Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
- Previous fractures of the knee joint
- Varus or valgus deformity of the knee joint exceeding 15 degrees
- Lack of full range of motion of the knee joint
- Clinically diagnosed instability of knee ligaments
- Earlier qualification for unilateral or total knee replacement
- Diabetes
- Hematologic disorders
- Rheumatic diseases
- Active oncological process.
2.1. Study and Control Group Description
2.2. Surgical Procedure
2.3. Post-Operative Care
2.4. Data Acquisition
2.5. Statistical Analysis
- measurability of the dependent variable on a scale of at least a range;
- normality of the variable distribution within each group;
- independence of measurements;
- uniformity of variance in all groups;
- homogeneity of covariance of measurements in the same object;
- sphericity (no correlation between consecutive measurements).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Glyn-Jones, S.; Palmer, A.; Agricola, R.; Price, A.; Vincent, T.; Weinans, H.; Carr, A. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Cibere, J.; Sayre, E.C.; Guermazi, A.; Nicolaou, S.; Kopec, J.A.; Esdaile, J.M.; Thorne, A.; Singer, J.; Wong, H. Natural history of cartilage damage and osteoarthritis progression on magnetic resonance imaging in a population-based cohort with knee pain. Osteoarthr. Cartil. 2011, 19, 683–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [Green Version]
- Johnson, V.; Hunter, D. The epidemiology of osteoarhtritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.; Garcia-Gil, M.; Elorza, J.; Mendez-Boo, L.; Hermosilla, E.; Javaid, M.; Cooper, C.; Diez-Perez, A.; Arden, N.; Bolibar, B.; et al. Socio-economic status and the risk of developing hand, hip or knee osteoarthritis: A region-wide ecological study. Osteoarthr. Cartil. 2015, 23, 1323–1329. [Google Scholar] [CrossRef] [Green Version]
- Castagnini, F.; Sudanese, A.; Bordini, B.; Tassinari, E.; Stea, S.; Toni, A. Total Knee Replacement in Young Patients: Survival and Causes of Revision in a Registry Population. J. Arthroplast. 2017, 32, 3368–3372. [Google Scholar] [CrossRef]
- Roberts, V.I.; Esler, C.N.A.; Harper, W.M. A 15-year follow-up study of 4606 primary total knee replacements. J. Bone Jt. Surg. Br. Vol. 2007, 89-B, 1452–1456. [Google Scholar] [CrossRef]
- Steadman, J.R.; Rodkey, W.G.; Briggs, K.K. Microfracture: Its History and Experience of the Developing Surgeon. Cartilage 2010, 1, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Feagin, J.A.; Steadman, J.R. The Crucial Principles in Care of the Knee; Wolters Kluwer: Alphen aan den Rijn, The Netherlands; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; ISBN 978-0-7817-7250-1. [Google Scholar]
- Steadman, J.R.; Rodkey, W.G.; Singleton, S.B.; Briggs, K.K. Microfracture technique forfull-thickness chondral defects: Technique and clinical results. Oper. Tech. Orthop. 1997, 7, 300–304. [Google Scholar] [CrossRef]
- Caplan, A.I. Bone development and repair. Bioessays 1987, 6, 171–175. [Google Scholar] [CrossRef]
- Miniaci, A.; Tytherleigh-Strong, G. Fixation of Unstable Osteochondritis Dissecans Lesions of the Knee Using Arthroscopic Autogenous Osteochondral Grafting (Mosaicplasty). Arthrosc. J. Arthrosc. Relat. Surg. 2007, 23, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, D.D.; Trotter, G.W.; Powers, B.E.; Rodkey, W.G.; Steadman, J.R.; Howard, R.D.; Park, R.D.; McIlwraith, C.W. Arthroscopic Subchondral Bone Plate Microfracture Technique Augments Healing of Large Chondral Defects in the Radial Carpal Bone and Medial Femoral Condyle of Horses. Vet. Surg. 1999, 28, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Eyre, D.R.; Koide, S.; Glimcher, M.J. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J. Bone Jt. Surg. Am. 1980, 62, 79–89. [Google Scholar] [CrossRef]
- Shapiro, F.; Koide, S.; Glimcher, M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J. Bone Jt. Surg. Am. 1993, 75, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.S.; Steadman, J.R.; Briggs, K.K.; Rodrigo, J.J.; Rodkey, W.G. Patient satisfaction and outcome after microfracture of the degenerative knee. J. Knee Surg. 2004, 17, 13–17. [Google Scholar] [CrossRef]
- Milano, G.; Sanna Passino, E.; Deriu, L.; Careddu, G.; Manunta, L.; Manunta, A.; Saccomanno, M.F.; Fabbriciani, C. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: An experimental study in a sheep model. Osteoarthr. Cartil. 2010, 18, 971–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolis, D.J.; Kantor, J.; Santanna, J.; Strom, B.L.; Berlin, J.A. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2001, 24, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Whitman, D.H.; Berry, R.L.; Green, D.M. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 1997, 55, 1294–1299. [Google Scholar] [CrossRef]
- Nikolidakis, D.; Jansen, J.A. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng. Part B Rev. 2008, 14, 249–258. [Google Scholar] [CrossRef]
- Rendu, F.; Brohard-Bohn, B. The platelet release reaction: Granules’ constituents, secretion and functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.P.; Hondke, S.; Endres, M.; Pruss, A.; Siclari, A.; Kaps, C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J. Orthop. Res. 2012, 30, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Blaney Davidson, E.N.; Vitters, E.L.; van der Kraan, P.M.; van den Berg, W.B. Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: Role in cartilage degradation, chondrogenesis and osteophyte formation. Ann. Rheum. Dis. 2006, 65, 1414–1421. [Google Scholar] [CrossRef] [Green Version]
- Hickey, D.G.; Frenkel, S.R.; Di Cesare, P.E. Clinical applications of growth factors for articular cartilage repair. Am. J. Orthop. 2003, 32, 70–76. [Google Scholar]
- Nixon, A.J.; Fortier, L.A.; Williams, J.; Mohammed, H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J. Orthop. Res. 1999, 17, 475–487. [Google Scholar] [CrossRef]
- Milano, G.; Deriu, L.; Sanna Passino, E.; Masala, G.; Manunta, A.; Postacchini, R.; Saccomanno, M.F.; Fabbriciani, C. Repeated platelet concentrate injections enhance reparative response of microfractures in the treatment of chondral defects of the knee: An experimental study in an animal model. Arthroscopy 2012, 28, 688–701. [Google Scholar] [CrossRef]
- Bauer, T.; Boisrenoult, P.; Jenny, J.-Y. Post-arthroscopy septic arthritis: Current data and practical recommendations. Orthop. Traumatol. Surg. Res. 2015, 101, S347–S350. [Google Scholar] [CrossRef] [Green Version]
- Barnett, A.J.; Toms, A.D. Revision Total Hip and Knee Replacement. Clin. Geriatr. Med. 2012, 28, 431–446. [Google Scholar] [CrossRef]
- Boffa, A.; Previtali, D.; Altamura, S.A.; Zaffagnini, S.; Candrian, C.; Filardo, G. Platelet-Rich Plasma Augmentation to Microfracture Provides a Limited Benefit for the Treatment of Cartilage Lesions: A Meta-analysis. Orthop. J. Sports Med. 2020, 8. [Google Scholar] [CrossRef]
- Cameron, M.L.; Briggs, K.K.; Steadman, J.R. Reproducibility and Reliability of the Outerbridge Classification for Grading Chondral Lesions of the Knee Arthroscopically. Am. J. Sports Med. 2003, 31, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Dhurat, R.; Sukesh, M. Principles and methods of preparation of platelet-rich plasma: A review and author′s perspective. J. Cutan. Aesthet Surg. 2014, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar] [PubMed]
- Tegner, Y.; Lysholm, J. Rating systems in the evaluation of knee ligament injuries. Clin. Orthop. Relat. Res. 1985, 43–49. [Google Scholar] [CrossRef]
- Rabiej, M. Analizy Statystyczne z Programami Statistica i Excel; Helion: Gliwice, Poland, 2018; ISBN 978-83-283-3922-4. [Google Scholar]
- Duif, C.; Vogel, T.; Topcuoglu, F.; Spyrou, G.; von Schulze Pellengahr, C.; Lahner, M. Does intraoperative application of leukocyte-poor platelet-rich plasma during arthroscopy for knee degeneration affect postoperative pain, function and quality of life? A 12-month randomized controlled double-blind trial. Arch. Orthop. Trauma Surg. 2015, 135, 971–977. [Google Scholar] [CrossRef]
- Guney, A.; Akar, M.; Karaman, I.; Oner, M.; Guney, B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2384–2389. [Google Scholar] [CrossRef]
- Lee, G.W.; Son, J.-H.; Kim, J.-D.; Jung, G.-H. Is platelet-rich plasma able to enhance the results of arthroscopic microfracture in early osteoarthritis and cartilage lesion over 40 years of age? Eur. J. Orthop. Surg. Traumatol. 2013, 23, 581–587. [Google Scholar] [CrossRef]
- Frank, R.M.; McCormick, F.; Rosas, S.; Amoo-Achampong, K.; Erickson, B.; Bach, B.R.; Cole, B.J. Reoperation Rates After Cartilage Restoration Procedures in the Knee: Analysis of a Large US Commercial Database. Am. J. Orthop. 2018, 47. [Google Scholar] [CrossRef] [Green Version]
- Piedade, S.R.; Pinaroli, A.; Servien, E.; Neyret, P. TKA outcomes after prior bone and soft tissue knee surgery. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2737–2743. [Google Scholar] [CrossRef]
- Piedade, S.R.; Pinaroli, A.; Servien, E.; Neyret, P. Is previous knee arthroscopy related to worse results in primary total knee arthroplasty? Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 328–333. [Google Scholar] [CrossRef]
- Hildner, F.; Eder, M.J.; Hofer, K.; Aberl, J.; Redl, H.; van Griensven, M.; Gabriel, C.; Peterbauer-Scherb, A. Human platelet lysate successfully promotes proliferation and subsequent chondrogenic differentiation of adipose-derived stem cells: A comparison with articular chondrocytes: Human platelet lysate promotes proliferation/chondrogenic differentiation of ASCs. J. Tissue Eng. Regen. Med. 2015, 9, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Mancò, A.; Goderecci, R.; Rughetti, A.; De Giorgi, S.; Necozione, S.; Bernardi, A.; Calvisi, V. Microfracture versus microfracture and platelet-rich plasma: Arthroscopic treatment of knee chondral lesions. A two-year follow-up study. Joints 2016, 04, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Papalia, R.; Diaz Balzani, L.; Torre, G.; Tirindelli, M.C.; Nobile, C.; Maffulli, N.; Denaro, V. Intraoperative application Platelet rich fibrin, postoperative injections OF PRP or microfracture only for osteochondral lesions of the knee: A five-year retrospective evaluation. J. Biol. Regul. Homeost. Agents 2016, 30, 41–49. [Google Scholar] [PubMed]
- Manunta, A.F.; Manconi, A. The treatment of chondral lesions of the knee with the microfracture technique and platelet-rich plasma. Joints 2013, 1, 167–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Parameter | PRP Group N = 16 | Control Group N = 13 | p-Value |
|---|---|---|---|
| Age, year (mean ± SD) | 63 ± 3.8 | 59 ± 6.8 | n. s (p = 0.069) |
| Female gender, n (%) | 11 (68.7%) | 8 (61.5%) | n. s (p = 0.685) |
| BMI (mean ± SD) | 30.6 ± 2.85 | 31 ± 4.1 | n. s (p = 0.763) |
| Duration of symptoms, months | 53.2 ± 66.9 | 78 ± 81.7 | n. s (p = 0.539) |
| History of trauma, n (%) | 3 (18.75%) | 1 (7.7%) | n. s (p = 0.391) |
| Involvement of the dominant extremity, n (%) | 5 (31.25%) | 6 (46.15%) | n. s (p = 0.411) |
| Opposite extremity involvement, n (%) | 6 (37.5%) | 10 (77%) | p = 0.034 |
| Prior to surgery physiotherapy, n (%) | 12 (75%) | 11 (84.6%) | n. s (p = 0.525) |
| Medial meniscus lesions, n (%) | 10 (62.5%) | 8 (61.5%) | n. s (p = 0.958) |
| Lateral meniscus lesions, n (%) | 5 (31.25%) | 3 (23%) | n. s (p = 0.624) |
| Bilateral meniscus lesions, n (%) | 3 (18.75%) | 3 (23%) | n. s (p = 0.775) |
| MFC cartilage lesions grade III-IV, n (%) | 11 (68.75%) | 6 (46.15%) | n. s (p = 0.219) |
| LFC cartilage lesions grade III-IV, n (%) | 3 (18.75%) | 1 (7.6%) | n. s (p = 0.391) |
| MTC cartilage lesions grade III-IV, n (%) | 7 (43.75%) | 9 (69.2%) | n. s (p = 0.170) |
| LTC cartilage lesions grade III-IV, n (%) | 2 (12.5%) | 0 (0%) | n. s (p = 0.187) |
| PFJ cartilage lesions grade III-IV, n (%) | 8 (50%) | 2 (15%) | n. s (p = 0.051) |
| Scale | Test | Control Group | PRP |
|---|---|---|---|
| WOMAC Total | Wilks’, Pillai’s, Hotelln‘s, Roy’s | 0.014 | 0.004 |
| G-G, H-F | 0.000 | 0.000 | |
| WOMAC Stiffness | Wilks’, Pillai’s, Hotelln‘s, Roy’s | 0.028 | 0.002 |
| G-G, H-F | 0.012 | 0.000 | |
| WOMAC Function | Wilks’, Pillai’s, Hotelln‘s, Roy’s | 0.016 | 0.004 |
| G-G, H-F | 0.000 | 0.000 | |
| WOMAC Pain | Wilks’, Pillai’s, Hotelln‘s, Roy’s | 0.012 | 0.007 |
| G-G, H-F | 0.000 | 0.000 | |
| Lysholm | Univariate tests | 0.001 | 0.000 |
| WOMAC Score PRP Group TOTAL | ||||
|---|---|---|---|---|
| Preoperative | Postoperative 6 weeks | Postoperative 12 weeks | Postoperative 24 weeks | |
| Preoperative | - | 0.012 | 0.015 | 0.029 |
| Postoperative 6 weeks | 0.012 | - | 1.000 | 0.987 |
| Postoperative 12 weeks | 0.015 | 1.000 | - | 0.995 |
| Postoperative 24 weeks | 0.029 | 0.987 | 0.995 | - |
| WOMAC Control Group TOTAL | ||||
| Preoperative | - | 0.042 | 0.034 | 0.099 |
| Postoperative 6 weeks | 0.042 | - | 1.000 | 0.983 |
| Postoperative 12 weeks | 0.034 | 1.000 | - | 0.968 |
| Postoperative 24 weeks | 0.099 | 0.983 | 0.968 | - |
| WOMAC score PRP Group STIFFNESS | ||||
| Preoperative | - | 0.009 | 0.027 | 0.036 |
| Postoperative 6 weeks | 0.009 | - | 0.977 | 0.953 |
| Postoperative 12 weeks | 0.027 | 0.977 | - | 1.000 |
| Postoperative 24 weeks | 0.036 | 0.953 | 1.000 | - |
| WOMAC Control Group STIFFNESS | ||||
| Preoperative | - | 0.347 | 0.407 | 0.559 |
| Postoperative 6 weeks | 0.347 | - | 1.000 | 0.984 |
| Postoperative 12 weeks | 0.407 | 1.000 | - | 0.994 |
| Postoperative 24 weeks | 0.559 | 0.984 | 0.994 | - |
| WOMAC score PRP Group Function | ||||
| Preoperative | - | 0.015 | 0.017 | 0.034 |
| Postoperative 6 weeks | 0.015 | - | 1.000 | 0.990 |
| Postoperative 12 weeks | 0.017 | 1.000 | - | 0.994 |
| Postoperative 24 weeks | 0.034 | 0.990 | 0.994 | - |
| WOMAC Control Group Function | ||||
| Preoperative | - | 0.052 | 0.045 | 0.121 |
| Postoperative 6 weeks | 0.052 | - | 1.000 | 0.981 |
| Postoperative 12 weeks | 0.045 | 1.000 | - | 0.972 |
| Postoperative 24 weeks | 0.121 | 0.981 | 0.972 | - |
| WOMAC score PRP Group Pain | ||||
| Preoperative | - | 0.012 | 0.018 | 0.029 |
| Postoperative 6 weeks | 0.012 | - | 0.999 | 0.988 |
| Postoperative 12 weeks | 0.018 | 0.999 | - | 0.998 |
| Postoperative 24 weeks | 0.029 | 0.988 | 0.998 | - |
| WOMAC Control Group Pain | ||||
| Preoperative | - | 0.015 | 0.008 | 0.033 |
| Postoperative 6 weeks | 0.015 | - | 0.995 | 0.989 |
| Postoperative 12 weeks | 0.008 | 0.995 | - | 0.947 |
| Postoperative 24 weeks | 0.033 | 0.989 | 0.947 | - |
| Lysholm score PRP Group | ||||
| Preoperative | - | - | - | 0.000 |
| Postoperative 24 weeks | 0.000 | - | - | - |
| Lysholm score Control Group | ||||
| Preoperative | - | - | - | 0.001 |
| Postoperative 24 weeks | 0.001 | - | - | - |
| Parameter | PRP Group | Control Group | p-Value |
|---|---|---|---|
| WOMAC Total mean (±SD) | |||
| Preoperative | 1354.2 (±627.3) | 1122.6 (±542.9) | n. s (p = 0.327) |
| Postoperative 6 weeks | 602.6 (±604.9) | 487.8 (±574.6) | n. s (p = 0.625) |
| Postoperative 12 weeks | 626.4 (±686.5) | 467.7 (±581.1) | n. s (p = 0.534) |
| Postoperative 24 weeks | 681.8 (±731.3) | 573.5 (±665.3) | n. s (p = 0.698) |
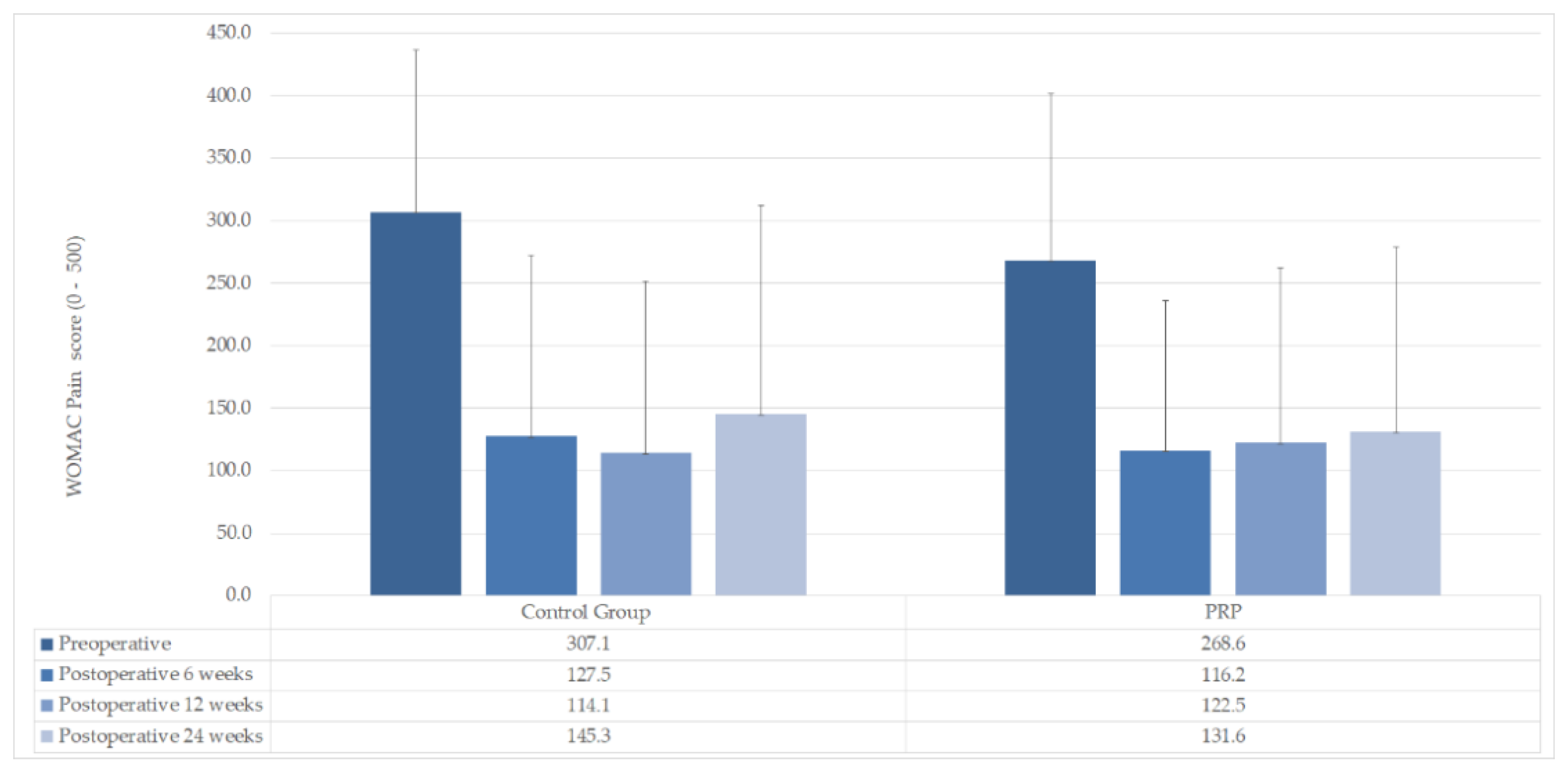
| WOMAC Pain mean (±SD) | |||
| Preoperative | 268.6 (±133.5) | 307.1 (±129.7) | n. s (p = 0.463) |
| Postoperative 6 weeks | 116.2 (±120.1) | 127.5 (±145.1) | n. s (p = 0.828) |
| Postoperative 12 weeks | 122.5 (±139.7) | 114.1 (±137.7) | n. s (p = 0.878) |
| Postoperative 24 weeks | 131.6 (±147.2) | 145.3 (±167.1) | n. s (p = 0.825) |
| WOMAC Stiffness mean (±SD) | |||
| Preoperative | 110.2 (±61.2) | 71.5 (±38.4) | n. s (p = 0.070) |
| Postoperative 6 weeks | 41.8 (±49.3) | 39.5 (±47.7) | n. s (p = 0.903) |
| Postoperative 12 weeks | 50.3 (±59.2) | 41.5 (±51.6) | n. s (p = 0.692) |
| Postoperative 24 weeks | 52.7 (±64.0) | 46.4 (±55.8) | n. s (p = 0.793) |
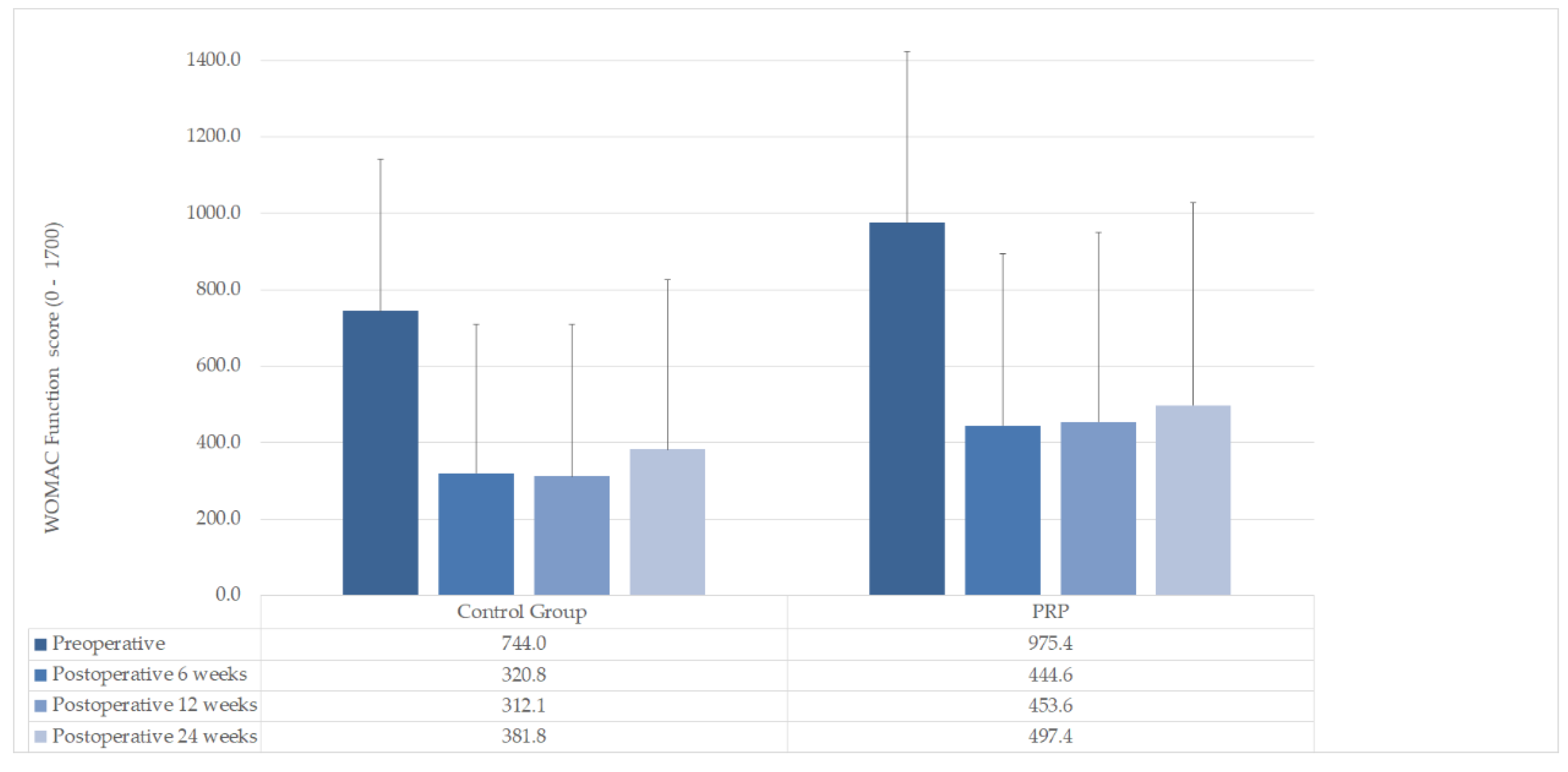
| WOMAC Function mean (±SD) | |||
| Preoperative | 975.4 (±449.2) | 744.0 (±397.2) | n. s (p = 0.178) |
| Postoperative 6 weeks | 444.6 (±449.2) | 320.8 (±387.9) | n. s (p = 0.462) |
| Postoperative 12 weeks | 453.6 (±495.9) | 312.1 (±397.4) | n. s (p = 0.435) |
| Postoperative 24 weeks | 497.4 (±530.4) | 381.8 (±444.9) | n. s (p = 0.556) |
| Lysholm. mean (±SD) | |||
| Preoperative | 49.25 (±12.86) | 53.08 (±11.06) | n. s (p = 0.427) |
| Postoperative 24 weeks | 77.06 (±17.01) | 76.54 (±18.93) | n. s (p = 0.941) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J.; Jurkiewicz, A. Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis. Appl. Sci. 2020, 10, 8312. https://doi.org/10.3390/app10238312
Krakowski P, Karpiński R, Maciejewski R, Jonak J, Jurkiewicz A. Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis. Applied Sciences. 2020; 10(23):8312. https://doi.org/10.3390/app10238312
Chicago/Turabian StyleKrakowski, Przemysław, Robert Karpiński, Ryszard Maciejewski, Józef Jonak, and Andrzej Jurkiewicz. 2020. "Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis" Applied Sciences 10, no. 23: 8312. https://doi.org/10.3390/app10238312





