Monitoring Microarthropods Assemblages along a pH Gradient in a Forest Soil over a 60 Years’ Time Period
Abstract
:1. Introduction
2. Material and Methods
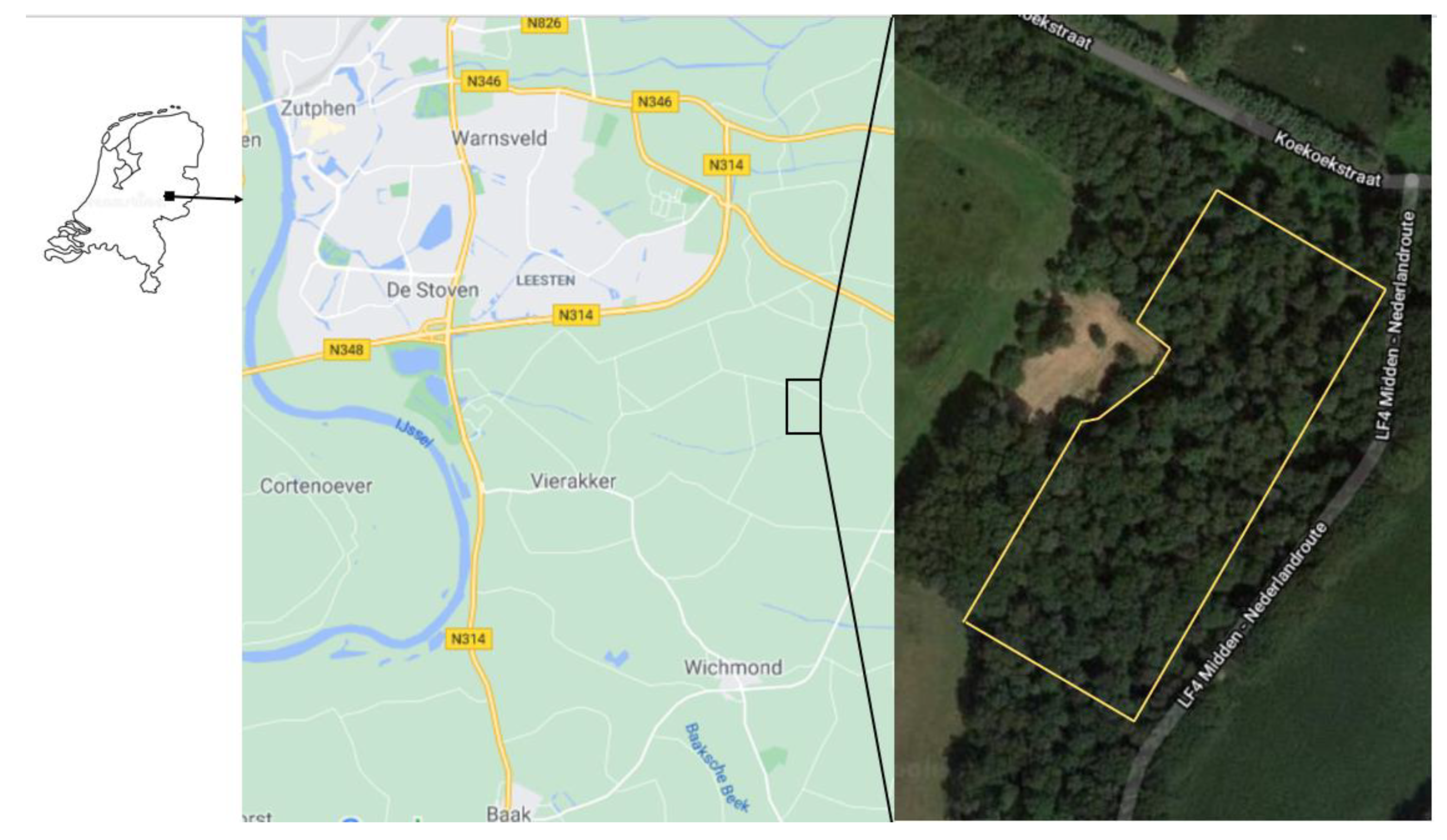
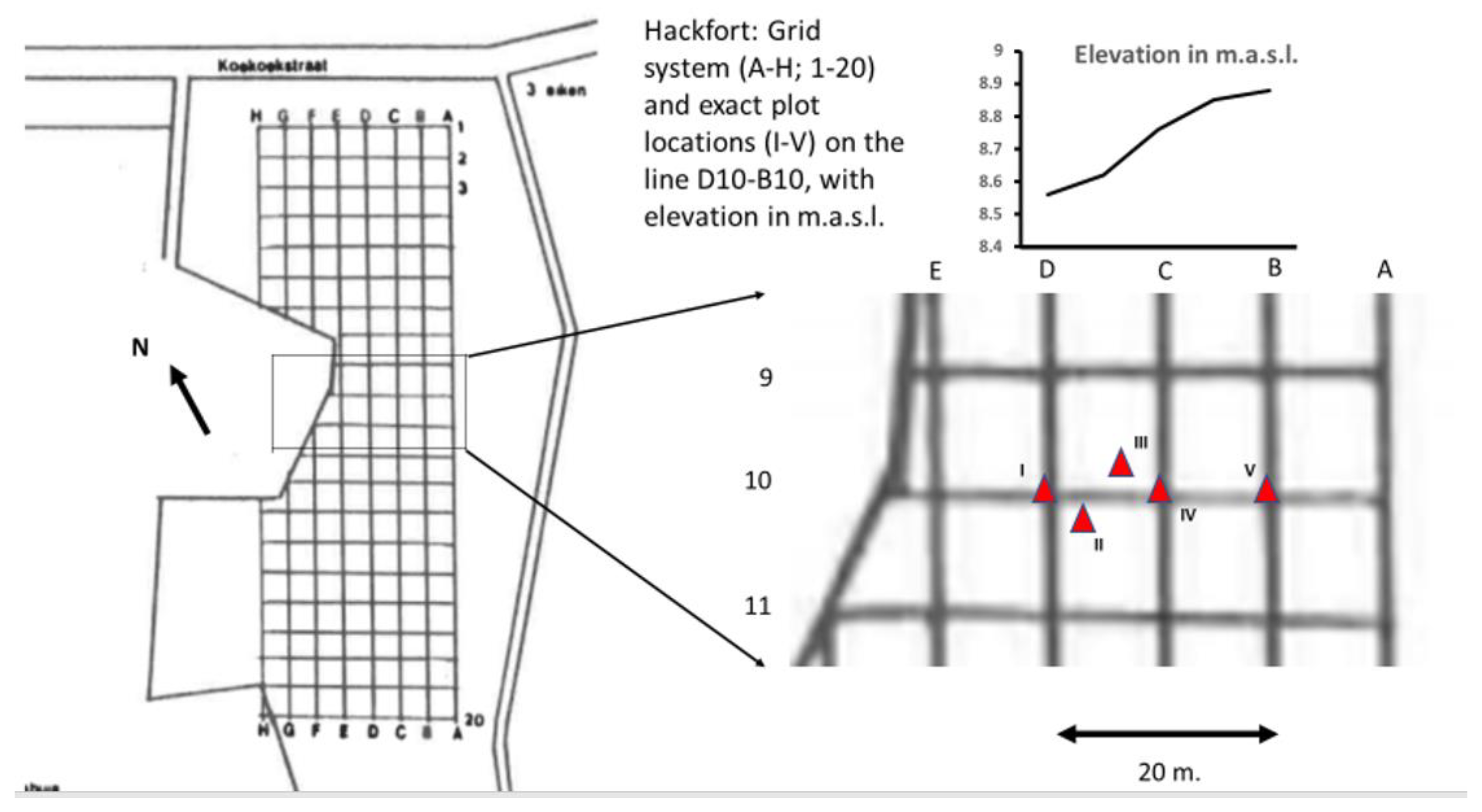
2.1. Site Description
2.2. Microarthropod Sampling and pH Measurement
2.3. Statistical Analysis
3. Results
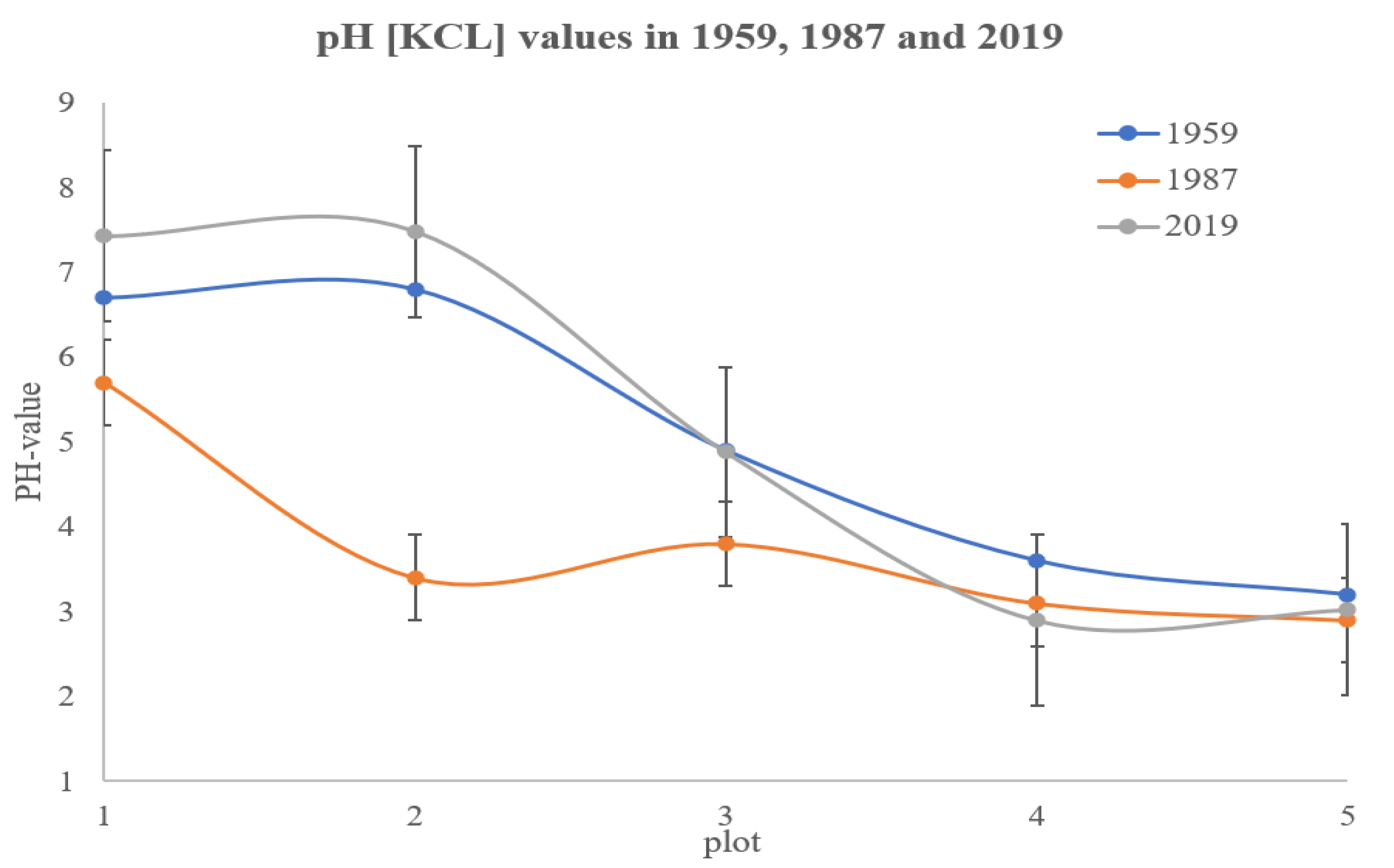
3.1. pH Values Variation During 60 Years Over the Gradient
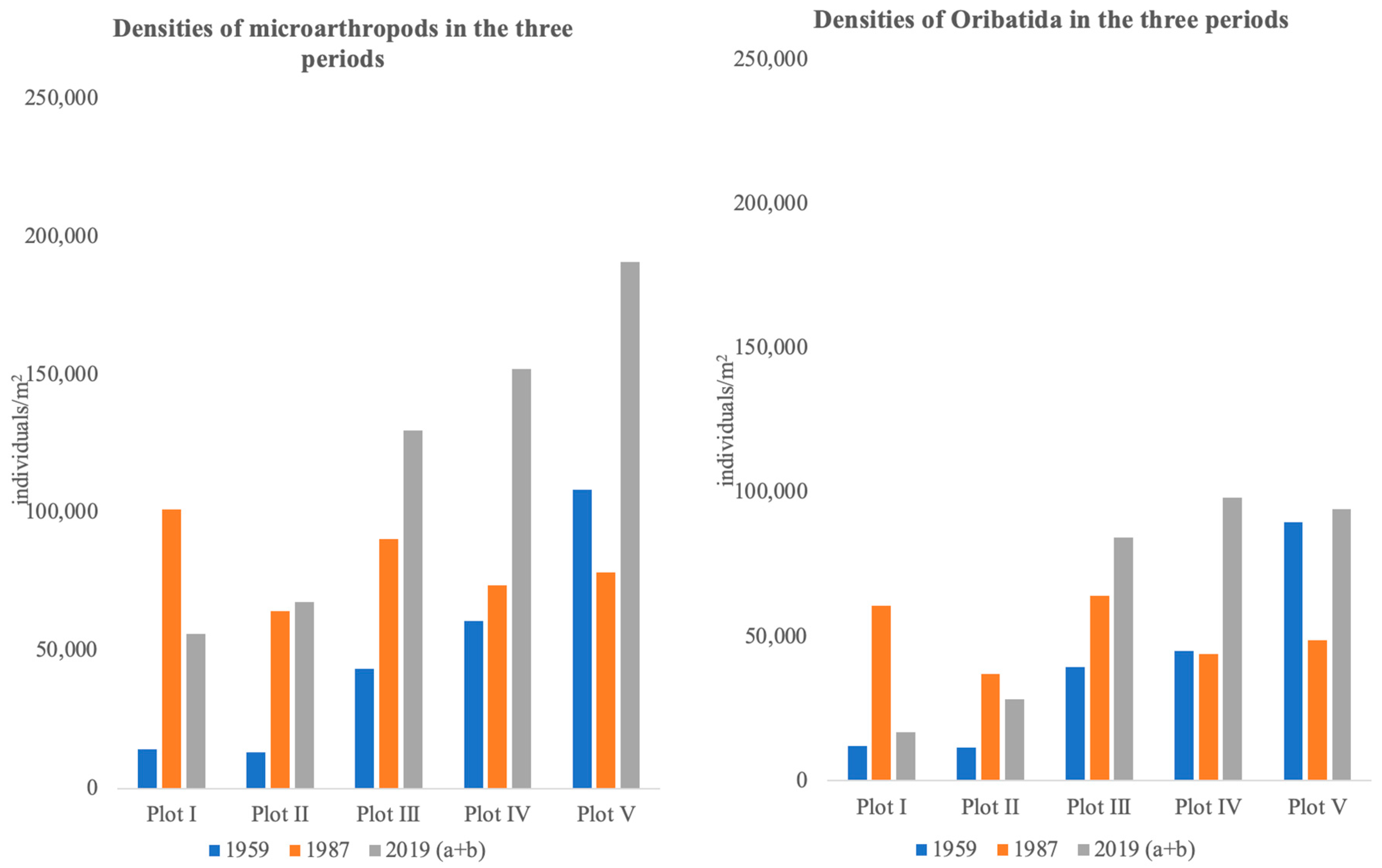
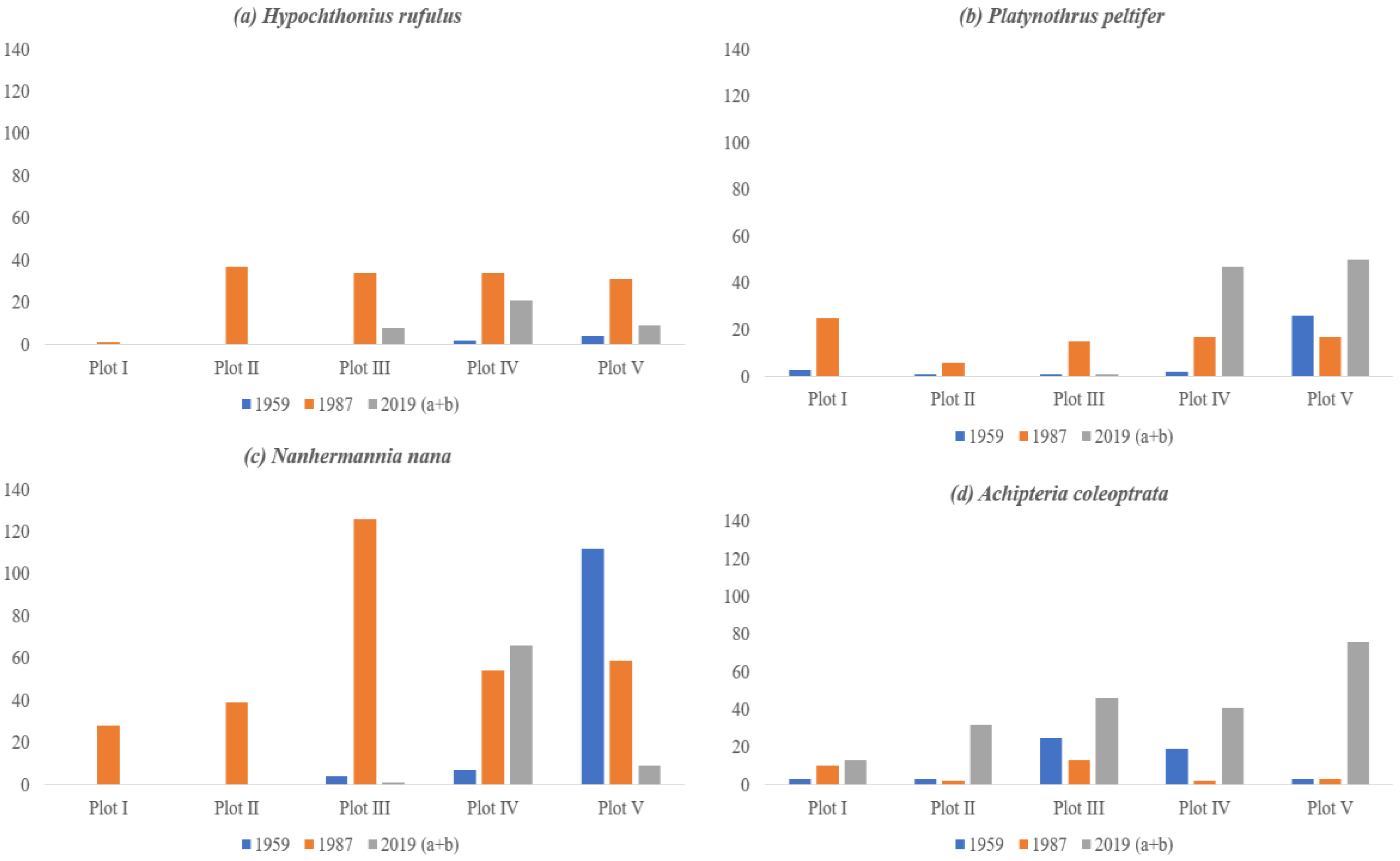
3.2. Abundance and Distribution of Microarthropods
3.3. The Relation between pH and Soil Microarthropods
4. Discussion
4.1. The Relation between pH with Soil Microarthropods
4.2. Changes in the pH Gradient Over Time
4.3. Methodological Aspects
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buol, S.W.; Southard, R.J.; Graham, R.C.; McDaniel, P.A. Soil Genesis and Classification, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Coleman, N.T.; Thomas, G.W. The basic chemistry of soil acidity. In Soil Acidity and Liming; American Society of Agronomy, Inc.: Madison, WI, USA, 1967; pp. 1–40. [Google Scholar]
- Jackson, M.L.; Sherman, G.D. Chemical Weathering of Minerals in Soils. Adv. Agron. 1953, 5, 219–318. [Google Scholar] [CrossRef]
- Hagvar, S. Effects of liming and artificial acid rain on Collembola and Protura in coniferous forest. Pedobiologia 1984, 27, 341–354. [Google Scholar]
- Minderman, G. Mull and mor (Müller-Hesselman) in relation to the soil water regime of a forest. Plant Soil 1960, 13, 1–27. [Google Scholar] [CrossRef]
- Went, J.C. Cellophane as a Medium to study the Cellulose Decomposition in Forest Soils. Acta Bot Neerl. 1959, 8, 490–491. [Google Scholar] [CrossRef]
- Went, J.C.; de Jong, F. Decomposition of Cellulose in Soils. Antonie Leeuwenhoek 1966, 32, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Minderman, G. Addition, Decomposition and Accumulation of Organic Matter in Forests. J. Ecol. 1968, 355–362. [Google Scholar] [CrossRef]
- van Grinsven, J.J.M.; Kros, J.; van Breemen, N.; van Riemsdijk, W.H.; van Eek, E. Simulated response of an acid forest soil to acid deposition and mitigation measures. NJAS Wagening. J. Life Sci. 1989, 37, 279–299. [Google Scholar]
- Stams, A.J.M.; Marinka Flameling, E.; Marnette, E.C.L. The importance of autotrophic versus heterotrophic oxidation of atmospheric ammonium in forest ecosystems with acid soil. FEMS Microbiol. Lett. 1990, 74, 337–344. [Google Scholar] [CrossRef]
- Mulder, J.; Stein, A. The solubility of aluminum in acidic forest soils: Long-term changes due to acid deposition. Geochim. Cosmochim. Acta 1994, 58, 85–94. [Google Scholar] [CrossRef] [Green Version]
- De Vries, W.; Leeters, E.; Hendriks, C.; Van Dobben, H.; Burg, J.V.D.; Boumans, L. Large scale impacts of acid deposition on forests and forest soils in the Netherlands. Stud. Environ. Sci. 1995, 64, 261–277. [Google Scholar] [CrossRef]
- Wesselink, L.; Breemen, N.; Mulder, J.; Janssen, P. A simple model of soil organic matter complexation to predict the solubility of aluminium in acid forest soils. Eur. J. Soil Sci. 1996, 47, 373–384. [Google Scholar] [CrossRef]
- Weng, L.; Temminghoff, E.J.; Van, W.H.; Van Riemsdijk, W.H. Interpretation of humic acid coagulation and soluble soil organic matter using a calculated electrostatic potential. Eur. J. Soil Sci. 2002, 53, 575–588. [Google Scholar] [CrossRef]
- Arnold, C. Invloed van Bodemverzuring op Mosmijten (Acari, Sarcoptiformes) in een Eikenhakhoutbosje te Hackfort. Internal Report Research Institute for Nature Management, Arnhem; Rijksinstituut voor Natuurbeheer: Arnhem, The Netherlands, 1989. [Google Scholar]
- Kampichler, C.; Geissen, V. Temporal predictability of soil microarthropod communities in temperate forests. Pedobiologia 2005, 49, 41–50. [Google Scholar] [CrossRef]
- Hoy, J.B. Long-Term Effects of Lindane on the Oribatid Community in a Pine Plantation. Environ. Entomol. 1990, 19, 898–900. [Google Scholar] [CrossRef]
- Koehler, H. Secondary succession of soil mesofauna: A thirteen year study. Appl. Soil Ecol. 1998, 9, 81–86. [Google Scholar] [CrossRef]
- Wolters, V. Long-term dynamics of a collembolan community. Appl. Soil Ecol. 1998, 9, 221–227. [Google Scholar] [CrossRef]
- Siepel, H.; van de Bund, C.F. The influence of management practices on the microarthropod community of grassland. Pedobiologia 1988, 31, 339–354. [Google Scholar]
- Siepel, H. Niche relationships between two panphytophagous soil mites, Nothrus silvestris Nicolet (Acari, Oribatida, Nothridae) and Platynothrus peltifer (Koch) (Acari, Oribatida, Camisiidae). Biol. Fertil. Soils 1990, 9, 139–144. [Google Scholar] [CrossRef]
- Weigmann, G. Acari, Actinochaetidae Hornmilben (Oribatida); Goecke and Evers: Keltern, Germany, 2006; Volume 520. [Google Scholar]
- Lehtinen, P. Review: Acari (Acarina), Milben. Parasitiformes (Anactinochaeta) Cohors Gamasina Leach. Raubmilben. Entomol. Fenn. 1994, 5, 10–12. [Google Scholar] [CrossRef]
- Karg, W. Acari (Acarina), Milben, Unterordnung Parasitiformes (Anactinochaeta), Uropodina Kramer, Schildkrötenmilben; VEB Gustav Fischer Verlag: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Hopkin, S.P. A key to the Collembola (springtails) of Britain and Ireland; Field Studies Council (AIDGAP Project): Telford, UK, 2007; p. 246. [Google Scholar]
- Siepel, H.; Zaitsev, A.S.; Berg, H. Checklist of the oribatid mites of the Netherlands (Acari: Oribatida). Ned. Faun. Meded. 2009, 30, 83–111. [Google Scholar]
- Siepel, H.; Cremers, H.; Vierbergen, B. Provisional checklist of the astigmatic mites of the Netherlands (Acari: Oribatida: Astigmatina). Ned. Faun. Meded. 2016, 47, 49–88. [Google Scholar]
- Siepel, H. Checklist of the mesostigmatic mites of the Netherlands. Ned. Faun. Meded. 2018, 51, 115–188. [Google Scholar]
- Hågvar, S.; Abrahamsen, G. Colonisation by Enchytraeidae, Collembola and Acari in Sterile Soil Samples with Adjusted pH Levels. Oikos 1980, 34, 245. [Google Scholar] [CrossRef]
- Francis, A.J. Effects of acidic precipitation and acidity on soil microbial processes. Water Air Soil Pollut. 1982, 18, 375–394. [Google Scholar] [CrossRef]
- Kandeler, E.; Kampichler, C.; Horak, O. Influence of heavy metals on the functional diversity of soil microbial communities. Biology Fertil. of Soils 1996, 23, 299–306. [Google Scholar] [CrossRef]
- Raubuch, M.; Beese, F. Interaction between microbial biomass and activity and the soil chemical conditions and the processes of acid load in coniferous forest soils. Zeitschrift für Pflanzenernährung und Bodenkunde 1998, 161, 59–65. [Google Scholar] [CrossRef]
- Tyler, S.W.; Wheatcraft, S.W. Fractal Scaling of Soil Particle-Size Distributions: Analysis and Limitations. Soil Sci. Soc. Am. J. 1992, 56, 362–369. [Google Scholar] [CrossRef]
- Brümmer, G.W. Heavy Metal Species, Mobility and Availability in Soils. In The Importance of Chemical “Speciation” in Environmental Processes; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1986; pp. 169–192. [Google Scholar]
- Geissen, V. Reactions of Soil Fauna (Lumbricidae, Annelida; Collembola, Arthropoda) to Varying Nutrient and Toxic Element Contents of Forest Soils After Liming and Fertilization; Institut für Bodenkunde: Hannover, Germany, 2000; p. 31. [Google Scholar]
- Ulrich, B. Soil Acidity and its Relations to Acid Deposition. In Effects of Accumulation of Air Pollutants in Forest Ecosystems; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 1983; pp. 127–146. [Google Scholar]
- Ulrich, B. Natural and anthropogenic components of soil acidification. J. Plant Nutr. Soil Sci. 1986, 149, 702–717. [Google Scholar] [CrossRef]
- Wilke, B.-M. Determination of Chemical and Physical Soil Properties. In Soil Biology; Springer Science and Business Media Springer: Berlin/Heidelberg, Germany, 2005; pp. 47–95. [Google Scholar]
- Zottl, H.W. Zur Frage der toxischen Wirkung von Aluminium auf Pflanzen. Allg. Forstz. 1983, 38, 206–208. [Google Scholar]
- Pannatier, E.G.; Walthert, L.; Blaser, P. Solution chemistry in acid forest soils: Are the BC: Al ratios as critical as expected in Switzerland? J. Plant Nutr. Soil Sci. 2004, 167, 160–168. [Google Scholar] [CrossRef]
- Zöttl, H.W.; Hüttl, R.F. Schadsymptome und Ernährungszustand von Fichtenbeständen im südwestdeutschen Alpenvorland. Allg. Forstz. 1985, 40, 197–199. [Google Scholar]
- Jaeger, G.; Eisenbeis, G. pH-dependent absorption of solutions by the ventral tube of Tomocerus flavescens (Tullberg, 1871) (Insecta, Collembola). Rev. Ecol. Biol. Sol 1984, 21, 519–531. [Google Scholar]
- Van Straalen, N.M.; Verhoef, H.A. The Development of a Bioindicator System for Soil Acidity Based on Arthropod pH Preferences. J. Appl. Ecol. 1997, 34, 217. [Google Scholar] [CrossRef]
- Dunger, W.; Voigtländer, K. Assessment of biological soil quality in wooded reclaimed mine sites. Geoderma 2005, 129, 32–44. [Google Scholar] [CrossRef]
- Franz, G.J. Splashes as Sources of Sound in Liquids. Cit. J. Acoust. Soc. Am. 1959, 31, 1080. [Google Scholar] [CrossRef]
- Hågvar, S.; Abrahamsen, G. Effect of artificial acid rain on Enchytraeidae, Collembola and Acarina in coniferous forest soil, and on Enchytraeidae in Sphagnum bog: Preliminary results. Ecol. Bull. 1977, 25, 568–570. [Google Scholar]
- Hauge, E.; Hagvar, S.; Ostbye, E. Pit-fall catches of surface-active arthropods in some high mountain habitats at Finse, south Norway. III. The species of Araneida. Nor. J. Entomol. 1978, 25, 207–220. [Google Scholar]
- Gisin, H. Ökologie und Lebensgemeinschaften der Collembolen im schweizerischen Exkursionsgebiet Basels. Rev. Suisse Zool. 1943, 50, 131–224. [Google Scholar]
- Hågvar, S. Reactions to soil acidification in microarthropods: Is competition a key factor? Biol. Fertil. Soils 1990, 9, 178–181. [Google Scholar] [CrossRef]
- Bobbink, R.; Hettelingh, J.-P.; Braun, S.; Nordin, A.; Power, S.; Schütz, K.; Strengbom, J.; Weijters, M.; Tomassen, H. Review and revision of empirical critical loads and dose-response relationships. In Proceedings of the An Expert Workshop, Noordwijkerhout, The Netherlands, 23–25 June 2010. [Google Scholar]
- Nijssen, M.; WallisDeVries, M.; Siepel, H. Pathways for the effects of increased nitrogen deposition on fauna. Biol. Conserv. 2017, 212, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Buijsman, E.; Aben, J.M.M.; Hetteling, J.P.; Van Hinsberg, A.; Koelemeijer, R.B.A.; Maas, R.J.M. Zure regen: Een analyse van dertig jaar verzuringsproblematiek in Nederland | Hydrotheek. Available online: https://library.wur.nl/WebQuery/hydrotheek/1949388 (accessed on 11 November 2020).
- Zehetner, F.; Lair, G.J.; Maringer, F.; Gerzabek, M.H.; Hein, T. From sediment to soil: Floodplain phosphorus transformations at the Danube River. Biogeochemistry 2008, 88, 117–126. [Google Scholar] [CrossRef]
- Siepel, H.; Bobbink, R.; Van De Riet, B.P.; Burg, A.B.V.D.; Jongejans, E. Long-term effects of liming on soil physico-chemical properties and micro-arthropod communities in Scotch pine forest. Biol. Fertil. Soils 2019, 55, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Sakchoowong, W.; Nomura, S.; Ogata, K.; Chanpaisaeng, J. Comparison of Extraction Efficiency between Winkler and Tullgren Extractors for Tropical Leaf Litter Macroarthropods. Thai J. Agric. Sci. 2007, 40, 97–105. [Google Scholar]


| 1959 | 1987 | 2019 a | 2019 a + b | |
|---|---|---|---|---|
| Oribatida | 330 | 637 | 317 | 645 |
| Astigmata | 31 | 34 | 2 | 5 |
| Endeostigmata # | 1 | 48 | ||
| Mesostigmata | 16 | 126 | 98 | 121 |
| Prostigmata | 2 | 80 | 14 | 56 |
| Collembola | 19 | 142 | 128 | 266 |
| Protura | 2 | 2 | 7 | |
| Diplura | 1 | 3 | 5 | |
| Pauropoda | 1 | 5 | ||
| Symphyla | 1 | 1 | ||
| Pseudoscorpionida | 1 | 1 | ||
| Species richness * | 48 | 43 | 122 | 133 |
| Positive | Negative | ||||
|---|---|---|---|---|---|
| Species | r | p | Species | r | p |
| Hypochthonius luteus | 0.735 | <0.001 | Hypochthonius rufulus | −0.755 | <0.001 |
| Rhodacarus calcarulatus | 0.789 | <0.001 | Rhodacarus coronatus | −0.592 | 0.002 |
| Nothrus anauniensis | 0.544 | 0.005 | Platynothrus peltifer | −0.705 | <0.001 |
| Panthelozetes paollii | 0.525 | 0.007 | Poecilophysis pseudoreflexa | −0.707 | <0.001 |
| Nanorchestes arboriger | 0.500 | 0.011 | Oppiella nova | −0.608 | 0.001 |
| Friesea mirabilis | 0.494 | 0.012 | Suctobelbella subcornigera | −0.565 | 0.003 |
| Pseudosinella alba | 0.440 | 0.028 | Veigaia nemorensis | −0.552 | 0.004 |
| Folsomia manolachei | −0.546 | 0.005 | |||
| Pergamasus celticus | −0.542 | 0.005 | |||
| Nanhermannia nana | −0.508 | 0.010 | |||
| Parisotoma notabilis | −0.507 | 0.010 | |||
| Dissorhina ornata | −0.500 | 0.011 | |||
| Malaconothrus monodactylus | −0.490 | 0.013 | |||
| Ceratophysella denticulata | −0.440 | 0.028 | |||
| Eniochthonius minutissimus | −0.438 | 0.029 | |||
| Oribatula tibialis | −0.428 | 0.033 | |||
| Achipteria coleoptrata | −0.423 | 0.035 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Siepel, H. Monitoring Microarthropods Assemblages along a pH Gradient in a Forest Soil over a 60 Years’ Time Period. Appl. Sci. 2020, 10, 8202. https://doi.org/10.3390/app10228202
Guo Y, Siepel H. Monitoring Microarthropods Assemblages along a pH Gradient in a Forest Soil over a 60 Years’ Time Period. Applied Sciences. 2020; 10(22):8202. https://doi.org/10.3390/app10228202
Chicago/Turabian StyleGuo, Yuxi, and Henk Siepel. 2020. "Monitoring Microarthropods Assemblages along a pH Gradient in a Forest Soil over a 60 Years’ Time Period" Applied Sciences 10, no. 22: 8202. https://doi.org/10.3390/app10228202





