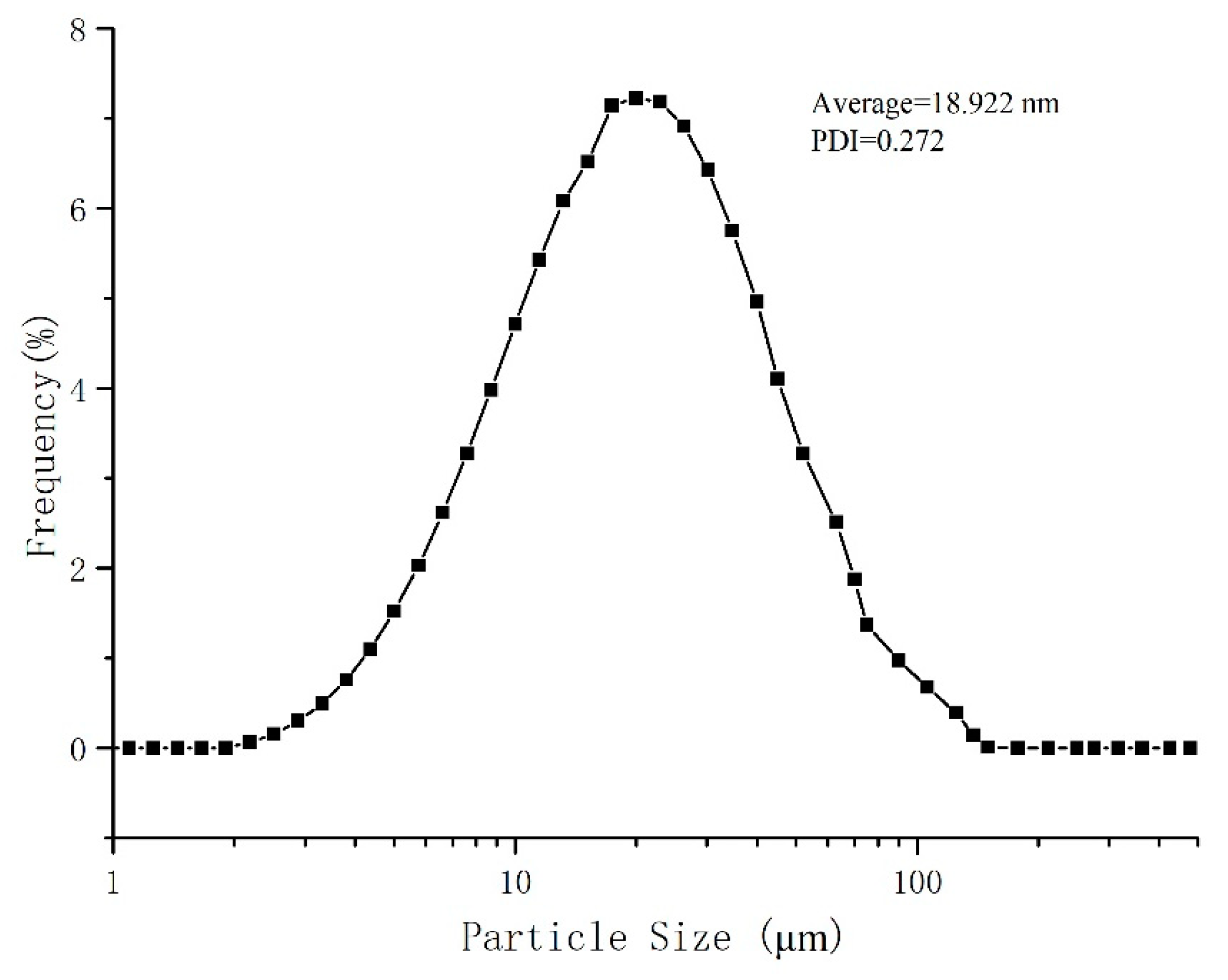
2.5.3. Particle Size
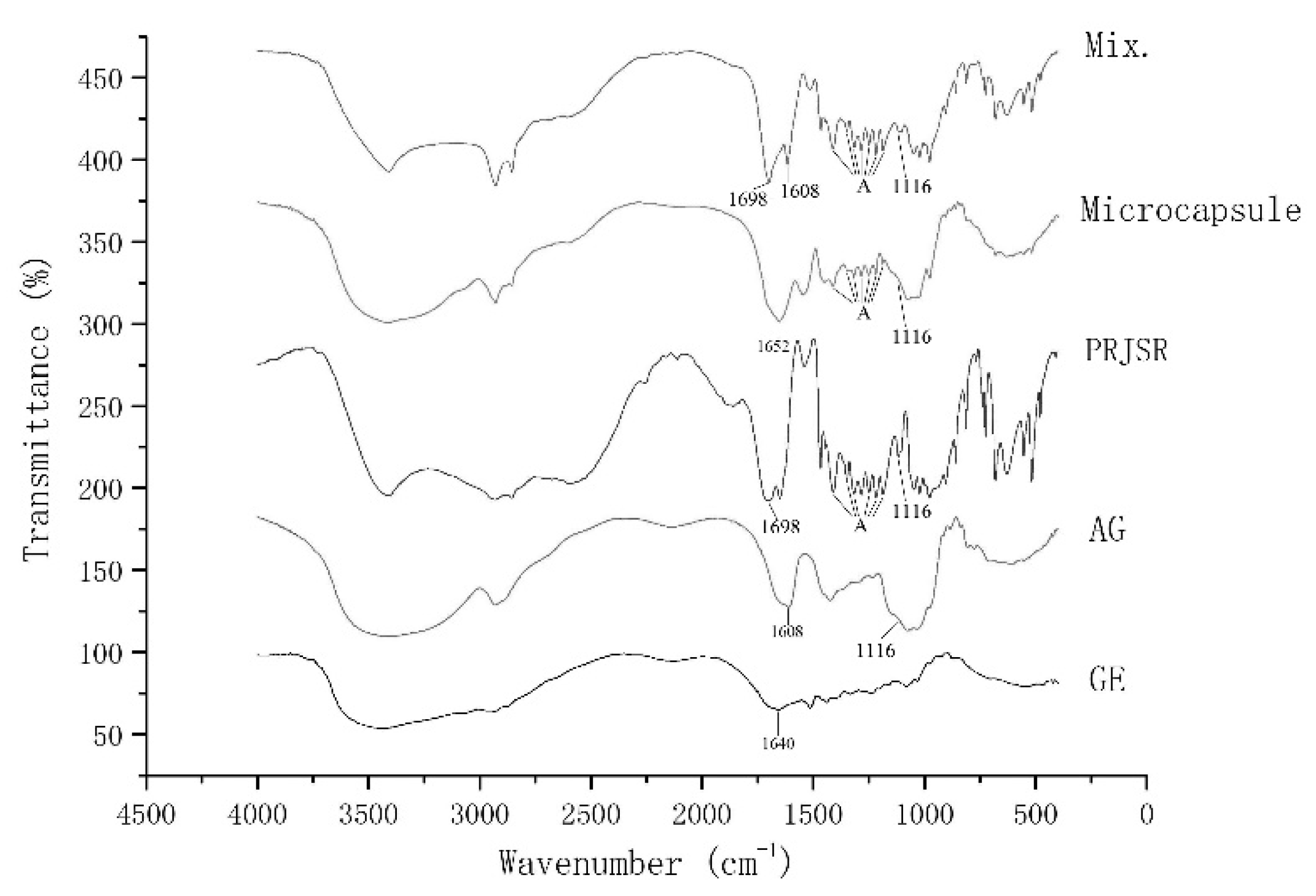
2.5.4. FTIR Analysis
2.6. Sensory Evaluation
2.6.1. Electronic Nose
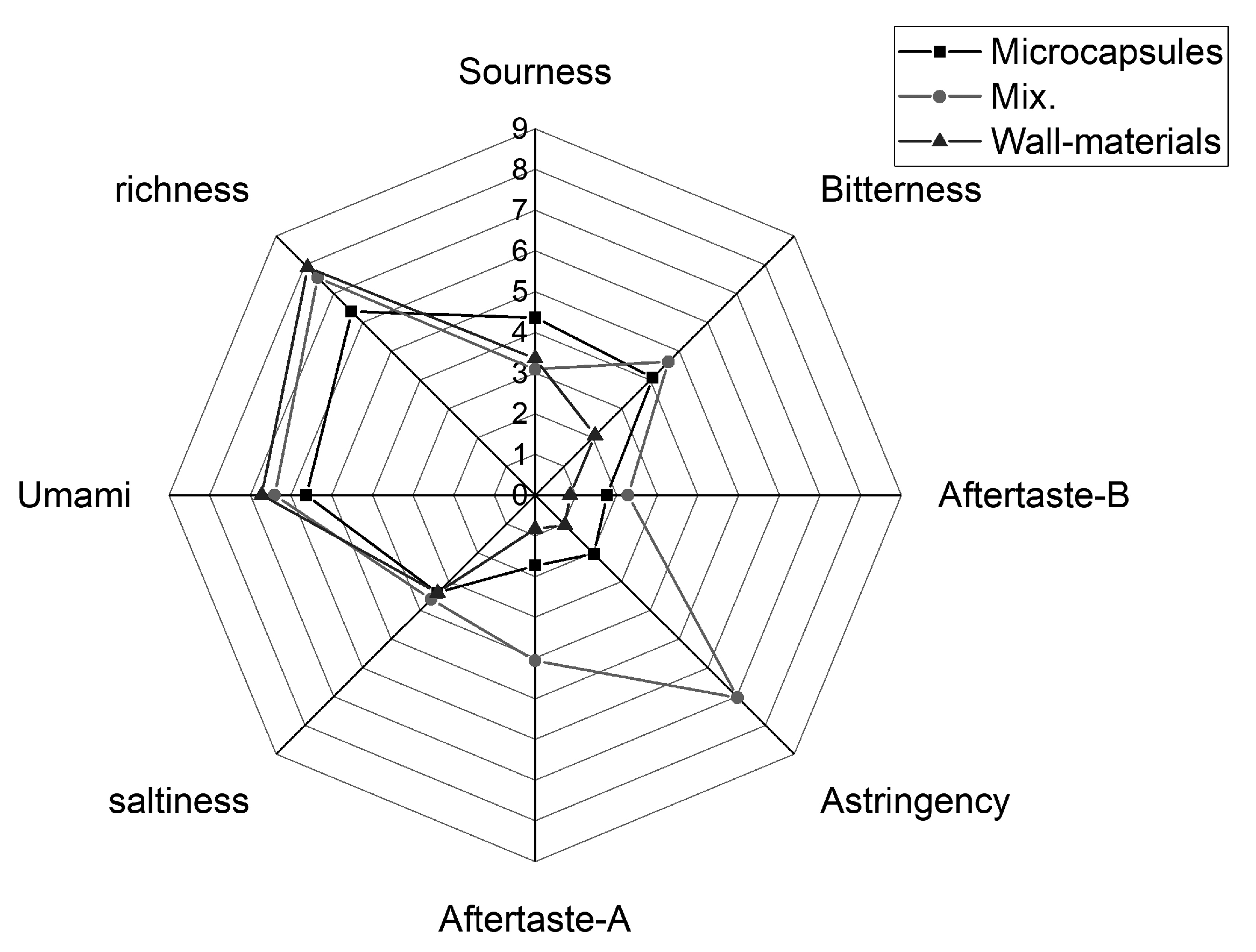
2.6.2. Electronic Tongue
2.7. Kinetics of Release during In Vitro Digestion
2.8. Statistical Analysis
3. Results and Discussion
3.1. Efficiency of Microencapsulation
3.2. Characterization of Microcapsules
3.2.1. Optical Microscope
3.2.2. SEM
3.2.3. Particle Size
3.2.4. FTIR Analysis
3.3. Sensory Evaluation
3.3.1. Electronic Nose
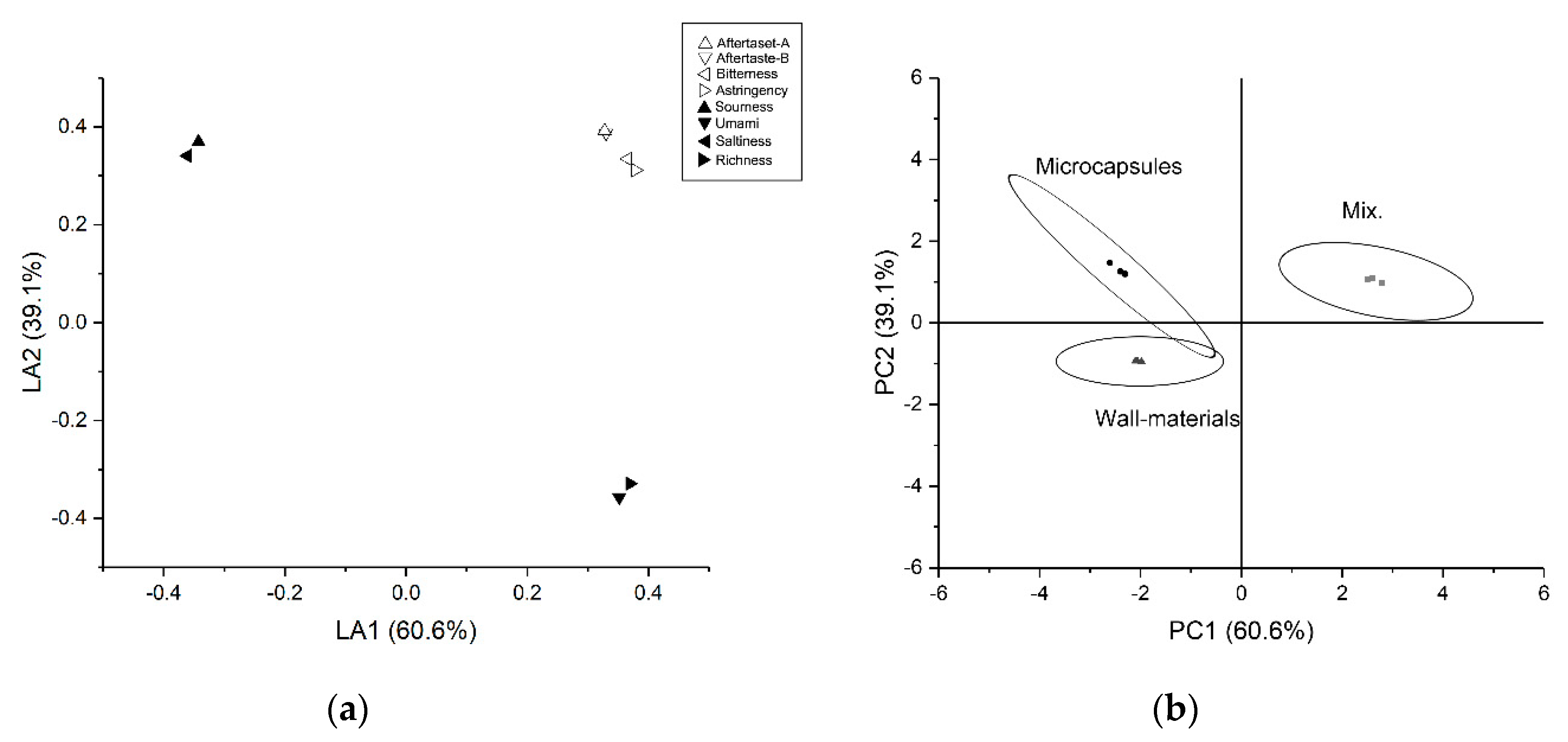
3.3.2. Electronic Tongue
3.4. Kinetics of Release during In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RJ | Royal jelly |
| RJSR | royal jelly sieve residue |
| PRJSR | pretreated royal jelly sieve residue |
| GE | gelatin |
| AG | Arabic gum |
| Mix. | the mixture of GE, AG and PRJSR |
| PCA | principal component analysis |
| LA | loading analysis |
References
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on Royal Jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Grzech, I. Gas chromatographic–mass spectrometric investigation of volatile and extractable compounds of crude royal jelly. J. Chromatogr. B 2012, 885–886, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; You, M.M.; Liu, Y.C.; Shi, Y.Z.; Wang, K.; Lu, Y.Y.; Hu, F.L. Potential protective effect of Trans-10-hydroxy-2-decenoic acid on the inflammation induced by Lipoteichoic acid. J. Funct. Foods 2018, 45, 491–498. [Google Scholar] [CrossRef]
- Mihajlovic, D.; Rajkovic, I.; Chinou, I.; Colic, M. Dose-dependent immunomodulatory effects of 10-hydroxy-2-decenoic acid on human monocyte-derived dendritic cells. J. Funct. Foods 2013, 5, 838–846. [Google Scholar] [CrossRef]
- Watanabe, T.; Terada, Y. Food Compounds Activating Thermosensitive TRP Channels in Asian Herbal and Medicinal Foods. J. Nutr. Sci. Vitam. 2015, 61, S86–S88. [Google Scholar] [CrossRef]
- Barker, S.A.; Foster, A.B.; Lamb, D.C.; Hodgson, N. Identification of 10-hydroxy-delta 2-decenoic acid in royal jelly. Nature 1959, 183, 996–997. [Google Scholar] [CrossRef]
- Costa, A.M.M.; Moretti, L.K.; Simões, G.; Silva, K.A.; Calado, V.; Tonon, R.V.; Torres, A.G. Microencapsulation of pomegranate (Punica granatum L.) seed oil by complex coacervation: Development of a potential functional ingredient for food application. LWT 2020, 131, 109519. [Google Scholar] [CrossRef]
- Ren, W.; Tian, G.; Zhao, S.; Yang, Y.; Gao, W.; Zhao, C.; Zhang, H.; Lian, Y.; Wang, F.; Du, H.; et al. Effects of spray-drying temperature on the physicochemical properties and polymethoxyflavone loading efficiency of citrus oil microcapsules. LWT 2020, 133, 109954. [Google Scholar] [CrossRef]
- Quan, J.; Kim, S.M.; Pan, C.H.; Chung, D. Characterization of fucoxanthin-loaded microspheres composed of cetyl palmitate-based solid lipid core and fish gelatin–gum arabic coacervate shell. Food Res. Int. 2013, 50, 31–37. [Google Scholar] [CrossRef]
- De Almeida Paula, D.; Martins, E.M.F.; de Almeida Costa, N.; de Oliveira, P.M.; de Oliveira, E.B.; Ramos, A.M. Use of gelatin and gum arabic for microencapsulation of probiotic cells from Lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation. Int. J. Biol. Macromol. 2019, 133, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, N.; Hu, L.; Li, J.; Sun, Y. Development and evaluation of novel microcapsules containing poppy-seed oil using complex coacervation. J. Food Eng. 2015, 161, 87–93. [Google Scholar] [CrossRef]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Use of gelatin and gum Arabic for encapsulation of black raspberry anthocyanins by complex coacervation. Int. J. Biol. Macromol. 2018, 107, 1800–1810. [Google Scholar] [CrossRef]
- Da Silva, T.M.; Jacob Lopes, E.; Codevilla, C.F.; Cichoski, A.J.; de Moraes Flores, É.M.; Motta, M.H.; de Bona da Silva, C.; Grosso, C.R.F.; de Menezes, C.R. Development and characterization of microcapsules containing Bifidobacterium Bb-12 produced by complex coacervation followed by freeze drying. LWT 2018, 90, 412–417. [Google Scholar] [CrossRef]
- Li, Y.; Dou, X.; Pang, J.; Liang, M.; Feng, C.; Kong, M.; Liu, Y.; Cheng, X.; Wang, Y.; Chen, X. Improvement of fucoxanthin oral efficacy via vehicles based on gum Arabic, gelatin and alginate hydrogel: Delivery system for oral efficacy enhancement of functional food ingredients. J. Funct. Foods 2019, 63, 103573. [Google Scholar] [CrossRef]
- Tu, X.; Sun, F.; Wu, S.; Liu, W.; Gao, Z.; Huang, S.; Chen, W. Comparison of salting-out and sugaring-out liquid–liquid extraction methods for the partition of 10-hydroxy-2-decenoic acid in royal jelly and their co-extracted protein content. J. Chromatogr. B 2018, 1073, 90–95. [Google Scholar] [CrossRef]
- Pitigraisorn, P.; Srichaisupakit, K.; Wongpadungkiat, N.; Wongsasulak, S. Encapsulation of Lactobacillus acidophilus in moist-heat-resistant multilayered microcapsules. J. Food Eng. 2017, 192, 11–18. [Google Scholar] [CrossRef]
- Du, Y.L.; Huang, G.Q.; Wang, H.O.; Xiao, J.X. Effect of high coacervation temperature on the physicochemical properties of resultant microcapsules through induction of Maillard reaction between soybean protein isolate and chitosan. J. Food Eng. 2018, 234, 91–97. [Google Scholar] [CrossRef]
- Benedetti, S.; Drusch, S.; Mannino, S. Monitoring of autoxidation in LCPUFA-enriched lipid microparticles by electronic nose and SPME-GCMS. Talanta 2009, 78, 1266–1271. [Google Scholar] [CrossRef]
- Yi, E.-J.; Kim, J.-Y.; Rhee, Y.-S.; Kim, S.-H.; Lee, H.-J.; Park, C.-W.; Park, E.-S. Preparation of sildenafil citrate microcapsules and in vitro/in vivo evaluation of taste masking efficiency. Int. J. Pharm. 2014, 466, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, J.; Gu, S. Rapid identification of tea quality by E-nose and computer vision combining with a synergetic data fusion strategy. J. Food Eng. 2019, 241, 10–17. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Low-temperature and long-time heating regimes on non-volatile compound and taste traits of beef assessed by the electronic tongue system. Food Chem. 2020, 320, 126656. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. In-vitro digestion of refined kenaf seed oil microencapsulated in β-cyclodextrin/gum arabic/sodium caseinate by spray drying. J. Food Eng. 2018, 225, 34–41. [Google Scholar] [CrossRef]
- Ryu, D.; Koh, E. Stability of anthocyanins in bokbunja (Rubus occidentalis L.) under in vitro gastrointestinal digestion. Food Chem. 2018, 267, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Double emulsion followed by complex coacervation as a promising method for protection of black raspberry anthocyanins. Food Hydrocoll. 2018, 77, 803–816. [Google Scholar] [CrossRef]
- Samakradhamrongthai, R.S.; Thakeow Angeli, P.; Kopermsub, P.; Utama-ang, N. Optimization of gelatin and gum arabic capsule infused with pandan flavor for multi-core flavor powder encapsulation. Carbohydr. Polym. 2019, 226, 115262. [Google Scholar] [CrossRef]
- Pham, L.B.; Wang, B.; Zisu, B.; Truong, T.; Adhikari, B. Microencapsulation of flaxseed oil using polyphenol-adducted flaxseed protein isolate-flaxseed gum complex coacervates. Food Hydrocoll. 2020, 107, 105944. [Google Scholar] [CrossRef]
- Shewan, H.M.; Stokes, J.R.; Smyth, H.E. Influence of particle modulus (softness) and matrix rheology on the sensory experience of ‘grittiness’ and ‘smoothness’. Food Hydrocoll. 2020, 103, 105662. [Google Scholar] [CrossRef]
- Hossain, M.K.; Keidel, J.; Hensel, O.; Diakité, M. The impact of extruded microparticulated whey proteins in reduced-fat, plain-type stirred yogurt: Characterization of physicochemical and sensory properties. LWT 2020, 134, 109976. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Liu, Y. Temperature-dependent hygroscopic behaviors of atmospherically relevant water-soluble carboxylic acid salts studied by ATR-FTIR spectroscopy. Atmos. Environ. 2018, 191, 312–319. [Google Scholar] [CrossRef]
- Lu, R.; Mori, S.; Tani, H.; Tagawa, N.; Koganezawa, S. Low friction properties of associated carboxylic acids induced by molecular orientation. Tribol. Int. 2017, 113, 36–42. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhao, Y.; Ding, J.; Lin, S. Investigation on complex coacervation between fish skin gelatin from cold-water fish and gum arabic: Phase behavior, thermodynamic, and structural properties. Food Res. Int. 2018, 107, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Rousi, Z.; Malhiac, C.; Fatouros, D.G.; Paraskevopoulou, A. Complex coacervates formation between gelatin and gum Arabic with different arabinogalactan protein fraction content and their characterization. Food Hydrocoll. 2019, 96, 577–588. [Google Scholar] [CrossRef]
- Gu, S.Q.; Wang, X.C.; Tao, N.P.; Wu, N. Characterization of volatile compounds in different edible parts of steamed Chinese mitten crab (Eriocheir sinensis). Food Res. Int. 2013, 54, 81–92. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Tian, J.; Chu, Z. Effect of a new shell material—Jackfruit seed starch on novel flavor microcapsules containing vanilla oil. Ind. Crop. Prod. 2018, 112, 47–52. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Liu, H.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, H.; Brennan, M.; Guan, W.; Liu, J.; Wang, M.; Wen, X.; He, J.; Brennan, C. In vitro gastric digestion antioxidant and cellular radical scavenging activities of wheat-shiitake noodles. Food Chem. 2020, 330, 127214. [Google Scholar] [CrossRef]
- Mao, Y.; Krischke, M.; Hengst, C.; Kulozik, U. Comparison of the influence of pH on the selectivity of free and immobilized trypsin for β-lactoglobulin hydrolysis. Food Chem. 2018, 253, 194–202. [Google Scholar] [CrossRef]
- Souza, C.J.F.; Comunian, T.A.; Kasemodel, M.G.C.; Favaro-Trindade, C.S. Microencapsulation of lactase by W/O/W emulsion followed by complex coacervation: Effects of enzyme source, addition of potassium and core to shell ratio on encapsulation efficiency, stability and kinetics of release. Food Res. Int. Int. 2019, 121, 754–764. [Google Scholar] [CrossRef]


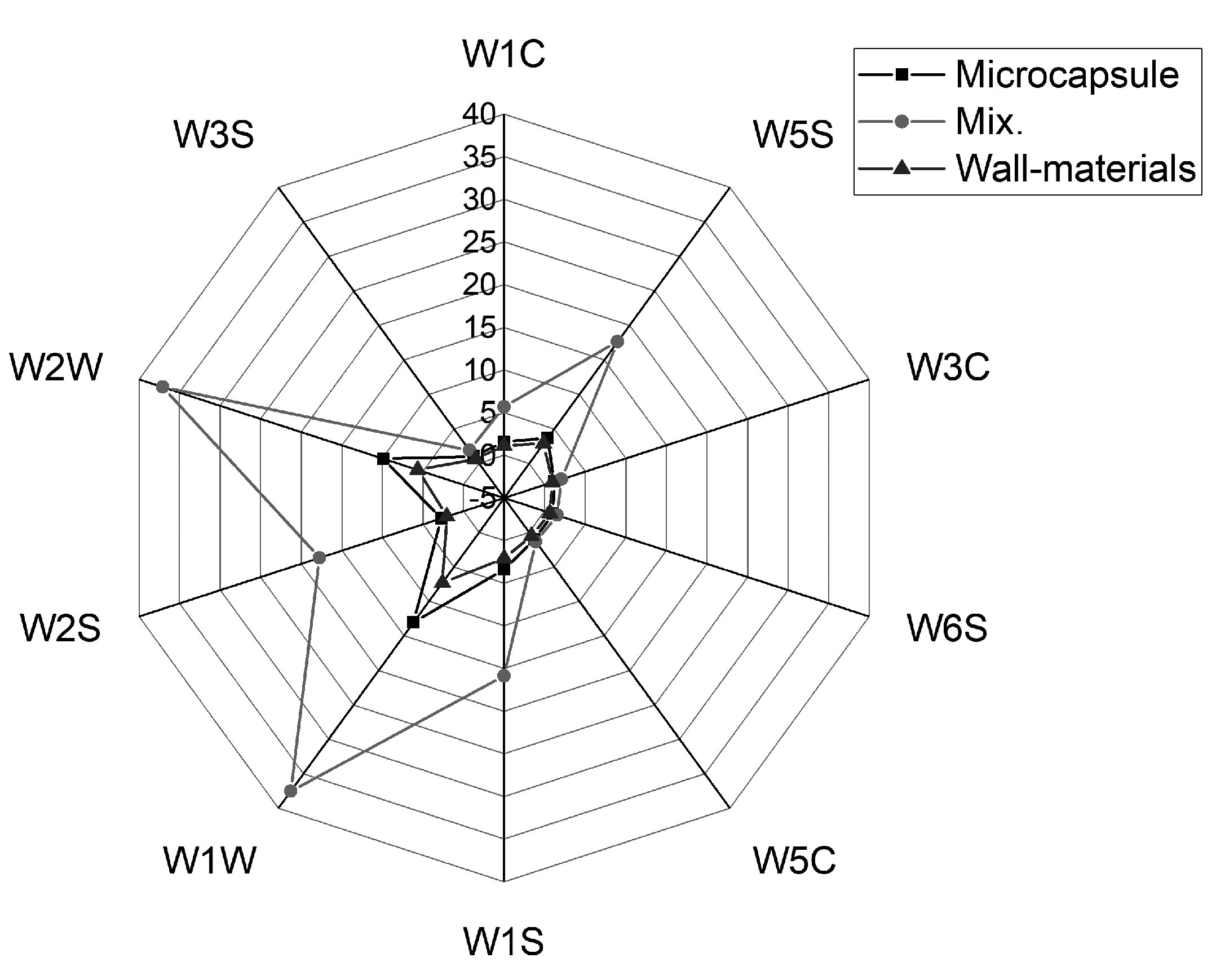


| Number | Name | Main Performance | Reference |
|---|---|---|---|
| R1 | W1C | Aromatic compounds | Toluene, 10 mg/L |
| R2 | W5S | Very sensitive, broad range sensitivity, reacts to nitrogen oxides, sensitive with a negative signal | NO2, l mg/L |
| R3 | W3C | Ammonia, used as a sensor for aromatic compounds | Benzene, 19 mg/L |
| R4 | W6S | Mainly hydrogen, selectively | H2, 100 mg/L |
| R5 | W5C | Alkenes, aromatic compounds, fewer polar compounds | Propane, 100 mg/L |
| R6 | W1S | Sensitive to methane | CH4, 100 mg/L |
| R7 | W1W | Reacts to sulfur compounds. Otherwise, sensitive to many terpenes and sulfur organic compounds, which are important for smell, limonene, pyrazine | H2S, l mg/L |
| R8 | W2S | Detects alcohols, partially aromatic compounds, broad range | CO, 100 mg/L |
| R9 | W2W | Aromatic compounds, sulfur organic compounds | H2S, l mg/L |
| R10 | W3S | Long chain alkanes | CH3, 100 mg/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.; Ye, J.; Wang, L.; Sun, P. Preparation and Evaluation of Microcapsules Encapsulating Royal Jelly Sieve Residue: Flavor and Release Profile. Appl. Sci. 2020, 10, 8126. https://doi.org/10.3390/app10228126
He R, Ye J, Wang L, Sun P. Preparation and Evaluation of Microcapsules Encapsulating Royal Jelly Sieve Residue: Flavor and Release Profile. Applied Sciences. 2020; 10(22):8126. https://doi.org/10.3390/app10228126
Chicago/Turabian StyleHe, Rongjun, Jiahao Ye, Lina Wang, and Peilong Sun. 2020. "Preparation and Evaluation of Microcapsules Encapsulating Royal Jelly Sieve Residue: Flavor and Release Profile" Applied Sciences 10, no. 22: 8126. https://doi.org/10.3390/app10228126






