The Perspective of Large-Scale Production of Algae Biodiesel
Abstract
1. Introduction
2. Materials and Methods
3. Production of Algal Biodiesel
3.1. Algae Farming Systems
3.2. The Production Process of Biodiesel from Algae
- Separation and thickening of microalgae from bulk suspension by micro strainers, by electrophoresis process, and by sedimentation, flotation, and flocculation;
- A large amount of chemical solvent is required for efficient lipid extraction.
- The problem of solvent toxicity and safety should be addressed.
- Additional energy is required to recover the solvent.
- Wastewater treatment is required which is an additional cost
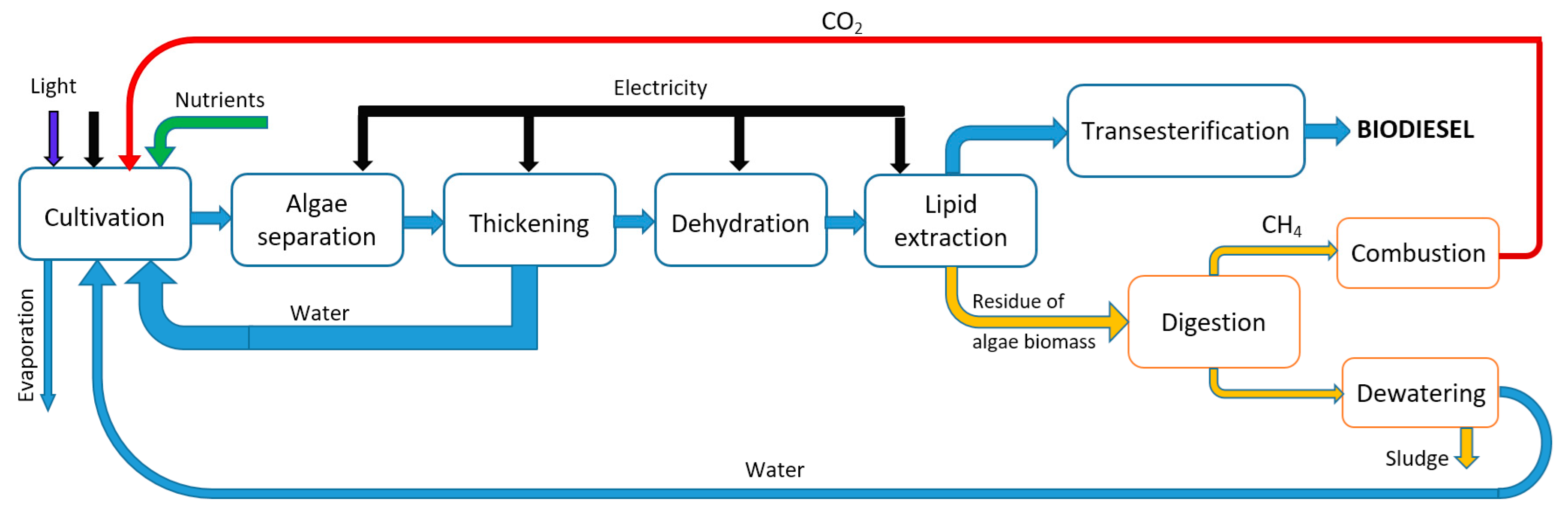
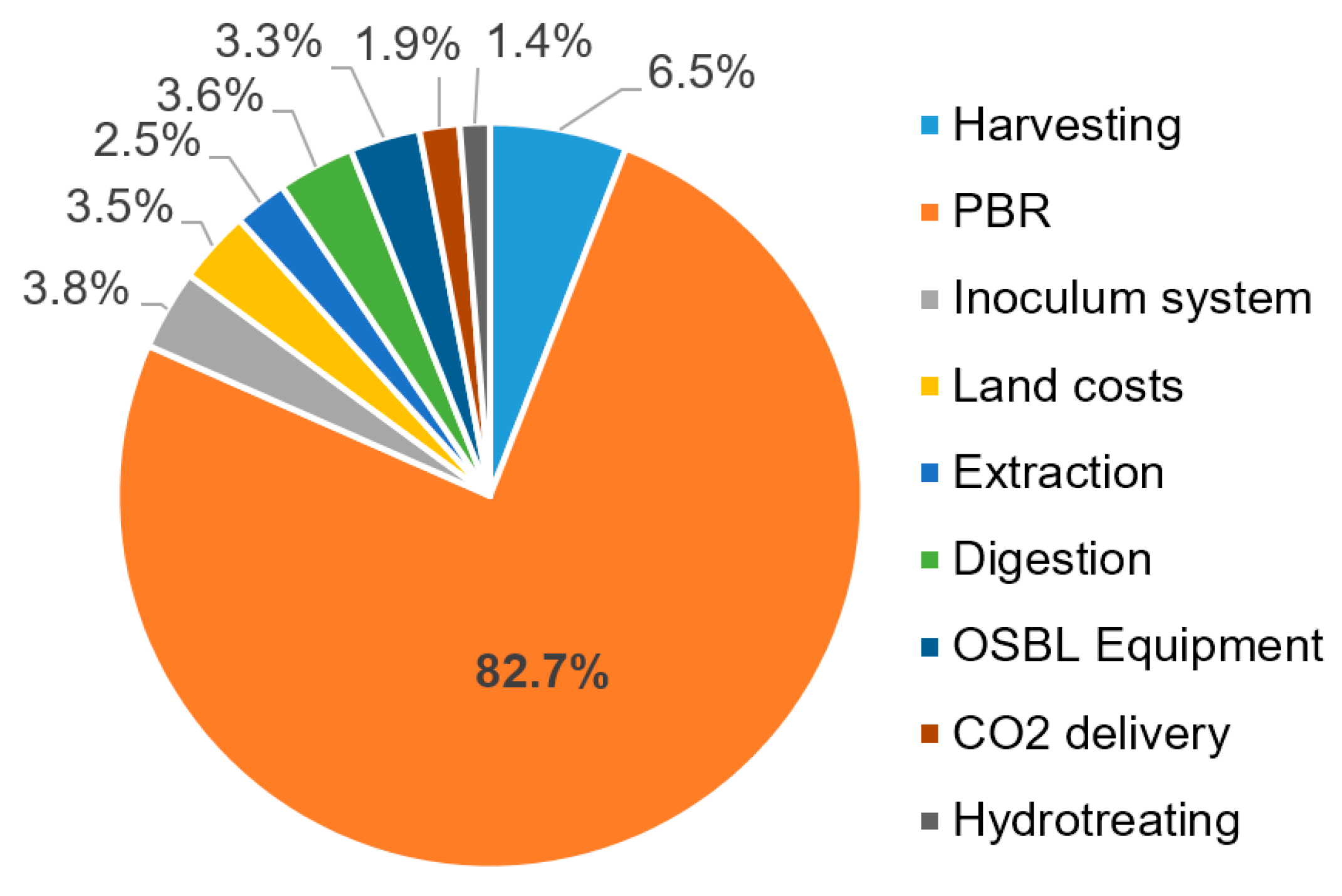
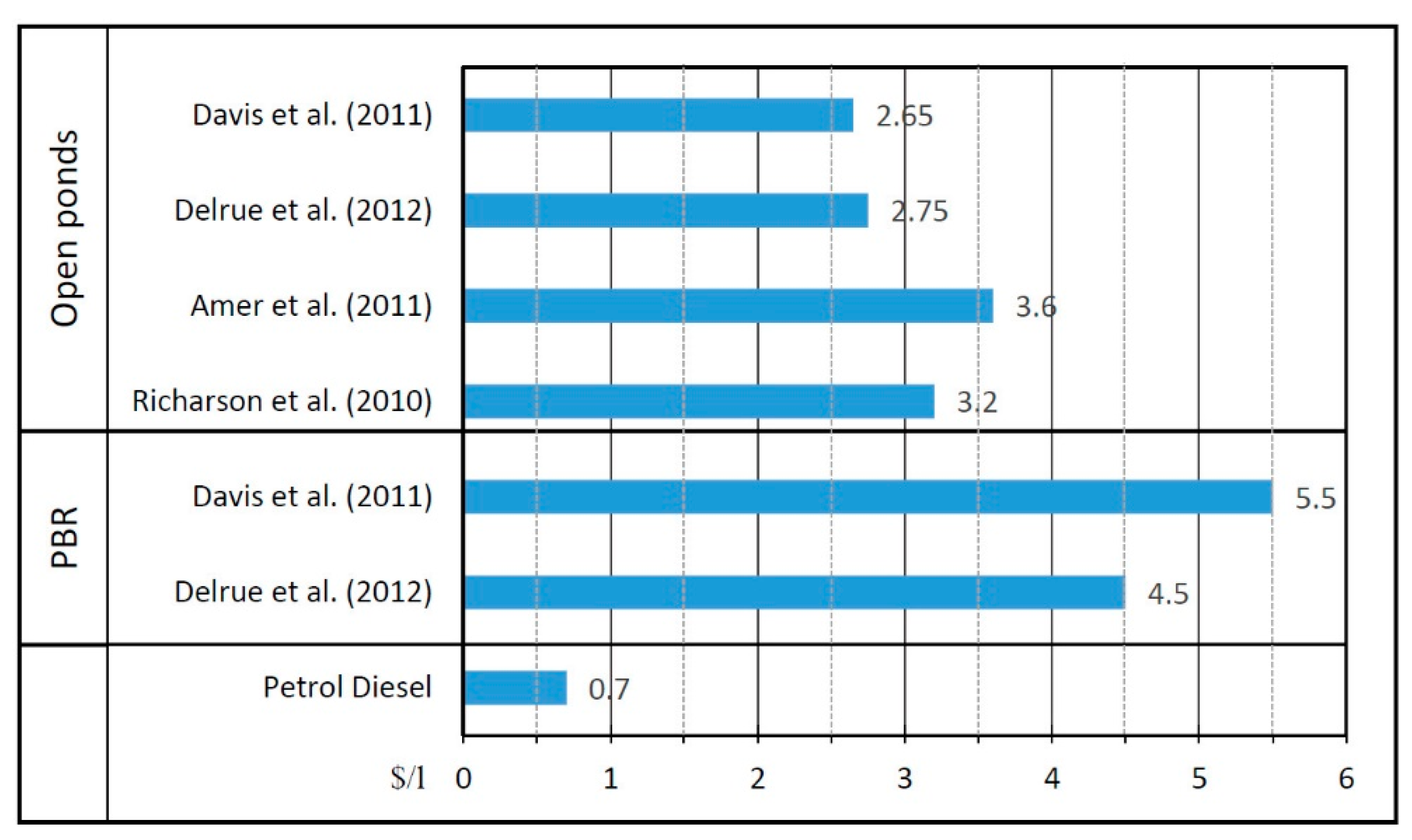
3.3. Production Cost Analysis
3.4. The Environmental Impact of Algal Biodiesel Production
3.4.1. GHG Emission
3.4.2. Land Use and Location
3.4.3. Use of Nutrients and Fertilizers
3.5. Biodiesel Production from Algae in the World
4. Results and Discussion
4.1. Strengths
4.1.1. Technical
4.1.2. Environmental
4.1.3. Diversity in Resources Harnessing
4.1.4. Sustainable Developments
4.2. Weaknesses
4.2.1. Technical
4.2.2. Economic
4.2.3. Environmental
4.3. Opportunities
4.3.1. Technical
4.3.2. Environmental
4.3.3. Economical
4.3.4. Social
4.3.5. Political
4.4. Threats
4.4.1. Technical
4.4.2. Economic
4.4.3. Market
4.4.4. Environmental
4.4.5. Political
5. Conclusions
- Essential strengths are as follows:
- Algae productivity is higher compared to most effective crops.
- A large number of algae species can be farmed.
- The CO2 footprint of algae biodiesel is smaller than conventional diesel.
- Essential weaknesses are as follows:
- Biodiesel production from algae is an extremely energy-intensive process that, in some cases, results in a negative energy balance.
- Production costs are significantly higher compared to the production of conventional diesel.
- The water footprint is large.
- Essential opportunities are as follows:
- Optimization of the biodiesel production process by introducing less energy-intensive technologies.
- Application of post-harvest water recycling.
- Application of CO2 from flue gases for algae cultivation.
- Linking algae cultivation and wastewater treatment and biogas production from algal biomass residues.
- Increasing local employability.
- Essential threats are as follows:
- Promotion of the use of other renewable energy sources in transport such as hydrogen.
- Encouraging the use of battery–electric cars.
- The production of biogas from algae is economically more profitable than the production of biodiesel.
- Production of biodiesel from lignocellulose raw materials has ecological advantages over production from algae.
- The policies of many countries are to reduce the production and sales of diesel cars.
- Linking biodiesel production and wastewater treatment,
- Algal biomass-based co-products can provide the necessary revenue to reduce the net cost of biodiesel production,
- Developing and applying less energy-intensive technologies for the biodiesel production process,
- Application of algal biodiesel for blending aviation fuel which would lead to a reduction of CO2 emissions from air transport.
Author Contributions
Funding
Conflicts of Interest
References
- EUROSTAT. Statistics Explained: Energy Statistics—An Overview. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/pdfscache/29046.pdf (accessed on 13 November 2020).
- EUROSTAT. Statistics Explained: Greenhouse Gas Emission Statistics—Emission Inventories. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/pdfscache/30599.pdf (accessed on 13 November 2020).
- European Environment Agency (EEA). Emissions of the Main Air Pollutants in Europe; EEA: Copenhagen, Denmark, 2019. [Google Scholar]
- UFOP Report on Global Market Supply 2018/2019, Union zur Förderung von Oel- und Proteinpflanzen. Available online: https://www.ufop.de/files/4815/4695/8891/WEB_UFOP_Report_on_Global_Market_Supply_18-19.pdf (accessed on 13 November 2020).
- Adeniyi, O.M.; Azimov, U.; Burluka, A. Algae biofuel: Current status and future applications. Renew. Sustain. Energy Rev. 2018, 90, 316–335. [Google Scholar] [CrossRef]
- Alaswad, A.; Dassisti, M.; Prescott, T.; Olabi, A. Technologies and developments of third generation biofuel production. Renew. Sustain. Energy Rev. 2015, 51, 1446–1460. [Google Scholar] [CrossRef]
- Luangpipat, T.; Chisti, Y. Biomass and oil production by Chlorella vulgaris and four other microalgae—Effects of salinity and other factors. J. Biotechnol. 2017, 257, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal Biofuels: Current Status and Key Challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef]
- Kirrolia, A.; Bishnoi, N.R.; Singh, R. Microalgae as a boon for sustainable energy production and its future research & development aspects. Renew. Sustain. Energy Rev. 2013, 20, 642–656. [Google Scholar] [CrossRef]
- Faried, M.; Samer, M.; Abdelsalam, E.; Yousef, R.; Attia, Y.; Ali, A. Biodiesel production from microalgae: Processes, technologies and recent advancements. Renew. Sustain. Energy Rev. 2017, 79, 893–913. [Google Scholar] [CrossRef]
- Musa, M.; Ayoko, G.A.; Ward, A.; Rösch, C.; Brown, R.J.; Rainey, T.J. Factors Afecting Microalgae Production for Biofuels and the Potentials of Chemometric Methods in Assessing and Optimizing Productivity. Cells 2019, 8, 851. [Google Scholar] [CrossRef]
- Remmers, I.M.; Wijffels, R.H.; Barbosa, M.J.; Lamers, P.P. Can We Approach Theoretical Lipid Yields in Microalgae? Trends Biotechnol. 2018, 36, 265–276. [Google Scholar] [CrossRef]
- Naeini, M.A.; Zandieh, M.; Najafi, S.E.; Sajadi, S.M. Analyzing the development of the third-generation biodiesel production from microalgae by a novel hybrid decision-making method: The case of Iran. Energy 2020, 195, 116895. [Google Scholar] [CrossRef]
- Weihrich, H. The TOWS matrix—A tool for situational analysis. Long Range Plan. 1982, 15, 54–66. [Google Scholar] [CrossRef]
- IRENA. Innovation Outlook: Advanced liquid Biofuels; International Renewable Energy Agency: Abu Dhabi, UAE, 2016. [Google Scholar]
- IRENA. Innovation Technology Outlook for Advanced Liquid Biofuels; International Renewable Energy Agency: Abu Dhabi, UAE, 2016. [Google Scholar]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strat. Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Namugenyi, C.; Nimmagadda, S.L.; Reiners, T. Design of a SWOT Analysis Model and its Evaluation in Diverse Digital Business Ecosystem Contexts. Procedia Comput. Sci. 2019, 159, 1145–1154. [Google Scholar] [CrossRef]
- Iasimone, F.; Panico, A.; De Felice, V.; Fantasma, F.; Iorizzi, M.; Pirozzi, F. Effect of light intensity and nutrients supply on microalgae cultivated in urban wastewater: Biomass production, lipids accumulation and settleability characteristics. J. Environ. Manag. 2018, 223, 1078–1085. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Darvehei, P.; Bahri, P.A.; Moheimani, N.R. Modeling the Effect of Temperature on Microalgal Growth under Outdoor Conditions. Comput. Aided Chem. Eng. 2018, 43, 55–60. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Weyer, K.M.; Bush, D.R.; Darzins, A.; Willson, B.D. Theoretical Maximum Algal Oil Production. BioEnergy Res. 2009, 3, 204–213. [Google Scholar] [CrossRef]
- Formighieri, C.; Franck, F.; Bassi, R. Regulation of the pigment optical density of an algal cell: Filling the gap between photosynthetic productivity in the laboratory and in mass culture. J. Biotechnol. 2012, 162, 115–123. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Shen, Y.; Yuan, W.; Pei, Z.J.; Wu, Q.; Mao, E. Microalgae Mass Production Methods. Trans. ASABE 2009, 52, 1275–1287. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Talent, M.; Burgess, G.; Fernández-Velasco, J.G. Protocol to compensate net evaporation and net precipitation in open-pond microalgal massive cultures and permit maximal steady-state productivities. Biomass Bioenergy 2014, 64, 81–90. [Google Scholar] [CrossRef]
- Tang, S.; Qin, C.; Wang, H.; Li, S.; Tian, S. Study on supercritical extraction of lipids and enrichment of DHA from oil-rich microalgae. J. Supercrit. Fluids 2011, 57, 44–49. [Google Scholar] [CrossRef]
- Chia, S.R.; Show, P.L.; Chew, K.W.; Chen, W.-H.; Phang, S.-M.; Ling, T.C.; Nagarajan, D.; Lee, D.; Chang, J.-S. Sustainable approaches for algae utilisation in bioenergy production. Renew. Energy 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Adam, F.; Abert-Vian, M.; Peltier, G.; Chemat, F. “Solvent-free” ultrasound-assisted extraction of lipids from fresh microalgae cells: A green, clean and scalable process. Bioresour. Technol. 2012, 114, 457–465. [Google Scholar] [CrossRef]
- Zhu, Y.; Jones, S.B.; Anderson, D.B. Algae Farm Cost Model: Considerations for Photobioreactors. In Algae Farm Cost Model: Considerations for Photobioreactors; Pacific Northwest National Lab.: Richland, WA, USA, 2018. [Google Scholar]
- Pankratz, S.; Oyedun, A.O.; Kumar, A. Development of cost models of algae production in a cold climate using different production systems. Biofuels Bioprod. Biorefining 2019, 13, 1246–1260. [Google Scholar] [CrossRef]
- Deng, X.-Y.; Gao, K.; Addy, M.; Li, D.; Zhang, R.-C.; Lu, Q.; Ma, Y.-W.; Cheng, Y.-L.; Chen, P.; Liu, Y.-H.; et al. Cultivation of Chlorella vulgaris on anaerobically digested swine manure with daily recycling of the post-harvest culture broth. Bioresour. Technol. 2018, 247, 716–723. [Google Scholar] [CrossRef]
- Ye, Y.; Huang, Y.; Xia, A.; Fu, Q.; Liao, Q.; Zeng, W.; Zheng, Y.; Zhu, X. Optimizing culture conditions for heterotrophic-assisted photoautotrophic biofilm growth of Chlorella vulgaris to simultaneously improve microalgae biomass and lipid productivity. Bioresour. Technol. 2018, 270, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Přibyl, P.; Cepák, V.; Zachleder, V. Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2012, 94, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, X.; Guo, D.; Ye, T.; Xiong, M.; Zhu, L.; Liu, C.; Jin, S.; Hu, Z. Operation of a vertical algal biofilm enhanced raceway pond for nutrient removal and microalgae-based byproducts production under different wastewater loadings. Bioresour. Technol. 2018, 253, 323–332. [Google Scholar] [CrossRef]
- Meng, Y.; Jiang, J.; Wang, H.; Cao, X.; Xue, S.; Yang, Q.; Wang, W. The characteristics of TAG and EPA accumulation in Nannochloropsis oceanica IMET1 under different nitrogen supply regimes. Bioresour. Technol. 2015, 179, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Ivanov, I.N.; Oshima, K.; Ishii, K.; Kawamoto, H.; Ota, S.; Yamazaki, T.; Hirata, A.; Kazama, Y.; Abe, T.; et al. Comparison of lipid productivity of Parachlorella kessleri heavy-ion beam irradiation mutant PK4 in laboratory and 150-L mass bioreactor, identification and characterization of its genetic variation. Algal Res. 2018, 35, 416–426. [Google Scholar] [CrossRef]
- Li, X.; Přibyl, P.; Bišová, K.; Kawano, S.; Cepák, V.; Zachleder, V.; Čížková, M.; Brányiková, I.; Vítová, M. The microalgaParachlorella kessleri--A novel highly efficient lipid producer. Biotechnol. Bioeng. 2012, 110, 97–107. [Google Scholar] [CrossRef]
- Shin, Y.S.; Jeong, J.; Nguyen, T.H.T.; Kim, J.Y.H.; Jin, E.; Sim, S.J. Targeted knockout of phospholipase A2 to increase lipid productivity in Chlamydomonas reinhardtii for biodiesel production. Bioresour. Technol. 2019, 271, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Pei, H. The growth and lipid accumulation of Scenedesmus quadricauda during batch mixotrophic/heterotrophic cultivation using xylose as a carbon source. Bioresour. Technol. 2018, 263, 525–531. [Google Scholar] [CrossRef]
- Rahman, D.Y.; Rachmayati, R.; Widyaningrum, D.N.; Susilaningsih, D. Enhancement of lipid production of Chlorella sp. 042 by mutagenesis. IOP Conf. Ser. Earth Environ. Sci. 2020, 439, 012021. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Alvaro, J.; Hyden, B.; Zienkiewicz, K.; Benning, N.; Zienkiewicz, A.; Bonito, G.; Benning, C. Enhancing oil production and harvest by combining the marine alga Nannochloropsis oceanica and the oleaginous fungus Mortierella elongata. Biotechnol. Biofuels 2018, 11, 174. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Zhu, M.; Yu, C.; Cao, Y.; Zhang, D.; Zhou, G. Increased lipid productivity and TAG content in Nannochloropsis by heavy-ion irradiation mutagenesis. Bioresour. Technol. 2013, 136, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, H.; Li, X.; Zhao, Q.; Yin, Y.; Xi, L.; Ge, B.; Qin, S. Enhanced biomass and lipid production by co-cultivation of Chlorella vulgaris with Mesorhizobium sangaii under nitrogen limitation. Environ. Boil. Fishes 2020, 32, 233–242. [Google Scholar] [CrossRef]
- Prabakaran, P.; Ravindran, A. A comparative study on effective cell disruption methods for lipid extraction from microalgae. Lett. Appl. Microbiol. 2011, 53, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Show, K.-Y.; Lee, D.; Mujumdar, A.S. Advances and Challenges on Algae Harvesting and Drying. Dry. Technol. 2015, 33, 386–394. [Google Scholar] [CrossRef]
- Kotasthane, T. Potential of Microalgae for Sustainable Biofuel Production. J. Mar. Sci. Res. Dev. 2017, 7, 223. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef]
- Pan, J.; Muppaneni, T.; Sun, Y.; Reddy, H.K.; Fu, J.; Lu, X.; Deng, S. Microwave-assisted extraction of lipids from microalgae using an ionic liquid solvent [BMIM][HSO4]. Fuel 2016, 178, 49–55. [Google Scholar] [CrossRef]
- Porphy, S.J.; Farid, M.M. Feasibility study for production of biofuel and chemicals from marine microalgae Nannochloropsis sp. based on basic mass and energy analysis. ISRN Renew. Energy 2012, 2012, 156824. [Google Scholar]
- Al-Ameri, M.; Al-Zuhair, S. Using switchable solvents for enhanced, simultaneous microalgae oil extraction-reaction for biodiesel production. Biochem. Eng. J. 2019, 141, 217–224. [Google Scholar] [CrossRef]
- Larrosa, A.; Comitre, A.; Vaz, L.; Pinto, L.A. Influence of Air Temperature on Physical Characteristics and Bioactive Compounds in Vacuum Drying of Arthrospira spirulina. J. Food Process. Eng. 2016, 40, e12359. [Google Scholar] [CrossRef]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae—A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Samorì, C.; Barreiro, D.L.; Vet, R.; Pezzolesi, L.; Brilman, D.W.F.; Galletti, P.; Tagliavini, E. Effective lipid extraction from algae cultures using switchable solvents. Green Chem. 2013, 15, 353–356. [Google Scholar] [CrossRef]
- Karmakar, B.; Halder, G. Progress and future of biodiesel synthesis: Advancements in oil extraction and conversion technologies. Energy Convers. Manag. 2019, 182, 307–339. [Google Scholar] [CrossRef]
- Couto, R.M.; Simões, P.; Reis, A.; Da Silva, M.T.L.; Martins, V.H.; Sánchez-Vicente, Y. Supercritical fluid extraction of lipids from the heterotrophic microalga Crypthecodinium cohnii. Eng. Life Sci. 2010, 10, 158–164. [Google Scholar] [CrossRef]
- Clarens, A.F.; Nassau, H.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental Impacts of Algae-Derived Biodiesel and Bioelectricity for Transportation. Environ. Sci. Technol. 2011, 45, 7554–7560. [Google Scholar] [CrossRef]
- Davis, R.; Aden, A.; Pienkos, P.T. Techno-economic analysis of autotrophic microalgae for fuel production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Grima, E.M.; Belarbi, E.-H.; Fernández, F.A.; Medina, A.R.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Delrue, F.; Setier, P.-A.; Sahut, C.; Cournac, L.; Roubaud, A.; Peltier, G.; Froment, A.-K. An economic, sustainability, and energetic model of biodiesel production from microalgae. Bioresour. Technol. 2012, 111, 191–200. [Google Scholar] [CrossRef]
- Araujo, V.K.W.S.; Hamacher, S.; Scavarda, L.F. Economic assessment of biodiesel production from waste frying oils. Bioresour. Technol. 2010, 101, 4415–4422. [Google Scholar] [CrossRef] [PubMed]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment with CO2 biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Dasan, Y.K.; Lam, M.K.; Yusup, S.; Lim, J.W.; Lee, K.T. Life cycle evaluation of microalgae biofuels production: Effect of cultivation system on energy, carbon emission and cost balance analysis. Sci. Total Environ. 2019, 688, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Jegathese, S.J.P.; Farid, M. Microalgae as a Renewable Source of Energy: A Niche Opportunity. J. Renew. Energy 2014, 2014, 430203. [Google Scholar] [CrossRef]
- Nhat, P.V.H.; Ngo, H.H.; Guo, W.; Chang, S.; Nguyen, D.; Bui, X.; Zhang, X.; Guo, J. Can algae-based technologies be an affordable green process for biofuel production and wastewater remediation? Bioresour. Technol. 2018, 256, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Gendy, T.S.; El-Temtamy, S.A. Commercialization potential aspects of microalgae for biofuel production: An overview. Egypt. J. Pet. 2013, 22, 43–51. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Chang, C.; Bai, J.; Liu, L.; Fang, S. Techno-Economic Analysis of Biodiesel Production from Microalgae: A Review. Trends Renew. Energy 2017, 3, 141–152. [Google Scholar] [CrossRef][Green Version]
- Singh, J.; Gu, S. Commercialization potential of microalgae for biofuels production. Renew. Sustain. Energy Rev. 2010, 14, 2596–2610. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Dong, W.; Zhang, X.; Tyagi, R.D.; Drogui, P.; Surampalli, R.Y. The potential of microalgae in biodiesel production. Renew. Sustain. Energy Rev. 2018, 90, 336–346. [Google Scholar] [CrossRef]
- Tredici, M.R.; Rodolfi, L.; Biondi, N.; Bassi, N.; Sampietro, G. Techno-economic analysis of microalgal biomass production in a 1-ha Green Wall Panel (GWP®) plant. Algal Res. 2016, 19, 253–263. [Google Scholar] [CrossRef]
- Amer, L.D.; Adhikari, B.; Pellegrino, J. Technoeconomic analysis of five microalgae-to-biofuels processes of varying complexity. Bioresour. Technol. 2011, 102, 9350–9359. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.W.; Outlaw, J.L.; Allison, M. The Economics of Microalgae Oil. AgBioForum. 2010, 13, 119–130. [Google Scholar]
- Bošnjaković, M. Biodiesel from Algae. J. Mech. Eng. Autom. 2013, 3, 179–188. [Google Scholar] [CrossRef]
- Wang, Z.; Calderon, M.M.; Lu, Y. Lifecycle assessment of the economic, environmental and energy performance of Jatropha curcas L. biodiesel in China. Biomass Bioenergy 2011, 35, 2893–2902. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, T.; Chen, B. A LCA Based Biofuel Supply Chain Analysis Framework. Chin. J. Chem. Eng. 2014, 22, 669–681. [Google Scholar] [CrossRef]
- Azari, A.; Noorpoor, A.; Bozorg-Haddad, O. Carbon footprint analyses of microalgae cultivation systems under autotrophic and heterotrophic conditions. Int. J. Environ. Sci. Technol. 2018, 16, 6671–6684. [Google Scholar] [CrossRef]
- Science Advisory Council EA. GHG Footprints of Different Oil Feedstocks; Science Advisory Council EA: Brussels, Belgium, 2016; pp. 1–12. [Google Scholar]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Jacob, A.; Xia, A.; Murphy, J.D. A perspective on gaseous biofuel production from micro-algae generated from CO2 from a coal-fired power plant. Applied Energy 2015, 148, 396–402. [Google Scholar] [CrossRef]
- Zhu, L.; Ketola, T. Microalgae production as a biofuel feedstock: Risks and challenges. Int. J. Sustain. Dev. World Ecol. 2011, 19, 268–274. [Google Scholar] [CrossRef]
- Baldev, E.; MubarakAli, D.; Saravanakumar, K.; Arutselvan, C.; Alharbi, N.S.; Alharbi, S.A.; Sivasubramanian, V.; Thajuddin, N. Unveiling algal cultivation using raceway ponds for biodiesel production and its quality assessment. Renew. Energy 2018, 123, 486–498. [Google Scholar] [CrossRef]
- Rafiqul, I.; Weber, C.; Lehmann, B.; Voss, A. Energy efficiency improvements in ammonia production—perspectives and uncertainties. Energy 2005, 30, 2487–2504. [Google Scholar] [CrossRef]
- Demirbas, A. Use of algae as biofuel sources. Energy Convers. Manag. 2010, 51, 2738–2749. [Google Scholar] [CrossRef]
- Dai, R.; Wang, P.; Jia, P.; Zhang, Y.; Chu, X.; Wang, Y. A review on factors affecting microcystins production by algae in aquatic environments. World J. Microbiol. Biotechnol. 2016, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lavrinovičs, A.; Juhna, T. Review on Challenges and Limitations for Algae-Based Wastewater Treatment. Constr. Sci. 2017, 20, 17–25. [Google Scholar] [CrossRef]
- Moejes, F.W.; Moejes, K.B. Algae for Africa: Microalgae as a source of food, feed and fuel in Kenya. Afr. J. Biotechnol. 2017, 16, 288–301. [Google Scholar] [CrossRef]
- Grubišić, M.; Šantek, M.I.; Šantek, B. Potential of Microalgae for the Production of Different Biotechnological Products. Chem. Biochem. Eng. Q. 2019, 33, 161–181. [Google Scholar] [CrossRef]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental Life Cycle Comparison of Algae to Other Bioenergy Feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef]
- Yang, J.; Xu, M.; Zhang, X.; Hu, Q.; Sommerfeld, M.; Chen, Y. Life-cycle analysis on biodiesel production from microalgae: Water footprint and nutrients balance. Bioresour. Technol. 2011, 102, 159–165. [Google Scholar] [CrossRef]
- Trivedi, J.; Aila, M.; Bangwal, D.; Kaul, S.; Garg, M. Algae based biorefinery—How to make sense? Renew. Sustain. Energy Rev. 2015, 47, 295–307. [Google Scholar] [CrossRef]
- Ge, F.; Xiao, Y.; Yang, Y.; Wang, W.; Moe, B.; Li, X.-F. Formation of water disinfection byproduct 2,6-dichloro-1,4-benzoquinone from chlorination of green algae. J. Environ. Sci. 2018, 63, 1–8. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sustain. Energy Rev. 2019, 115. [Google Scholar] [CrossRef]
- Dale, B.E.; Anderson, J.E.; Brown, R.C.; Csonka, S.; Dale, V.H.; Herwick, G.; Jackson, R.D.; Jordan, N.; Kaffka, S.; Kline, K.L.; et al. Take a Closer Look: Biofuels Can Support Environmental, Economic and Social Goals. Environ. Sci. Technol. 2014, 48, 7200–7203. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Huo, S.; Qin, L. A Microalgae-Based Biodiesel Refinery: Sustainability Concerns and Challenges. Int. J. Green Energy 2015, 12, 595–602. [Google Scholar] [CrossRef]
- European Environment Agency. Transport: Increasing Oil Consumption and Greenhouse Gas Emissions Hamper EU Progress towards Environment and Climate Objectives; EEA: Copenhagen, Denmark, 2020. [Google Scholar]
- Jard, G.; Marfaing, H.; Carrère, H.; Delgenes, J.; Steyer, J.; Dumas, C. French Brittany macroalgae screening: Composition and methane potential for potential alternative sources of energy and products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef]
- Campbell, J.E.; Lobell, D.B.; Field, C.B. Greater Transportation Energy and GHG Offsets from Bioelectricity Than Ethanol. Science 2009, 324, 1055–1057. [Google Scholar] [CrossRef]
- Nijs, S.D. Reducing the Emissions of GHGes from Ships by Using Biofuel Made from Microalgae. Master’s Thesis, Ghent University, Ghent, Belgium, 2018. [Google Scholar]
- Jacquin, A.-G.; Brulé-Josso, S.; Cornish, M.L.; Critchley, A.T.; Gardet, P. Selected Comments on the Role of Algae in Sustainability. In Advances in Botanical Research; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 1–30. [Google Scholar]


| Crop | Oil Yield (L/ha/year) | Biodiesel Productivity (kg/ha/year) | Crop | Oil Yield (L/ha/year) | Biodiesel Productivity (kg/ha/year) |
|---|---|---|---|---|---|
| Rapeseed | 1190 | 862 | Sunflower | 952 | 946 |
| Oil palm | 5950 | 4747 | Jatropha | 1892 | 656 |
| Corn | 172 | 152 | Microalgae * | 58,700 | 51,927 |
| Soybean | 446 | 562 | Microalgae ** | 136,900 | 121,104 |
| Microalgae Species | Lipid Productivity (g/L/day) | Lipid Content (% Dry Weight Biomass) | Biomass Productivity (g/L/day) | Reference |
|---|---|---|---|---|
| Chlorella vulgaris | 0.1837 | 32.5 | 0.2341–0.5322 | (Deng et al., 2018) [37] |
| Chlorella vulgaris | 0.0421 | 47.53 | - | (Ye et al., 2018) [38] |
| Chlorella vulgaris | 0.300 | 31 | 0.71 | (Pribyl et a., 2012) [39] |
| Desmodesmus sp. S81 | 0.01983 | 48.41 | - | (Zhang et al., 2016) [40] |
| Nannochloropsis oceanica | 0.05691 | 46.14 | 0.91 | (Meng et al., 2015) [41] |
| Parachlorella kessleri | 0.590 | 66 | 0.82 | (Takeshita et al., 2018) [42] |
| Parachlorella kessleri | 0.500 | 25 | 0.64 | (Li et al., 2013) [43] |
| Chlamydomonas reinhardtii | 0.08092 | - | 3.3 | (Shin et al., 2019) [44] |
| Scenedesmus quadricauda | 0.13955 | 38.61 | 0.3614 | (Song and Pei, 2018) [45] |
| Feedstock | Technology | Energy Efficiency Ratio (EER) |
|---|---|---|
| Jatropha | Linked transesterification and biogas production. | 3.34 |
| Palm oil | Linked transesterification and biogas production. | 3.58 |
| Marine microalgae | Algae cultivation through PBR 1. Linked transesterification and biogas production. | 0.07 |
| Freshwater microalgae | Algae cultivation in PBR. | 0.35 |
| Freshwater microalgae | Algae cultivation in ORP 2. | 1.46 |
| Culture System | Raceway Ponds | Photobioreactors |
|---|---|---|
| Required space | High | Low |
| Area/Volume ratio (m−1) | Low (5–10) | High (20–200) |
| Water loss | Very high, may also cause salt precipitation | Low |
| CO2 loss | High, depending on pond depth | Low |
| Oxygen concentration | Usually low enough because of continuous spontaneous outgassing | The closed system requires gas exchange devices |
| Light utilization efficiency | Poor | Excellent |
| Temperature | Highly variable | Cooling often required |
| Cleaning | None | Required (wall-growth and dirt reduce light intensity), but causes abrasion, limiting photobioreactor life-time |
| Contamination risk | High (limiting the number of species that can be grown) | Low |
| Biomass quality | Variable | Reproducible |
| Biomass concentration (g/L) | Low, between 0.1 and 0.5 | High, generally between 0.5 and 8 |
| Process control | Limited (flow speed and mixing) | Possible |
| Weather dependence | High (light, temperature, rainfall) | Low |
| Start-up | 6–8 weeks | 2–4 weeks |
| Capital expenses | High | Very high |
| Operating costs | Low | Higher |
| Harvesting efficiency | Low | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bošnjaković, M.; Sinaga, N. The Perspective of Large-Scale Production of Algae Biodiesel. Appl. Sci. 2020, 10, 8181. https://doi.org/10.3390/app10228181
Bošnjaković M, Sinaga N. The Perspective of Large-Scale Production of Algae Biodiesel. Applied Sciences. 2020; 10(22):8181. https://doi.org/10.3390/app10228181
Chicago/Turabian StyleBošnjaković, Mladen, and Nazaruddin Sinaga. 2020. "The Perspective of Large-Scale Production of Algae Biodiesel" Applied Sciences 10, no. 22: 8181. https://doi.org/10.3390/app10228181
APA StyleBošnjaković, M., & Sinaga, N. (2020). The Perspective of Large-Scale Production of Algae Biodiesel. Applied Sciences, 10(22), 8181. https://doi.org/10.3390/app10228181






