Attenuation of Colonic Injury and Inflammation by Administration of a Phenolic Extract of Summer Savory (Satureja hortensis L.) in Experimental Inflammatory Bowel Disease in Mice
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Plant Related Material and Extract Preparation
2.3. Total Phenolic and Total Flavonoid Content
2.4. High-Performance Liquid Chromatography (HPLC)
2.5. Antioxidant Capacity
2.5.1. Assay for Reduction of Cupric Antioxidant Capacity (CUPRAC)
2.5.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.3. DPPH (2,2-diphenyl-1-picrylhydrazyl) Radical-Scavenging Assay
2.5.4. Superoxide Anion Radical-Scavenging Assay
2.6. Carrageenan-Induced Paw Edema in Rat
2.6.1. Animals
2.6.2. Edema Induction and Evaluation
2.6.3. Experimental Groups
2.7. TNBS-Induced Ulcerative Colitis Model in Mice
2.7.1. Animals
2.7.2. Induction of Colitis
2.7.3. Experimental Groups
- Sham group (n = 6): the colitis induction protocol was performed as stated previously, except for the intracolonic administration that was performed with 100 μL of saline solution. Animals were administered daily with 10 mL/kg of water by gastric gavage until the end of the experiment.
- Ethanol group (n = 6): the colitis induction protocol was performed as stated previously except for the intracolonic administration that was performed with 100 μL of 50% (v/v) ethanol solution. Animals were administered daily with 10 mL/kg of water by gastric gavage until the end of the experiment.
- TNBS group (n = 10): the colitis induction protocol was performed as previously described, with the administration of 100 μL of a TNBS solution (2.5% TNBS in 50% ethanol). Animals were administered daily with 10 mL/kg of water by gastric gavage until the end of the experiment.
- TNBS + Summer Savory group (n = 10): the colitis induction protocol was performed as described previously. Animals were administered daily with Summer Savory extract (15 mg/kg of phenolic acids by oral gavage) until the end of the experiment.
2.7.4. Evaluation of Colitis Severity (Macroscopic Analysis)
2.7.5. Histology/Immunohistochemistry Procedures
2.8. Animal Experiments
2.9. Statistical Analysis
3. Results
3.1. Chemical Characterization
3.2. Paw Edema Evaluation
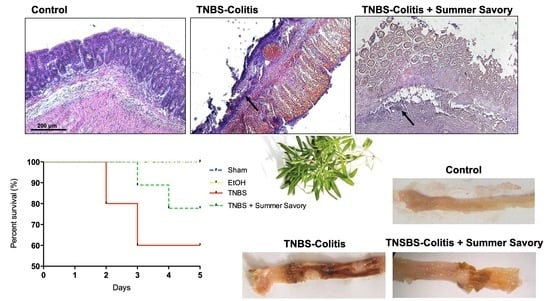
3.3. Macroscopic and Functional Signs of Colitis Injury
3.4. Colon Injury: Histological Features and Inflammatory Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fierascu, I.; Dinu-Pirvu, C.E.; Fierascu, R.; Velescu, B.S.; Anuta, V.; Ortan, A.; Jinga, V. Phytochemical Profile and Biological Activities of Satureja hortensis L.: A Review of the Last Decade. Molecules 2018, 23, 2458. [Google Scholar] [CrossRef]
- Güllüce, M.; Sökmen, M.; Daferera, D.; Aǧar, G.; Özkan, H.; Kartal, N.; Polissiou, M.; Sökmen, A.; Şahi̇n, F. In Vitro Antibacterial, Antifungal, and Antioxidant Activities of the Essential Oil and Methanol Extracts of Herbal Parts and Callus Cultures of Satureja hortensis L. J. Agric. Food Chem. 2003, 51, 3958–3965. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Lee, S.-C.; Huh, M.-J.; Seo, S.-M.; Kwon, J.H.; Park, I.-K. Fumigant Antifungal Activity via Reactive Oxygen Species of Thymus vulgaris and Satureja hortensis Essential Oils and Constituents Against Raffaelea quercus-mongolicae and Rhizoctonia solani. Biomolecules 2019, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Şahin, F.; Karaman, I.; Güllüce, M.; Öğütçü, H.; Şengül, M.; Adıgüzel, A.; Öztürk, S.; Kotán, R. Evaluation of antimicrobial activities of Satureja hortensis L. J. Ethnopharmacol. 2003, 87, 61–65. [Google Scholar] [CrossRef]
- Sharifi, A.; Mohammadzadeh, A.; Salehi, T.Z.; Mahmoodi, P. Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J. Appl. Microbiol. 2018, 124, 379–388. [Google Scholar] [CrossRef]
- Gomes, F.; Dias, M.I.; Lima, Â.; Barros, L.; Rodrigues, M.E.; Ferreira, I.; Henriques, M. Satureja montana L. and Origanum majorana L. Decoctions: Antimicrobial Activity, Mode of Action and Phenolic Characterization. Antibiotics 2020, 9, 294. [Google Scholar] [CrossRef]
- Tepe, B.; Cilkiz, M. A pharmacological and phytochemical overview onSatureja. Pharm. Biol. 2015, 54, 375–412. [Google Scholar] [CrossRef]
- Popovici, R.A.; Vaduva, D.; Pinzaru, I.; Dehelean, C.A.; Farcas, C.G.; Coricovac, D.; Danciu, C.; Popescu, I.; Alexa, E.; Lazureanu, V.; et al. A comparative study on the biological activity of essential oil and total hydro-alcoholic extract of Satureja hortensis L. Exp. Ther. Med. 2019, 18, 932–942. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Sadraei, H.; Ghannadi, A.R.; Mohseni, M. Antispasmodic and anti-diarrhoeal effect of Satureja hortensis L. essential oil. J. Ethnopharmacol. 2000, 71, 187–192. [Google Scholar] [CrossRef]
- Zeidán-Chuliá, F.; Keskin, M.; Könönen, E.; Uitto, V.-J.; Söderling, E.; Moreira, J.C.F.; Gursoy, U.K. Antibacterial and Antigelatinolytic Effects of Satureja hortensis L. Essential Oil on Epithelial Cells Exposed to Fusobacterium nucleatum. J. Med. Food 2015, 18, 503–506. [Google Scholar] [CrossRef]
- SSharifzadeh, A.; Khosravi, A.R.; Ahmadian, S. Chemical composition and antifungal activity of Satureja hortensis L. essentiall oil against planktonic and biofilm growth of Candida albicans isolates from buccal lesions of HIV+ individuals. Microb. Pathog. 2016, 96, 1–9. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G. New Tricks for Old Guys: Recent Developments in the Chemistry, Biochemistry, Applications and Exploitation of Selected Species from the Lamiaceae Family. In Chemistry and Biodiversity; Wiley-VCH Verlag: Weinheim, Germany, 2020. [Google Scholar] [CrossRef]
- Delgado, A.M.; Issaoui, M.; Chammem, N. Analysis of main and healthy Phenolic compounds in foods. J. AOAC Int. 2019, 102, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Direito, R.; Lima, A.; Mota, J.; Gonçalves, M.; Duarte, M.P.; Solas, J.; Peniche, B.F.; Fernandes, A.; Pinto, R.; et al. Reduction of inflammation and colon injury by a Pennyroyal phenolic extract in experimental inflammatory bowel disease in mice. Biomed. Pharmacother. 2019, 118. [Google Scholar] [CrossRef]
- Direito, R.; Rocha, J.; Lima, A.I.G.; Gonçalves, M.; Duarte, M.P.; Mateus, V.; Sousa, C.; Fernandes, A.; Pinto, R.M.A.; Ferreira, R.B.; et al. Reduction of Inflammation and Colon Injury by a Spearmint Phenolic Extract in Experimental Bowel Disease in Mice. Medicines 2019, 6, 65. [Google Scholar] [CrossRef]
- Exarchou, V.; Nenadis, N.; Tsimidou, M.; Gerothanassis, I.P.; Troganis, A.; Boskou, D. Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J. Agric. Food Chem. 2002, 50, 5294–5299. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Choulitoudi, E.; Bimpilas, A.; Mitropoulou, G.; Kourkoutas, Y.; Oreopoulou, V. Exploitation of the biological potential of Satureja thymbra essential oil and distillation by-products. J. Appl. Res. Med. Aromat. Plants 2017, 4, 12–20. [Google Scholar] [CrossRef]
- Boroja, T.; Katanić, J.; Rosić, G.; Selaković, D.; Joksimović, J.; Mišić, D.; Stanković, V.; Jovičić, N.; Mihailović, V. Summer savory (Satureja hortensis L.) extract: Phytochemical profile and modulation of cisplatin-induced liver, renal and testicular toxicity. Food Chem. Toxicol. 2018, 118, 252–263. [Google Scholar] [CrossRef]
- Mašković, P.; Veličković, V.; Mitić, M.N.; Đurović, S.; Zeković, Z.; Radojković, M.; Cvetanović, A.; Švarc-Gajić, J.; Vujić, J. Summer savory extracts prepared by novel extraction methods resulted in enhanced biological activity. Ind. Crop. Prod. 2017, 109, 875–881. [Google Scholar] [CrossRef]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef]
- Corridoni, D.; Arseneau, K.O.; Cominelli, F. Inflammatory bowel disease. Immunol. Lett. 2014, 161, 231–235. [Google Scholar] [CrossRef]
- Salaritabar, A.; Darvishi, B.; Hadjiakhoondi, F.; Manayi, A.; Sureda, A.; Nabavi, S.M.; Fitzpatrick, L.R.; Bishayee, A. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J. Gastroenterol. 2017, 23, 5097. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Shah, S.C. Diagnosis and management of inflammatory bowel disease-associated neoplasia: Considera. In Therapeutic Advances in Gastroenterology; SAGE Publications Ltd.: Thousand Oaks, CA, USA, 2020. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016, 22, 4794. [Google Scholar] [CrossRef] [PubMed]
- McDowell, C.; Haseeb, M. Bowel, Inflammatory Disease (IBD). StatPearls 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470312/ (accessed on 26 November 2020).
- Nasef, N.A.; Mehta, S. Role of inflammation in pathophysiology of colonic disease: An update. Int. J. Mol. Sci. 2020, 21, 4748. [Google Scholar] [CrossRef] [PubMed]
- Caprara, G.; Allavena, P.; Erreni, M. Intestinal Macrophages at the Crossroad between Diet, Inflammation, and Cancer. Int. J. Mol. Sci. 2020, 21, 4825. [Google Scholar] [CrossRef]
- Romano, M.; De Francesco, F.; Zarantonello, L.; Ruffolo, C.; Ferraro, G.A.; Zanus, G.; Giordano, A.; Bassi, N.; Cillo, U. From Inflammation to Cancer in Inflammatory Bowel Disease: Molecular Perspectives. Anticancer Res. 2016, 36, 1447–1460. [Google Scholar]
- Rogler, G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014, 345, 235–241. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Rubin, D.C.; Shaker, A.; Levin, M.S. Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef]
- Shawki, S.; Ashburn, J.; Signs, S.A.; Huang, E. Colon Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 269–287. [Google Scholar] [CrossRef]
- Donovan, M.G.; Selmin, O.I.; Doetschman, T.C.; Romagnolo, D.F. Mediterranean Diet: Prevention of Colorectal Cancer. Front. Nutr. 2017, 4, 59. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed]
- Kapinova, A.; Kubatka, P.; Golubnitschaja, O.; Kello, M.; Zubor, P.; Solar, P.; Pec, M. Dietary phytochemicals in breast cancer research: Anticancer effects and potential utility for effective chemoprevention. Environ. Health Prev. Med. 2018, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Koşar, M.; Göger, F.; Can Başer, K.H. In Vitro Antioxidant Properties and Phenolic Composition of Salvia virgata Jacq. from Turkey. J. Agric. Food Chem. 2008, 56, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterisation in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Ramful, D.; Bahorun, T.; Bourdon, E.; Tarnus, E.; Aruoma, O.I. Bioactive phenolics and antioxidant propensity of flavedo extracts of Mauritian citrus fruits: Potential prophylactic ingredients for functional foods application. Toxicology 2010, 278, 75–87. [Google Scholar] [CrossRef]
- Miceli, N.; Trovato, A.; Dugo, P.; Cacciola, F.; Donato, P.A.E.; Marino, A.; Bellinghieri, V.; La Barbera, T.M.; Güvenç, A.; Taviano, M.F. Comparative Analysis of Flavonoid Profile, Antioxidant and Antimicrobial Activity of the Berries of Juniperus communis L. var. communis and Juniperus communis L. var. saxatilis Pall. from Turkey. J. Agric. Food Chem. 2009, 57, 6570–6577. [Google Scholar] [CrossRef]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidant activity of Centaurium erythraea infusion evidenced by its superoxide radical scavenging and xanthine oxidase inhibitory activity. J. Agric. Food Chem. 2001, 49, 3476–3479. [Google Scholar] [CrossRef]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.M.; Serra, A.T.; Pinto, R.; Freitas, M.; et al. Anti-inflammatory effect of rosmarinic acid and an extract of rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 398–413. [Google Scholar] [CrossRef]
- Direito, R.; Lima, A.; Rocha, J.; Ferreira, R.B.; Mota, J.; Rebelo, P.; Fernandes, A.; Pinto, R.M.A.; Alves, P.C.; Bronze, R.; et al. Dyospiros kaki phenolics inhibit colitis and colon cancer cell proliferation, but not gelatinase activities. J. Nutr. Biochem. 2017, 46, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Figueira, M.-E.; Camara, M.B.; Direito, R.; Rocha, J.; Serra, A.-T.; Duarte, C.M.; Fernandes, A.; Freitas, M.; Fernandes, E.; Marques, M.C.; et al. Chemical characterization of a red raspberry fruit extract and evaluation of its pharmacological effects in experimental models of acute inflammation and collagen-induced arthritis. Food Funct. 2014, 5, 3241–3251. [Google Scholar] [CrossRef] [PubMed]
- Figueira, M.-E.; Oliveira, M.; Direito, R.; Rocha, J.; Alves, P.; Serra, A.-T.; Duarte, C.; Bronze, R.; Fernandes, A.; Brites, D.; et al. Protective effects of a blueberry extract in acute inflammation and collagen-induced arthritis in the rat. Biomed. Pharmacother. 2016, 83, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Abou Baker, D.H.; Al-Moghazy, M.; ElSayed, A.A.A. The in vitro cytotoxicity, antioxidant and antibacterial potential of Satureja hortensis L. essential oil cultivated in Egypt. Bioorg. Chem. 2020, 95. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M.; Sohraby, M. Summer savory: From the selection of traditional applications to the novel effect in relief, prevention, and treatment of a number of serious illnesses such as diabetes, cardiovascular disease, Alzheimer’s disease, and cancer. J. Tradit. Complement. Med. 2014, 4, 140–144. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem. 2004, 87, 307–311. [Google Scholar] [CrossRef]
- Fatiha, B.; Didier, H.; Naima, G.; Khodir, M.; Martin, K.; Léocadie, K.; Caroline, S.; Mohamed, C.; Pierre, D. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae). Ind. Crop. Prod. 2015, 74, 722–730. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: Thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 2008, 108, 1039–1053. [Google Scholar] [CrossRef]
- Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.R.; Hamedani, M.P. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn. Mag. 2012, 8, 37–41. [Google Scholar] [CrossRef]
- Rastegarpa, M.; Omidzohour, N.; Vahedi, H.; Malekzadeh, R.; Hashemian, F.; Safarnavad, T.; Abdollahi, M. Management of human ulcerative colitis by saturexTM: A randomized controlled trial. Int. J. Pharmacol. 2011, 7, 516–521. [Google Scholar] [CrossRef]
- Direito, R.; Rocha, J.; Serra, A.T.; Fernandes, A.; Freitas, M.; Fernandes, E.; Pinto, R.M.A.; Bronze, M.D.R.; Sepodes, B.; Figueira, M.-E. Anti-inflammatory Effects of Persimmon (Diospyros kaki L.) in Experimental Rodent Rheumatoid Arthritis. J. Diet. Suppl. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.-R.; Chung, K.-S.; Cheon, S.-Y.; Lee, M.; Hwang, S.; Hwang, S.N.; Rhee, K.-J.; An, H.-J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, Z.; Kalantar, K.; Iraji, A.; Amirghofran, Z. Inhibition of LPS-induced inflammatory responses by Satureja hortensis extracts in J774.1 macrophages. J. Immunoass Immunochem. 2018, 39, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Uslu, C.; Karasen, R.M.; Sahin, F.; Taysi, S.; Akcay, F. Effects of aqueous extracts of Satureja hortensis L. on rhinosinusitis treatment in rabbit. J. Ethnopharmacol. 2003, 88, 225–228. [Google Scholar] [CrossRef]
- Kraus, S.; Arber, N. Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 2009, 9, 405–410. [Google Scholar] [CrossRef]
- Esmaeilbeig, M.; Kouhpayeh, S.A.; Amirghofran, Z. An investigation of the growth inhibitory capacity of several medicinal plants from Iran on tumor cell lines. Int. J. Cancer Manag. 2015, 8. [Google Scholar] [CrossRef]
- Zeidán-Chuliá, F.; De Oliveira, B.-H.N.; Gursoy, M.; Könönen, E.; Moreira, J.C.F.; Gursoy, U.K.; Uitto, V.-J. MMP-REDOX/NO Interplay in Periodontitis and Its Inhibition with Satureja hortensis L. Essential Oil. Chem. Biodivers. 2013, 10, 507–523. [Google Scholar] [CrossRef]
- Jurjus, A.; Eid, A.; Al Kattar, S.; Zeenny, M.N.; Gerges-Geagea, A.; Haydar, H.; Hilal, A.; Oueidat, D.; Matar, M.; Tawilah, J.F.; et al. Inflammatory bowel disease, colorectal cancer and type 2 diabetes mellitus: The links. BBA Clin. 2016, 5, 16–24. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Salvia fruticosa, salvia officinalis, and rosmarinic acid induce apoptosis and inhibit proliferation of human colorectal cell lines: The role in MAPK/ERK pathway. Nutr. Cancer 2009, 61, 564–571. [Google Scholar] [CrossRef]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Gunasekaran, S.; Jesudoss, V.A.S.; Namasivayam, N. The effect of rosmarinic acid on 1,2-dimethylhydrazine induced colon carcinogenesis. Exp. Toxicol. Pathol. 2013, 65, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Scheckel, K.A.; Degner, S.C.; Romagnolo, D.F. Rosmarinic acid antagonizes activator protein-1-dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J. Nutr. 2008, 138, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.K.; Wilson, K.T. Nitric oxide in inflammatory bowel disease. Inflamm. Bowel Dis. 2003, 9, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Hellström, P.M.; Fagerhol, M.K.; Weitzberg, E.; Roseth, A.G. Technology Insight: Calprotectin, lactoferrin and nitric oxide as novel markers of inflammatory bowel disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef]
- Azer, S.A. Overview of molecular pathways in inflammatory bowel disease associated with colorectal cancer development. Eur. J. Gastroenterol. Hepatol. 2013, 25, 271–281. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Xie, Z.; Zhou, S.; Li, Y.; Zhou, Y.; Sun, M.-Y. Nitric oxide (NO) and NO synthases (NOS)-based targeted therapy for colon cancer. Cancers 2020, 12, 1881. [Google Scholar] [CrossRef]
- Watanabe, K.; Kawamori, T.; Nakatsugi, S.; Wakabayashi, K. COX-2 and iNOS, good targets for chemoprevention of colon cancer. BioFactors 2000, 12, 129–133. [Google Scholar] [CrossRef]










| Score | Feces Consistency |
|---|---|
| 0 | Normal (hard pellets) |
| 1 | Slightly mucous |
| 2 | Soft |
| 3 | Liquid |
| Total Phenolics (mg GAE/L) | Total Flavonoids (mmol CE/L) | |
|---|---|---|
| Summer Savory | 3028.131 ± 55.197 | 9.116 ± 0.246 |
| Antioxidant Assay | Result |
|---|---|
| CUPRAC assay (μmol AAE/mL) | 14.9 ± 1.20 |
| FRAP assay (µmol Fe2+/mL) | 39.1 ± 2.24 |
| DPPH assay (μmol AAE/L) | 3.06 ± 0.05 |
| Superoxide anion radical-scavenging assay (μmol GAE/L) | 39.1 ± 0.00 |
| Colon Length (cm) | Injury Extent | Consistency/Presence of Diarrhea | Mortality Rate (%) | |
|---|---|---|---|---|
| Sham | 14.51 ± 0.082 | 0 | 0 | 0 |
| EtOH 50% | 14.12 ± 0.20 | 0 | 0 | 0 |
| TNBS + EtOH 50% | 11.81 ± 0.19 # | 3.6 ± 0.14 # | 3 # | 40 |
| TNBS + Summer Savory | 14.33 ± 0.09 * | 0.5 ± 0.14 * | 0.25 * | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, J.; Leandro, R.; Direito, R.; Gonçalves, M.; Duarte, M.P.; Fernandes, A.; Sepodes, B.; Figueira, M.-E. Attenuation of Colonic Injury and Inflammation by Administration of a Phenolic Extract of Summer Savory (Satureja hortensis L.) in Experimental Inflammatory Bowel Disease in Mice. Appl. Sci. 2020, 10, 8465. https://doi.org/10.3390/app10238465
Rocha J, Leandro R, Direito R, Gonçalves M, Duarte MP, Fernandes A, Sepodes B, Figueira M-E. Attenuation of Colonic Injury and Inflammation by Administration of a Phenolic Extract of Summer Savory (Satureja hortensis L.) in Experimental Inflammatory Bowel Disease in Mice. Applied Sciences. 2020; 10(23):8465. https://doi.org/10.3390/app10238465
Chicago/Turabian StyleRocha, João, Raquel Leandro, Rosa Direito, Margarida Gonçalves, Maria Paula Duarte, Adelaide Fernandes, Bruno Sepodes, and Maria-Eduardo Figueira. 2020. "Attenuation of Colonic Injury and Inflammation by Administration of a Phenolic Extract of Summer Savory (Satureja hortensis L.) in Experimental Inflammatory Bowel Disease in Mice" Applied Sciences 10, no. 23: 8465. https://doi.org/10.3390/app10238465
APA StyleRocha, J., Leandro, R., Direito, R., Gonçalves, M., Duarte, M. P., Fernandes, A., Sepodes, B., & Figueira, M.-E. (2020). Attenuation of Colonic Injury and Inflammation by Administration of a Phenolic Extract of Summer Savory (Satureja hortensis L.) in Experimental Inflammatory Bowel Disease in Mice. Applied Sciences, 10(23), 8465. https://doi.org/10.3390/app10238465