Optimization of Microwave and Ultrasound Extraction Methods of Açai Berries in Terms of Highest Content of Phenolic Compounds and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction Process
2.2. Total Phenolic Analysis
2.3. Total Anthocyanin Analysis
2.4. Total Flavonoid Analysis
2.5. DPPH Radical Scavenging Activity
2.6. ABTS Radical Cation Scavenging Activity
2.7. FRAP Antioxidant Assay Activity
2.8. Color and pH Measurements
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Ultrasound and Microwave Extraction on the Content of Bioactive Compounds
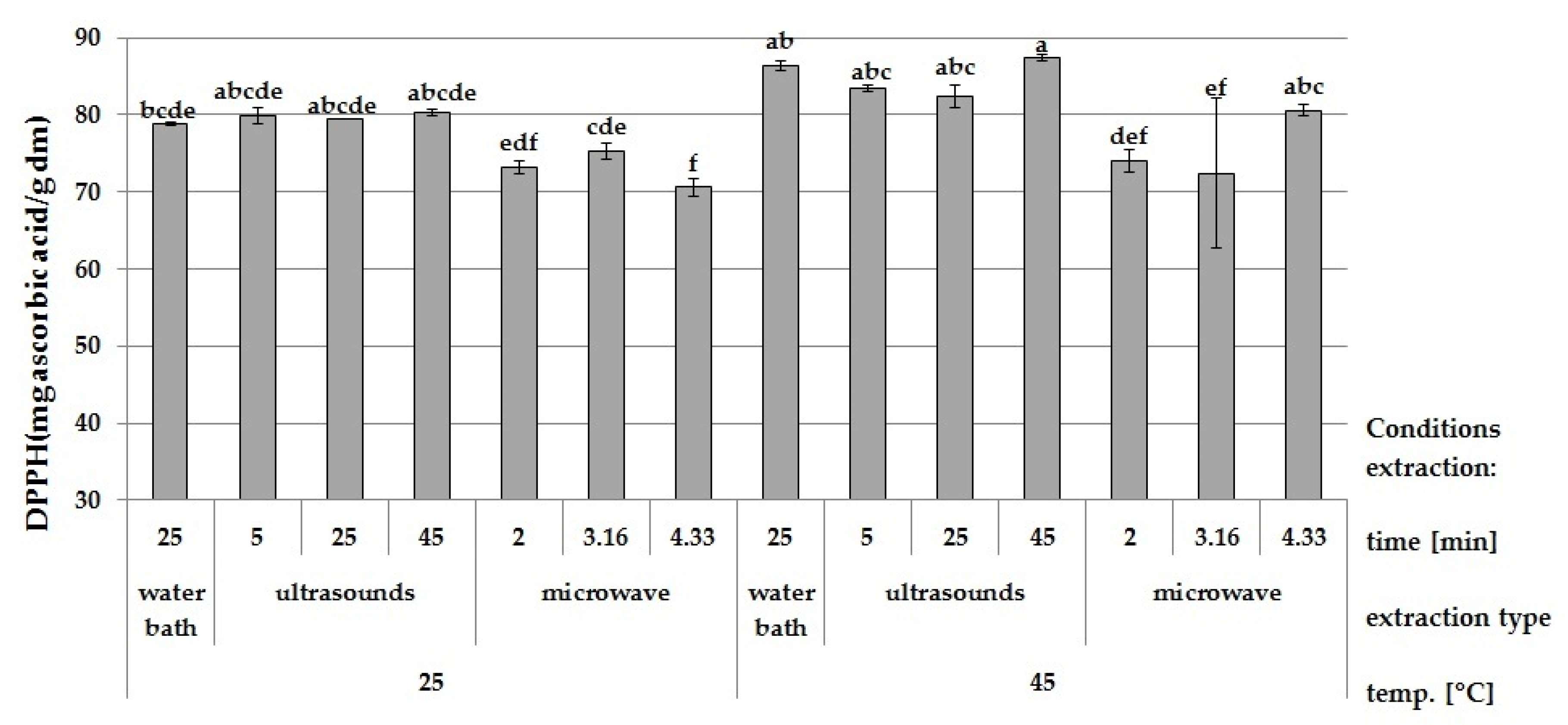
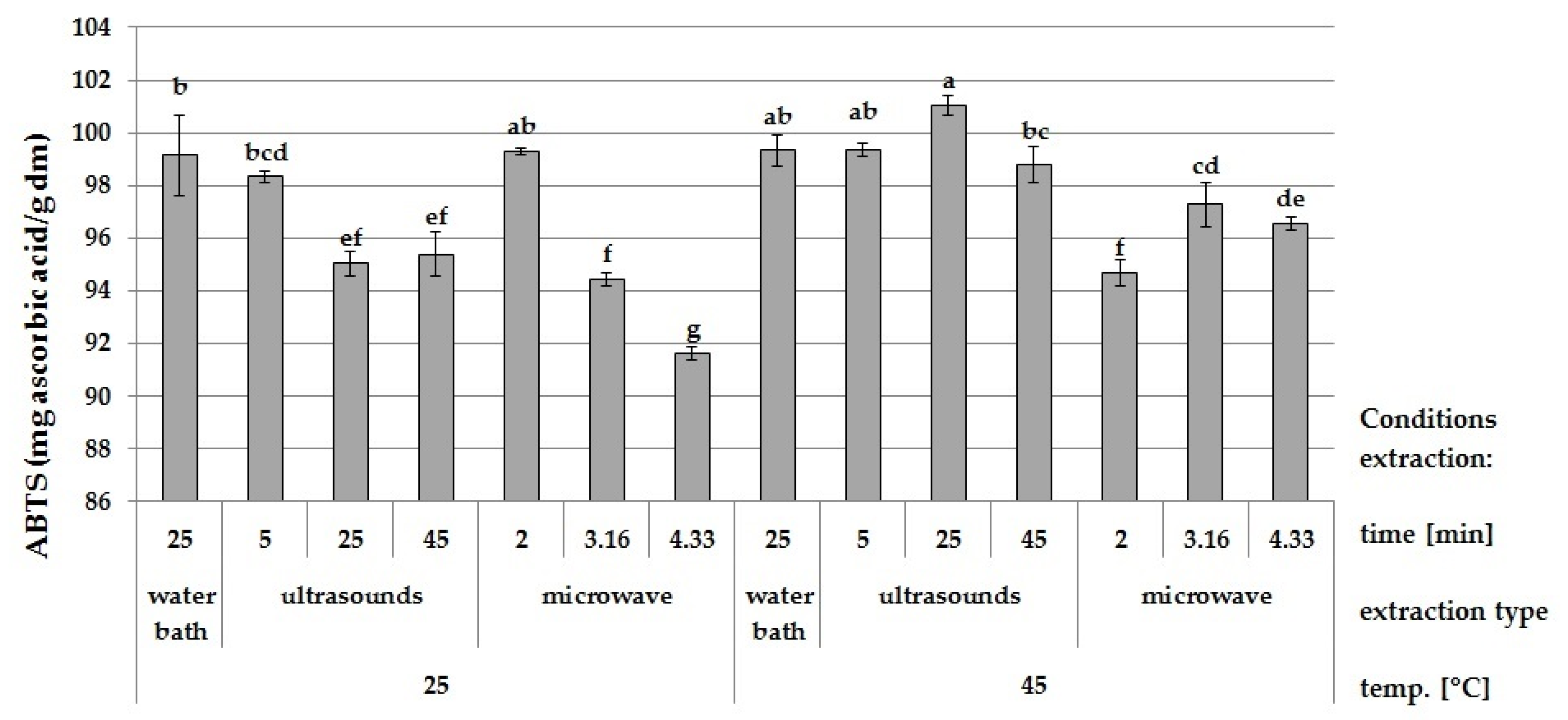
3.2. Effect of Ultrasound and Microwave Extraction on the Antioxidant Activity
3.3. Effect of Ultrasound and Microwave Extraction on the Physicochemical Properties of the Extract
3.4. Effect of Extraction Conditions on Correlation Coefficients between Antioxidant Activity, pH, Color and Content of Phenolic Compounds, Anthocyanins, and Flavonoids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lucas, B.F.; Zambiazi, R.C.; Costa, J.A.V. Biocompounds and physical properties of açaí pulp dried by different methods. LWT 2018, 98, 335–340. [Google Scholar] [CrossRef]
- Oliveira, S.R.; Chacón-Madrid, K.; Arruda, M.A.Z.; Barbosa Junior, F. In Vitro Gastrointestinal Digestion to Evaluate the Total; Bioaccessible and Bioavailable Concentrations of Iron and Manganese in Açaí (Euterpe Oleracea Mart.) Pulps. J. Trace Elem. Med. Biol. 2019, 53, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Gantner, M.; Stokowska, A. Nie tylko przyprawy przedłużają trwałość mięss. Tech. Przetwórstwa Spożywczego 2015, 1, 86–90. [Google Scholar]
- Guo, Q.; Gao, S.; Sun, Y.; Gao, Y.; Wang, X.; Zhang, Z. Antioxidant efficacy of rosemary ethanol extract in palm oil during frying and accelerated storage. Ind. Crop. Prod. 2016, 94, 82–88. [Google Scholar] [CrossRef]
- Yeᶊilsu, F.A.; Özyurt, G. Oxidative stability of microencapsulated fish oil with rosemary; thyme and laurel extracts: A kinetic assessment. J. Food Eng. 2019, 240, 171–182. [Google Scholar] [CrossRef]
- Yamaguchi, K.K.; Pereira, L.F.; Lamarão, C.V.; Lima, E.S.; da Vejga-Junior, V.F. Amazon açai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- Sangronis, E.; Sanabria, N. Impact of solar dehydration on composition and antioxidant properties of acai (Euterpe oleracea Mart.). Arch. Latinoam. Nutr. 2011, 61, 74–80. [Google Scholar]
- Spada, P.D.; Dani, C.; Bortolini, G.V.; Funchal, C.; Henrigues, J.A.; Salvador, M. Frozen Fruit Pulp of Euterpe oleraceae Mart. (Açai) Prevents Hydrogen Peroxide-Induced Damage in the Cerebral Cortex; Cerebellum; and Hippocampus of Rats. J. Med. Food 2009, 12, 1084–1088. [Google Scholar]
- Canuto, G.A.B.; Xavier, A.A.O.; Neves, L.C.; Benassi, M.T. Physical and chemical characterization of fruit pulps from Amazonia and their correlation to free radical scavenger activity. Rev. Bras. Frutic. 2010, 32, 1196–1205. [Google Scholar] [CrossRef] [Green Version]
- Avila-Sosa, R.; Montero-Rodríguez, A.F.; Aguilar-Alonso, P.; Vera-López, O.; Lazcano-Hernández, M.; Morales-Medina, J.C.; Navarro-Cruz, A.R. Antioxidant Properties of Amazonian Fruits: A Mini Review of In Vivo and In Vitro Studies. Oxidative Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Mamindla, S.; Prasad, K.V.S.R.G.; Bharathi, K. Herb-drug interactions: An overview of mechanisms and clinical aspects. Int. J. Pharm. Sci. Res. 2016, 7, 3576. [Google Scholar]
- Viana, D.S.; de Carvalho, L.M.J.; Moura, M.R.L.; Peixoto, J.C.; de Carvalho, J.L.V. Biochemical assessment of oxidative stress by the use of açai (Euterpe oleracea Martius) gel in physically active individuals. Food Sci. Technol. 2017, 37, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Chriboga, J.V.; Elst, K.; Camp, J.V.; Vera, E.; Ruales, J. Valorization of byproducts from tropical fruits: Extraction methodologies, applications, environmental, and economic assessment: A review (Part 1: General overview of the byproducts, traditional biorefinery practices, and possible applications). Compr. Rev. Food Sci. Food Saf. 2020, 19, 405–447. [Google Scholar]
- Quintin, D.; Garcia-Gomez, P.; Ayuso, M.; Sanmartin, A.M. Active biocompounds to improve food nutritional value. Trends Food Sci. Technol. 2019, 84, 19–21. [Google Scholar] [CrossRef]
- Putnik, P.; Kovačević, D.B.; Ježek, D.; Šustić, I.; Zorić, Z.; Dragović-Uzelac, V. High-pressure recovery of anthocyanins from grape skin pomace (Vitis vinifera cv. Teran) at moderate temperature. J. Food Process. Preserv. 2017, 42, e13342. [Google Scholar]
- Zu, G.; Zhang, R.; Yang, L.; Ma, C.; Zu, Y.; Wang, W.; Zhao, C. Ultrasound-Assisted Extraction of Carnosic Acid and Rosmarinic Acid Using Ionic Liquid Solution from Rosmarinus officinalis. Int. J. Mol. Sci. 2012, 13, 11027–11043. [Google Scholar] [CrossRef] [Green Version]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Wang, L.; Boussetta, N.; Lebovka, N.; Lefebvre, C.; Vorobiev, E. Correlations between disintegration degree of fruit skin cells induced by ultrasound and efficiency of bio-compounds extraction. Ultrason. Sonochem. 2019, 52, 280–285. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Smyth, T.J.; O’Donnell, C.P. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason. Sonochem. 2014, 23, 308–316. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Galanakis, C.M.; Brnčić, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Knoerzer, K.; Tiwari, B.K.; Barba, F.J. Clean recovery of antioxidant compounds from plant foods; by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015, 42, 134–149. [Google Scholar] [CrossRef]
- Flórez, N.; Conde, E.; Dominguez, H. Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biotechnol. 2014, 90, 590–607. [Google Scholar] [CrossRef]
- Ramli, N.S.; Ismail, P.; Rahmat, A. Influence of Conventional and Ultrasonic-Assisted Extraction on Phenolic Contents; Betacyanin Contents; and Antioxidant Capacity of Red Dragon Fruit (Hylocereus polyrhizus). Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Amoun, O.; Khodir, M. Comparison of microwave; ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-H.; See, T.-Y.; Yusoff, R.; Nhoh, G.-C.; Kow, K.-W. Extraction of bioactives from Orthosiphon stamineus using microwave and ultrasound-assisted techniques: Process optimization and scale up. Food Chem. 2017, 221, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Aliaño-González, M.J.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Ayuso, J.; Álvarez, J.Á.; Barbero, G.F. Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 2: Ultrasound-Assisted Extraction. Agronomy 2020, 10, 326. [Google Scholar]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Carrera, C.; Palma, M.; Ayuso, J.; Barbero, G.F.; Álvarez, J.Á. Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 3: Microwave-Assisted Extraction. Agronomy 2020, 10, 179. [Google Scholar]
- Tungmunnithum, D.; Malika, A.E.; Drouet, S.; Kiani, R.; Garros, L.; Kabra, A.; Addi, M.; Hano, C. A Quick, Green and Simple Ultrasound-Assisted Extraction for the Valorization of Antioxidant Phenolic Acids from Moroccan Almond Cold-Pressed Oil Residues. Appl. Sci. 2020, 10, 3313. [Google Scholar] [CrossRef]
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of plant extracts upon human health: A review. Crit. Rev. Food Sci. Nutr. 2018, 60, 873–886. [Google Scholar] [CrossRef]
- Robya, M.H.H.; Sarhana, M.A.; Selima, K.A.-H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crop. Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Teng, H.; Lee, W.Y. Optimization of microwave-assisted extraction of polyphenols from mulberry fruits (Morus alba L.) using response surface methodology. J. Korean Soc. Appl. Biol. Chem 2013, 56, 317–324. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, S.R.; Pande, V. Optimization Extraction Conditions for Improving Phenolic Content and Antioxidant Activity in Berberis Asiatica Fruits Using Response Surface Methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- González-Centeno, M.R.; Comas-Serra, F.; Femenia, A.; Rosselló, C.; Simal, S. Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (Vitis vinifera L.): Experimental kinetics and modeling. Ultrason. Sonochem. 2015, 22, 506–514. [Google Scholar]
- Hossain, M.B.; Brunton, N.P.; Patras, A.; Tiwari, B.; O’Donnell, C.P.; Martin-Diana, A.B.; Barry-Ryan, C. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram (Origanum majorana L.) using response surface methodology. Ultrason. Sonochem. 2012, 19, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Microwave-assistance provides very rapid and efficient extraction of grape seed polyphenols. Food Chem. 2011, 129, 570–576. [Google Scholar] [CrossRef]
- Zhang, G.; He, L.; Hu, M. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innov. Food Sci. Emerg. Technol. 2011, 12, 18–25. [Google Scholar] [CrossRef]
- Dadi, D.W.; Emire, S.A.; Hagos, A.D.; Eun, J.B. Effect of Ultrasound-Assisted Extraction of Moringa stenopetala Leaves on Bioactive Compounds and Their Antioxidant Activity. Food Technol. Biotechnol. 2019, 57, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, X.; Liu, C.; Sun, Y.; Lin, Z.; Liu, H. Extraction characteristics and optimal parameters of anthocyanin from blueberry powder under microwave-assisted extraction conditions. Sep. Purif. Technol. 2013, 104, 17–25. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Giorgis, M.; Garella, D.; Cena, C.; Boffa, L.; Cravotto, G.; Marini, E. An evaluation of the antioxidant properties of Arthrospira maxima extracts obtained using non-conventional techniques. Eur. Food Res. Technol. 2017, 243, 227–237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Neida, S.; Elba, S. Caracterización del acai o manaca (Euterpe oleracea Mart.): Um fruto del Amazonas. Archivos Latinoamericanos de Nutrición. Arch. Latinoam. Nutr. 2007, 57, 94. [Google Scholar] [PubMed]
- Ahmed, M.; Jiang, G.H.; Lee, K.C.; Seok, Y.Y.; Eun, J.B. Effects of ultrasonication; agitation and stirring extraction techniques on the physicochemical properties; health-promoting phytochemicals and structure of cold-brewed coffee. J. Sci. Food Agric. 2019, 99, 290–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Acker, S.A.B.E.; Van Den Berg, D.J.; Tromp, M.N.J.L.; Griffioen, D.H.; Van Bennekom, W.P.; Van Der Vijgh, W.J.F.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Anand, S.; Pang, E.; Livanos, G.; Mantri, N. Characterization of Physico-Chemical Properties and Antioxidant Capacities of Bioactive Honey Produced from Australian Grown Agastache rugosa and its Correlation with Colour and Poly-Phenol Content. Molecules 2018, 23, 108. [Google Scholar] [CrossRef] [Green Version]
- Bridle, P.; Timberlake, C.F. Anthocyanins as natural food colours-selected aspects. Food Chem. 1997, 58, 103–109. [Google Scholar] [CrossRef]

| Extraction Type | Example |
|---|---|
| Conventional | hot extraction |
| maceration | |
| Soxhlet | |
| Green | microwaves |
| sonication | |
| pulsating electric field | |
| high voltage electric field |
| Temp. (°C) | Extraction Type | Time (min) | TPC (mg Gallic Acid/g dm) | TF (mg Quercetin/g dm) | TA (mg cyanidin-3-glucoside/g dm) |
|---|---|---|---|---|---|
| 25 | water bath | 25 | 33.87 (0.11) ab | 5.13 (0.10) cd | 4.60 (0.02) bc |
| ultrasound | 5 | 34.15 (0.09) a | 5.16 (0.03) c | 4.68 (0.02) bc | |
| 25 | 34.18 (0.49) a | 5.26 (0.01) c | 5.11 (0.02) a | ||
| 45 | 34.12 (0.20) ab | 5.16 (0.01) c | 4.77 (0.03) e | ||
| microwave | 2 | 34.36 (0.19) a | 5.04 (0.12) cd | 5.09 (0.07) ab | |
| 3.16 | 34.22 (0.15) a | 5.10 (0.07) cd | 4.94 (0.12) ab | ||
| 4.33 | 33.42 (0.30) b | 4.92 (0.09) d | 4.83 (0.05) abc | ||
| 45 | water bath | 25 | 35.26 (0.02) ab | 5.66 (0.08) b | 4.50 (0.03) cd |
| ultrasound | 5 | 34.35 (0.09) c | 5.67 (0.12) b | 4.24 (0.05) de | |
| 25 | 35.95 (0.06) a | 6.03 (0.02) a | 4.05 (0.18) e | ||
| 45 | 35.76 (0.07) a | 6.04 (0.09) a | 4.01 (0.03) e | ||
| microwave | 2 | 34.26 (0.30) c | 5.15 (0.07) c | 4.92 (0.13) ab | |
| 3.16 | 35.33 (0.11) ab | 5.19 (0.06) c | 4.88 (0.10) abc | ||
| 4.33 | 34.52 (0.77) bc | 5.22 (0.08) c | 4.72 (0.33) abc | ||
| Extraction type | NS | *** | *** | ||
| Temperature | *** | *** | *** | ||
| Extraction type × Temperature | NS | *** | *** | ||
| Temp (°C) | Extraction Type | Time (min) | L *(D65) | a *(D65) | b *(D65) | pH |
|---|---|---|---|---|---|---|
| 25 | water bath | 25 | 19.61 (0.02) b | 1.97 (0.14) d | 3.95 (0.11) a | 5.52 (0.01) a |
| ultrasound | 5 | 19.36 (0.01) c | 3.38 (0.07) a | 3.90 (0.03) a | 5.39 (0.01) b | |
| 25 | 19.39 (0.03) c | 3.15 (0.06) ab | 3.79 (0.07) a | 5.39 (0.01) b | ||
| 45 | 20.86 (0.19 a | 3.06 (0.09) b | 3.19 (0.06) b | 5.39 (0.02) b | ||
| microwave | 2 | 19.48 (0.05) bc | 2.44 (0.03) c | 3.79 (0.04) a | 5.39 (0.01) b | |
| 3.16 | 19.45 (0.02) bc | 2.23 (0.17) cd | 3.89 (0.14) a | 5.43 (0.04) b | ||
| 4.33 | 19.45 (0.02) bc | 2.50 (0.04) c | 3.79 (0.03) a | 5.42 (0.01) b | ||
| 45 | water bath | 25 | 19.53 (0.02) bc | 2.12 (0.13) b | 3.55 (0.01) b | 5.40 (0.01) ab |
| ultrasound | 5 | 20.39 (0.08) a | 2.19 (0.12) b | 2.99 (0.06) c | 5.44 (0.02) a | |
| 25 | 19.56 (0.03) bc | 2.17 (0.05) b | 3.80 (0.08) a | 5.44 (0.02) a | ||
| 45 | 19.75 (0.21) b | 2.26 (0.12) b | 3.66 (0.15) ab | 5.37 (0.01) b | ||
| microwave | 2 | 19.33 (0.02) c | 2.37 (0.10) b | 3.73 (0.09) ab | 5.43 (0.03) a | |
| 3.16 | 19.43 (0.02) c | 2.74 (0.04) a | 3.81 (0.04) a | 5.36 (0.02) b | ||
| 4.33 | 19.53 (0.12) bc | 3.03 (0.14) a | 3.76 (0.05) ab | 5.42 (0.03) a |
| TF | TPC | TA | |
|---|---|---|---|
| DPPH | 0.73 *** | 0.47 ** | −0.64 *** |
| ABTS | 0.65 *** | 0.59 *** | −0.58 *** |
| FRAP | −0.53 *** | −0.23 NS | 0.48 ** |
| L * | 0.17 NS | −0.07 NS | −0.27 NS |
| a * | −0.38 NS | −0.24 * | 0.41 ** |
| b * | −0.28 NS | 0.00 NS | 0.28 NS |
| pH | −0.09 NS | −0.28 NS | −0.18 NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanula, M.; Wyrwisz, J.; Moczkowska, M.; Horbańczuk, O.K.; Pogorzelska-Nowicka, E.; Wierzbicka, A. Optimization of Microwave and Ultrasound Extraction Methods of Açai Berries in Terms of Highest Content of Phenolic Compounds and Antioxidant Activity. Appl. Sci. 2020, 10, 8325. https://doi.org/10.3390/app10238325
Hanula M, Wyrwisz J, Moczkowska M, Horbańczuk OK, Pogorzelska-Nowicka E, Wierzbicka A. Optimization of Microwave and Ultrasound Extraction Methods of Açai Berries in Terms of Highest Content of Phenolic Compounds and Antioxidant Activity. Applied Sciences. 2020; 10(23):8325. https://doi.org/10.3390/app10238325
Chicago/Turabian StyleHanula, Monika, Jarosław Wyrwisz, Małgorzata Moczkowska, Olaf K. Horbańczuk, Ewelina Pogorzelska-Nowicka, and Agnieszka Wierzbicka. 2020. "Optimization of Microwave and Ultrasound Extraction Methods of Açai Berries in Terms of Highest Content of Phenolic Compounds and Antioxidant Activity" Applied Sciences 10, no. 23: 8325. https://doi.org/10.3390/app10238325




