Evaluation of Sub-Lethal Toxicity of Benzethonium Chloride in Cyprinus carpio Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Test Organisms and Experimental Procedure
2.3. Biochemical Analyses
2.3.1. Tissue Homogenate Preparation
2.3.2. Enzymatic Activities Measurement
2.3.3. GSH Level Measurement
2.3.4. Lipid Peroxidation Measurement
2.4. Histopathology Staining
2.5. Statistical Analysis
3. Results
3.1. Characterization of Water Parameters
3.2. Hepatosomatic and Gonadosomatic Index after Exposure to BEC
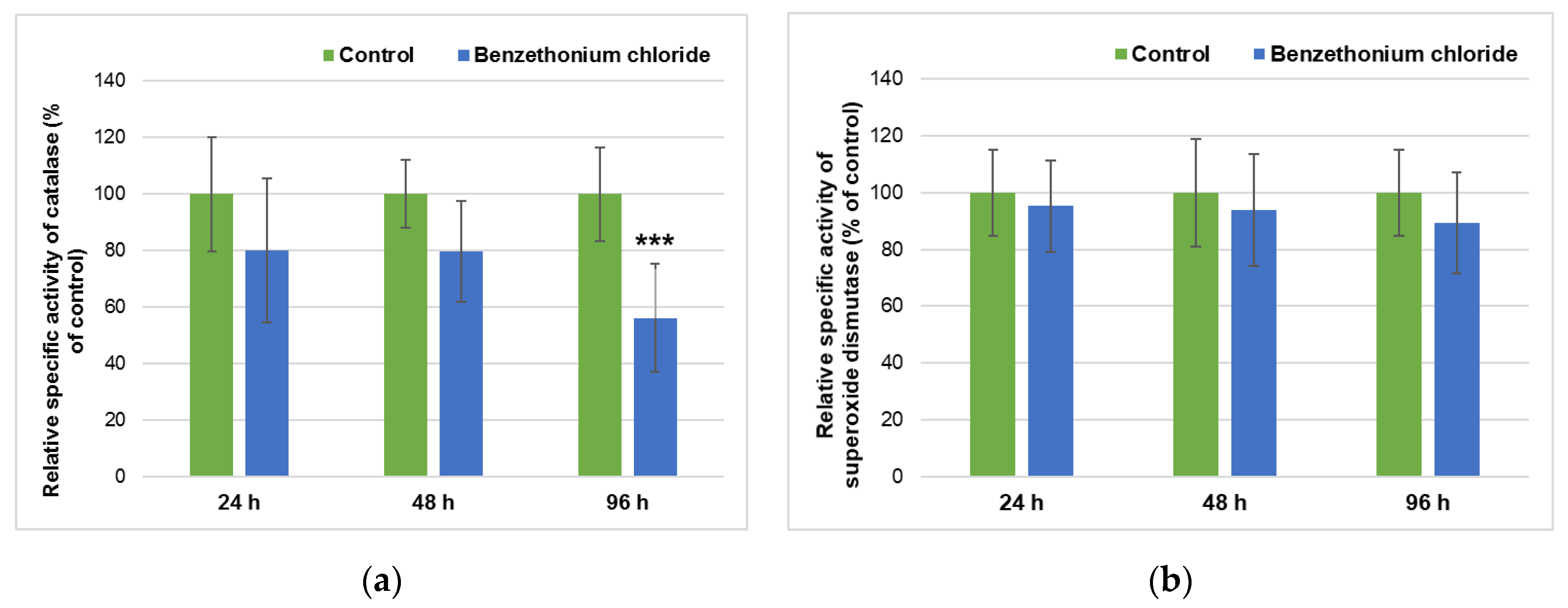
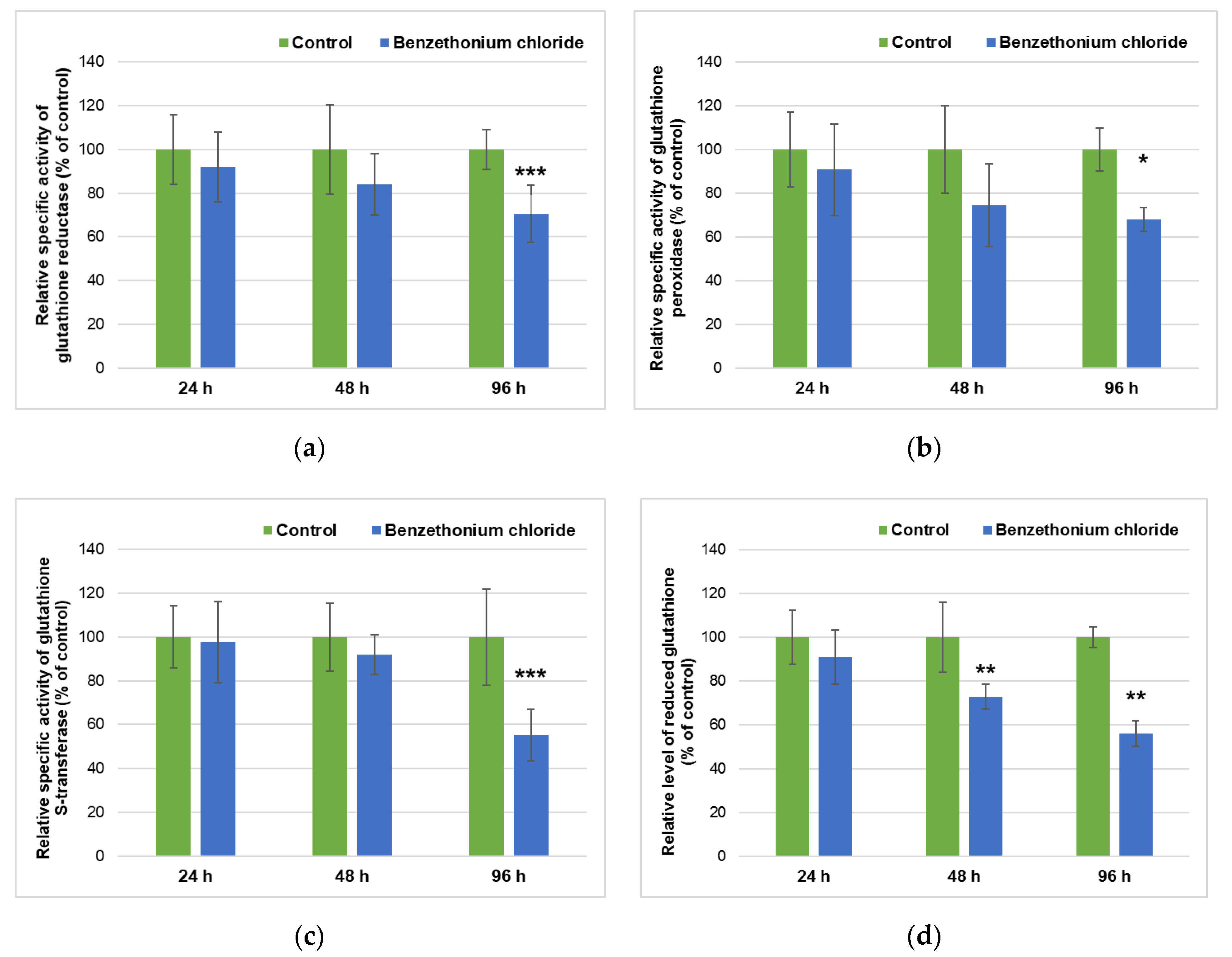
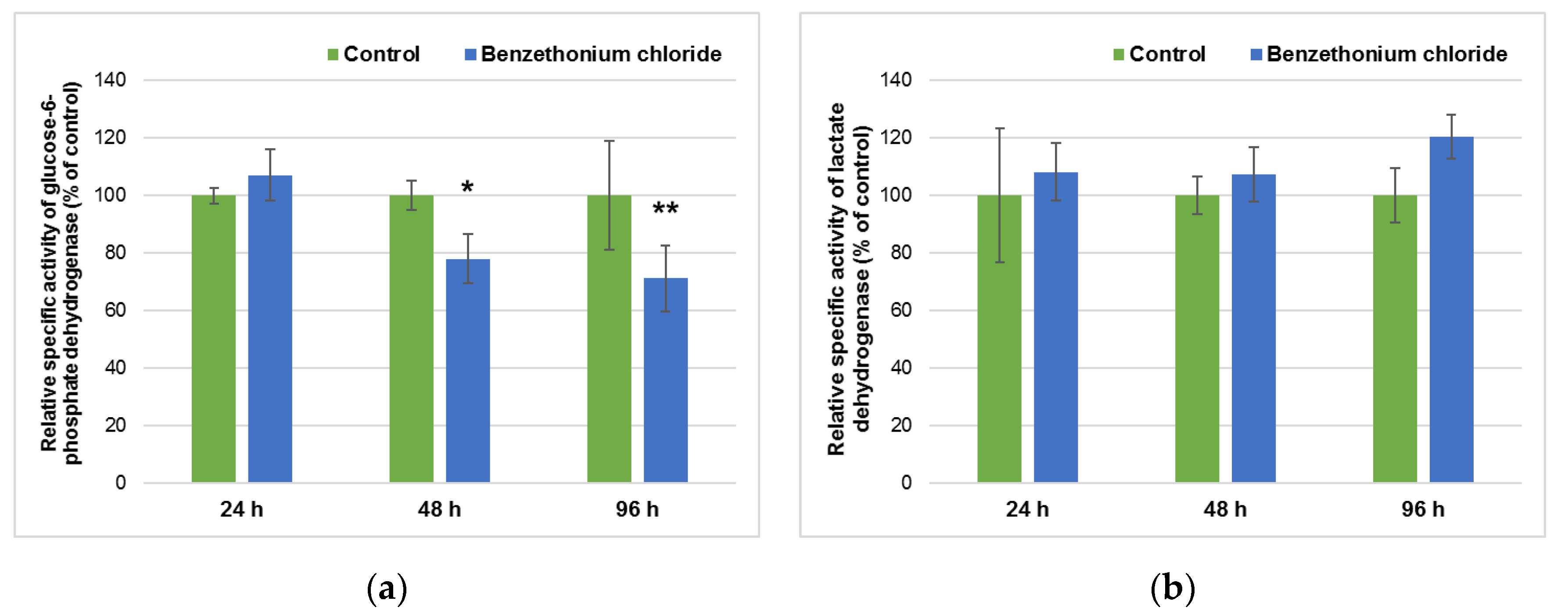
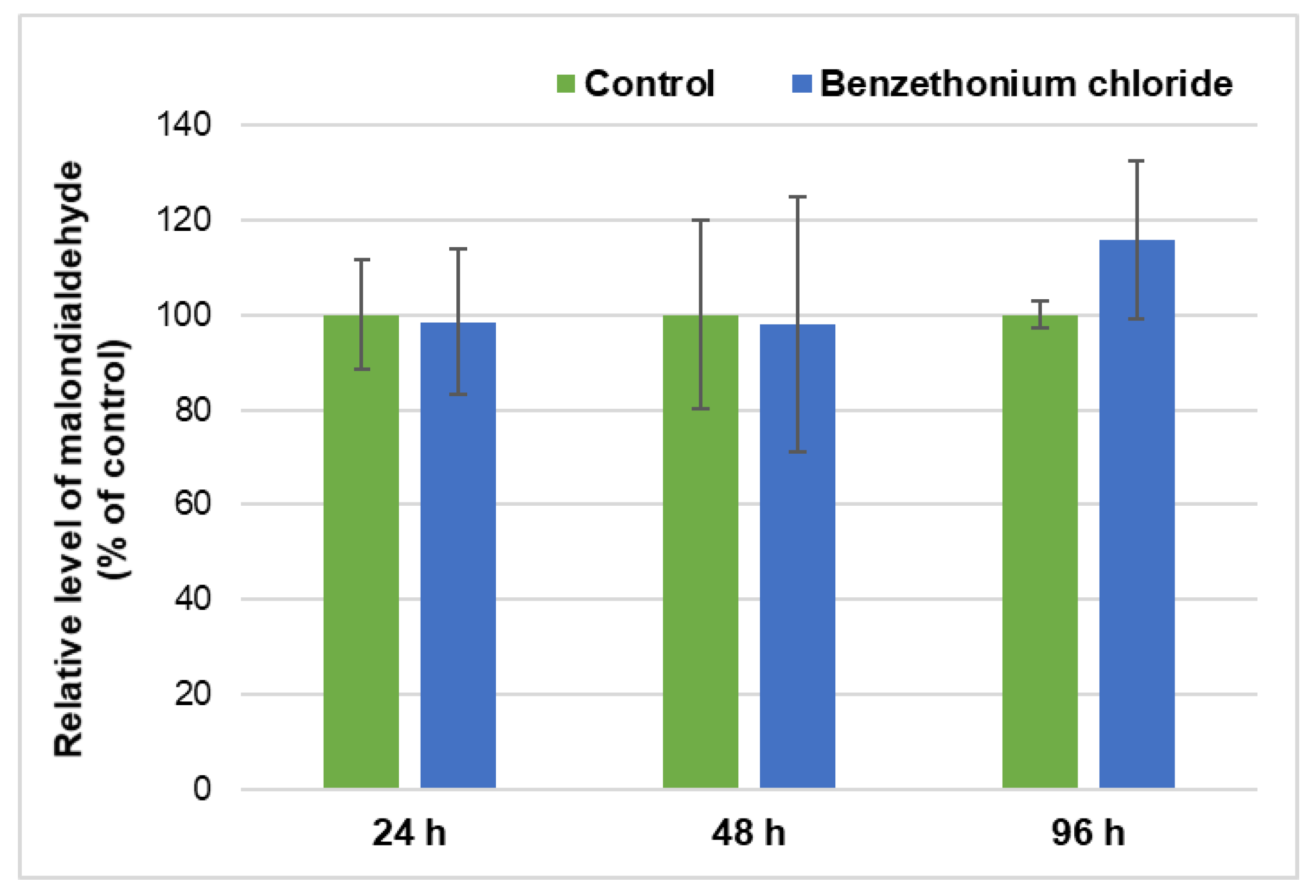
3.3. Effect of BEC Exposure on Liver Metabolism
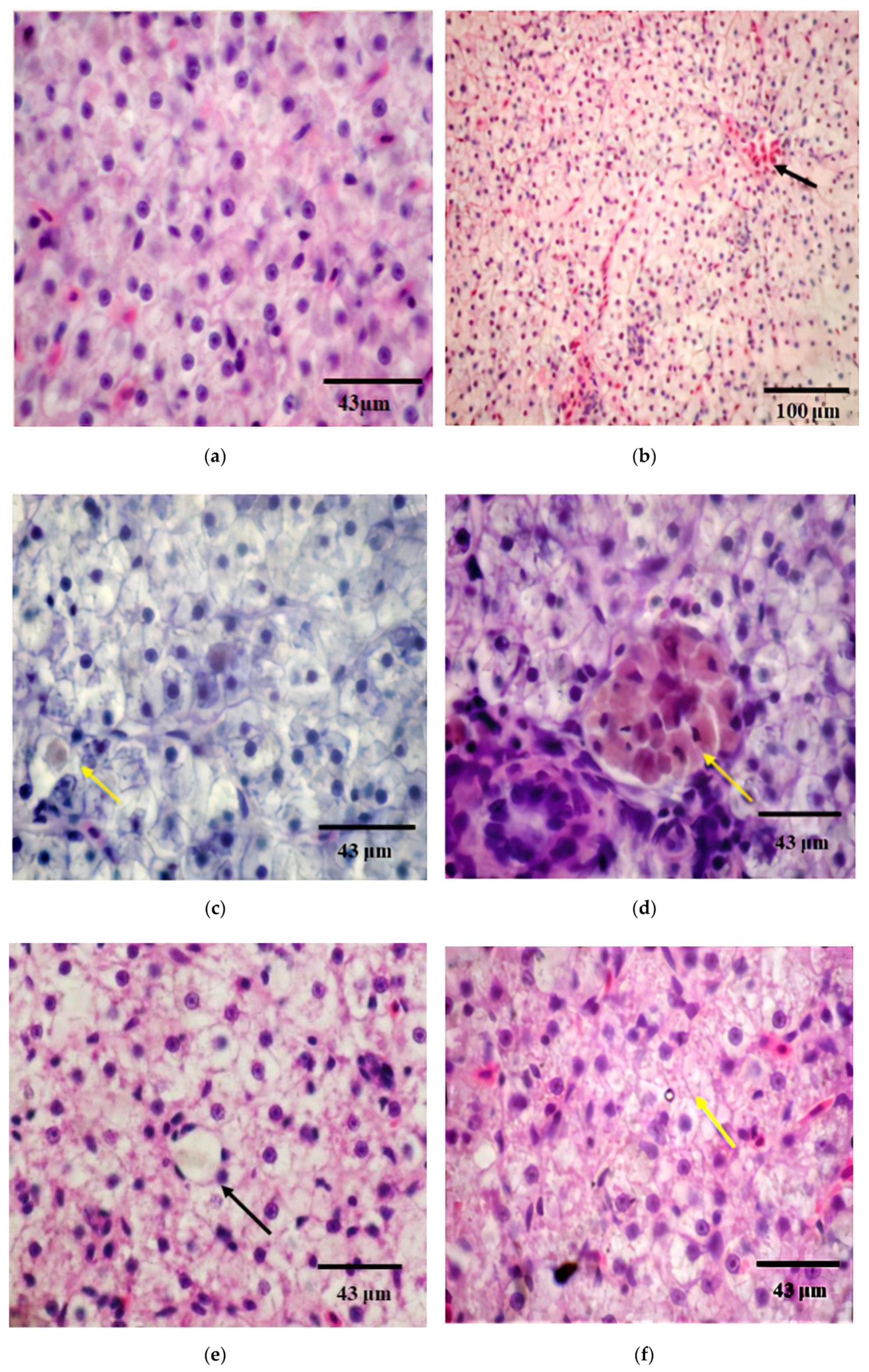
3.4. Histopathological Changes after Exposure to BEC
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaczerewska, O.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Environmental behavior and ecotoxicity of cationic surfactants towards marine organisms. J. Hazard. Mater. 2020, 392, 122299. [Google Scholar] [CrossRef]
- Ying, G.G. Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 2006, 32, 417–431. [Google Scholar] [CrossRef]
- Abel, P.D. Toxicity of synthetic detergents to fish and aquatic invertebrates. J. Fish Biol. 1974, 6, 279–298. [Google Scholar] [CrossRef]
- Lewis, M.A.; Suprenant, D. Comparative acute toxicities of surfactants to aquatic invertebrates. Ecotoxicol. Environ. Saf. 1983, 7, 313–322. [Google Scholar] [CrossRef]
- Lewis, M.A. Chronic and sub lethal toxicities of surfactants to aquatic animals: A review and risk assessment. Water Res. 1991, 25, 101–113. [Google Scholar] [CrossRef]
- Mandal, R.; Mandal, D.; Mishra, N.; Bahadur, A. Effect of surfactants on phosphatase level of fresh water fish Labeo rohita. J. Environ. Biol. 2010, 31, 395–398. [Google Scholar]
- Zhu, X.; Wang, Z.; Sun, Y.; Gu, L.; Zhang, L.; Wang, J.; Huang, Y.; Yang, Z. Surfactants at environmentally relevant concentrations interfere the inducible defense of Scenedesmus obliquus and the implications for ecological risk assessment. Environ. Pollut. 2020, 261, 114131. [Google Scholar] [CrossRef]
- Jackson, M.; Eadsforth, C.; Schowanek, D.; Delfosse, T.; Riddle, A.; Budgen, N. Comprehensive review of several surfactants in marine environments: Fate and ecotoxicity. Environ. Toxicol. Chem. 2016, 35, 1077–1086. [Google Scholar] [CrossRef]
- Pretti, C.; Chiappe, C.; Baldetti, I.; Brunini, S.; Monni, G.; Intorre, L. Acute toxicity of ionic liquids for three fresh water organisms: Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Ecotoxicol. Environ. Saf. 2009, 72, 1170–1176. [Google Scholar] [CrossRef]
- Clara, M.; Scharf, S.; Scheffknecht, C.; Gans, O. Occurrence of selected surfactants in untreated and treated sewage. Water Res. 2007, 41, 4339–4348. [Google Scholar] [CrossRef]
- Agrawal, K.; Agnihotri, G.; Shrivas, K.; Mundhara, G.L.; Patel, K.S.; Hoffmann, P. Determination of cationic surfactants in environmental samples by flow injection analysis. Microchim. Acta 2004, 147, 273–278. [Google Scholar] [CrossRef]
- Martínez-Carballo, E.; Sitka, A.; González-Barreiro, C.; Kreuzinger, N.; Fürhacker, M.; Scharf, S.; Gans, O. Determination of selected quaternary ammonium compounds by liquid chromatography with mass spectrometry. Part I. Application to surface, waste and indirect discharge water samples in Austria. Environ. Pollut. 2007, 145, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sreevidya, V.S.; Lenz, K.A.; Svoboda, K.R.; Ma, H. Benzalkonium chloride, benzethonium chloride, and chloroxylenol—Three replacement antimicrobials are more toxic than triclosan and triclocarban in two model organisms. Environ. Pollut. 2018, 235, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, F.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Yu, Z.G.; Zhu, M.Y.; Shen, L.Q. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 15, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Antunes, S.C.; Nunes, B.; Rodrigues, S.; Nunes, R.; Fernandes, J.; Correia, A.T. Effects of chronic exposure to benzalkonium chloride in Oncorhynchusmykiss: Cholinergic neurotoxicity, oxidative stress, peroxidativedamage and genotoxicity. Environ. Toxicol. Pharmacol. 2016, 45, 115–122. [Google Scholar] [CrossRef]
- Gheorghe, S.; Lucaciu, I.; Paun, I.; Stoica, C.; Stanescu, E. Ecotoxicological behavior of some cationic and amphoteric surfactants (biodegradation, toxicity and risk assessment). In Biodegradation—Life Science; Chamy, R., Rosenkranz, F., Eds.; IntechOpen: London, UK, 2013; pp. 83–114. [Google Scholar]
- CIR. Compendium, Containing Abstracts, Discussions, and Conclusions of CIR Cosmetic Ingredient Safety Assessments; Cosmetic Ingredient Review: Washington, DC, USA, 2006. [Google Scholar]
- Gheorghe, S.; Lucaciu, I.; Paun, I.; Stoica, C.; Stanescu, E. Environmental exposure and effects of some micropollutants found in the Romanian surface waters. J. Environ. Prot. Ecol. 2014, 15, 878–888. [Google Scholar]
- Pérez, P.; Fernández, E.; Beiras, R. Toxicity of benzalkonium chloride on monoalgal cultures and natural assemblages of marine phytoplankton. Water Air Soil Pollut. 2009, 201, 319–330. [Google Scholar] [CrossRef]
- Christen, V.; Faltermann, S.; Brun, N.R.; Kunz, P.Y.; Fent, K. Cytotoxicity and molecular effects of biocidal disinfectants (quaternary ammonia, glutaraldehyde, poly (hexamethylene biguanide) hydrochloride PHMB) and their mixtures in vitro and in zebrafish eleuthero-embryos. Sci. Total Environ. 2017, 586, 1204–1218. [Google Scholar] [CrossRef]
- Li, M.H. Effects of nonionic and ionic surfactants on survival, oxidative stress, and cholinesterase activity of planarian. Chemosphere 2008, 70, 1796–1803. [Google Scholar] [CrossRef]
- Cserhati, T.; Forgacs, E.; Oros, G. Biological activity and environmental impact of anionic surfactants. Environ. Int. 2002, 28, 337–348. [Google Scholar] [CrossRef]
- Jee, J.H.; Kang, J.C. Biochemical changes oif enzymatic defence system after phenanthrene exposure in olive flounder, Paralichthyus olivaceus. Physiol. Res. 2005, 54, 585–591. [Google Scholar]
- Bindu, P.C.; Babu, P. Surfactant-induced lipid peroxidation in a tropical euryhaline teleost Oreochromis mossambicus (Tilapia) adapted to fresh water. Indian J. Exp. Biol. 2001, 39, 1118–1122. [Google Scholar] [PubMed]
- Park, C.J.; Song, S.H.; Kim, D.H.; Gye, M.C. Developmental and acute toxicity of cetylpyridinium chloride in Bombina orientalis (Amphibia: Anura). Aquat. Toxicol. 2016, 177, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Sharples, F.; Anestidou, L.; Beil, K.; Fletcher, C.H.; Haycraft, R.; Zurlo, J. Guide for the Use and Care of Laboratory Animals, 8th ed. In National Research Council (US) Committee or the Update of the Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, B.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.V., Ed.; Academic Press: New York, NY, USA, 1984; pp. 673–677. [Google Scholar]
- Paoletti, F.; Mocali, A. Determination of superoxide dismutase activity by purely chemical system based on NADP(H) oxidation. Methods Enzymol. 1990, 186, 209–221. [Google Scholar]
- Beutler, E. Red Cell Metabolism: A Manual of Biochemical Method, 3rd ed.; Grune and Stratton: Orlando, FL, USA, 1984; pp. 68–73. [Google Scholar]
- Goldberg, D.M.; Spooner, R.J. Glutathione reductase. In Methods of Enzymatic Analysis, 3rd ed.; Bergmeyer, H.V., Ed.; Verlag Chemie: Weinheim, Germany, 1983; pp. 258–265. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Lohr, G.W.; Waller, H.D. Glucose-6-phosphate dehydrogenase. In Methods of Enzymatic Analysis; Bergmeyer, H.V., Ed.; Academic Press: London, UK, 1974; pp. 744–751. [Google Scholar]
- Korzeniewski, C.; Callewaert, D.M. An enzyme-release assay for natural cytotoxicity. J. Immunol. Methods 1983, 64, 313–320. [Google Scholar] [CrossRef]
- Stanca, L.; Petrache, S.N.; Serban, A.I.; Staicu, A.C.; Sima, C.; Munteanu, M.C.; Zărnescu, O.; Dinu, D.; Dinischiotu, A. Interaction of silicon-based quantum dots with gibel carp liver: Oxidative and structural modifications. Nanoscale Res. Lett. 2013, 8, 254. [Google Scholar] [CrossRef]
- Htun-han, M. The reproductive biology of the dab Limanda limanda (L) in the North Sea: Gonadosomatic index, hepatosomatic index and condition factor. J. Fish Biol. 1978, 13, 369–378. [Google Scholar] [CrossRef]
- King, M. Fisheries Biology, Assessment and Management; Blackwell Publishing: Oxford, UK, 1995; p. 341. [Google Scholar]
- Camargo, M.M.P.; Martinez, C.B.R. Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotrop. Ichthyol. 2007, 5, 327–336. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.M.; Al-Kahtani, M.A.; Elmenshawy, O.M. Histopathological biomarkers in gills and liver of Oreochromisniloticus from polluted wetland environments, Saudi Arabia. Chemosphere 2012, 88, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Dani, U.; Bahadur, A.; Kuperkar, K. Biotoxicity and tissue-specific oxidative stress induced by Gemini surfactant as a protocol on fingerlings of Cirrhinus mrigala (Ham.): An integrated experimental and theoretical methodology. Ecotoxicol. Environ. Saf. 2019, 183, 109478. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, S.; Lucaciu, I.; Grumaz, R.; Stoica, C. Acute toxicity assessment of several cationic and amphoteric surfactants on aquatic organisms. J. Environ. Prot. Ecol. 2012, 13, 541–553. [Google Scholar]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotox. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.M.; Wood, C.M.; McClelland, G.B. Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio). Am. J. Physiol. 2007, 293, R1882–R1892. [Google Scholar] [CrossRef] [PubMed]
- Ivan, Ş.; Lucaciu, I.; Rusu, G.; Iancu, V. Toxicity of some dangerous chemicals on Superoxide dismutase enzymatic activity. J. Environ. Prot. Ecol. 2010, 11, 247–252. [Google Scholar]
- Atli, G.; Canli, M. Response of antioxidant system of freshwater fish Oreochromis niloticus to acute and chronic metal (Cd, Cu, Cr, Zn, Fe) exposures. Ecotoxicol. Environ. Saf. 2010, 73, 1884–1889. [Google Scholar] [CrossRef]
- Dinu, D.; Marinescu, D.; Munteanu, M.C.; Staicu, A.C.; Costache, M.; Dinischiotu, A. Modulatory effects of deltamethrin on antioxidant defense mechanisms and lipid peroxidation in Carassius auratus gibelio liver and intestine. Arch. Environ. Contam. Toxicol. 2010, 58, 757–764. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Metodieva, D. The reactions superoxide with reduced glutathione. Arch. Biochem. Biophys. 1994, 314, 284–290. [Google Scholar] [CrossRef]
- Kletzien, R.F.; Harris, P.K.; Foellmi, L.A. Glucose-6-phosphate dehydrogenase: A ‘‘housekeeping’’ enzyme subject to tissue-specific regulation by hormones, nutrients and oxidant stress. FASEB J. 1994, 8, 174–181. [Google Scholar] [CrossRef]
- Inácio, A.S.; Costa, G.N.; Domingues, N.S.; Santos, M.S.; Moreno, A.J.M.; Vaz, W.L.C.; Vieira, O.V. Mitochondrial dysfunction is the focus of quaternary ammonium surfactant toxicity of mammalian epithelial cells. Antimicrob. Agents Chemother. 2013, 57, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Rogov, A.G.; Goleva, T.N.; Sukhanova, E.I.; Epremyan, K.K.; Trendeleva, T.A.; Ovchenkova, A.P.; Aliverdieva, D.A.; Zvyagilskaya, R.A. Mitochondrial dysfunctions may be one of the major causative factors underlaying detrimental effects of benzalkonium chloride. Oxid. Med. Cell Longev. 2020, 2020, 8956504. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.L.; Klaassen, C.D. Principles of toxicology. In Casarett & Doull’s Toxicology: The Basic Science of Poisons; Klaassen, C.D., Ed.; McGraw Hill Press: New York, NY, USA, 2001; pp. 11–32. [Google Scholar]
- Falfushynska, H.I.; Stolyar, O.B. Responses of biochemical markers in carp Cyprinus carpio from two field sites in Western Ukraine. Ecotoxicol. Environ. Saf. 2009, 72, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.; Santos, M.A. Biotransformation, genotoxic and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.). Ecotoxicol. Environ. Saf. 2002, 53, 331–347. [Google Scholar] [CrossRef]
- Fanta, E.; Rios, F.S.; Romão, S.; Vianna, A.C.; Freiberger, S. Histopathology of the fish Corydoras paleatus contaminated with sublethal levels of organophosphorus in water and food. Ecotoxicol Environ Saf. 2003, 54, 119–130. [Google Scholar] [CrossRef]
- Pauloin, T.; Dutot, M.; Warnet, J.M.; Rat, P. In vitro modulation of preservative toxicity: High molecular weight hyaluronan decreases apoptosis and oxidative stress induced by benzalkonium chloride. Eur. J. Pharm. Sci. 2008, 34, 263–273. [Google Scholar] [CrossRef]
- Hinton, D.; Segner, H.; Au, D.; Kullman, S.; Harman, R. Liver toxicity. In Toxicology of Fishes; Di Giulio, R.T., Hinton, D.E., Eds.; CRC Press Inc.: Florida, CA, USA, 2008; pp. 327–400. [Google Scholar]
- Haaparanta, A.; Valtonen, E.T.; Hoffaman, R.; Colmes, J. Do macrophages centers in freshwater fishes reflect the differences in water quality? Aquat. Toxicol. 1996, 34, 253–272. [Google Scholar] [CrossRef]
- Hermenean, A.; Damache, G.; Albu, P.; Ardelean, A.; Ardelean, G.; Ardelean, P.D.; Horge, M.; Nagy, T.; Braun, M.; Zsuga, M.; et al. Histopatological alterations and oxidative stress in liver and kidney of Leuciscus cephalus following exposure to heavy metals in the Tur River, North Western Romania. Ecotoxicol. Environ. Saf. 2015, 119, 198–205. [Google Scholar] [CrossRef]
- Wolf, J.C.; Wheeler, J.R. A critical review of histopathological findings associated with endocrine and non-endocrine hepatic toxicity in fish models. Aquat. Toxicol. 2018, 197, 60–78. [Google Scholar] [CrossRef]
- Moody, C.A.; Martin, J.W.; Kwan, W.C.; Muir, D.C.G.; Mabury, S.A. Monitoring perfluorinated surfactants in biota and surface water samples following an accidental release of fire-fighting foam into etobicoke creek. Environ. Sci. Technol. 2002, 36, 545–551. [Google Scholar] [CrossRef]
- Tolls, J.; Haller, M.; Seinen, W.; Sijm, D.T.H.M. LAS bioconcentration: Tissue distribution and effect of hardness-implications for processes. Environ. Sci. Technol. 2000, 34, 304–310. [Google Scholar] [CrossRef]
- Amelie, K.; Chang’er, C.; James, M.A.; Jon, A.A.; Steven, D.; Michael, S.M. Tissue distribution of several series of cationic surfactants in rainbow trout (Oncorhynchus mykiss) following exposure via water. Environ. Sci. Technol. 2020, 54, 4190–4199. [Google Scholar]
| Parameter | Mean Values | International Standards |
|---|---|---|
| pH | 6.94–7.86 | SR EN ISO 10523:2012 |
| Temperature | 20 ± 0.5 °C | - |
| Dissolved oxygen | 4–6 mg O2/L | SR EN ISO 5814:2013 |
| Total hardness as CaCO3 | 184 mg/L | SR ISO 6059:2008 |
| Suspended matter | 5.2–12.4 mg/L | SR EN 872:2005 |
| Chemical oxygen demand | 14.4–25.92 mg/L | SR ISO 6060:1996 |
| Parameter | Control Fish | Exposed Fish | ||
|---|---|---|---|---|
| 24 h | 96 h | 24 h | 96 h | |
| Body mass (g/fish) | 58.5 ± 6.95 | 59 ± 11.40 | 64.1 ±1 4.43 | 65.4 ± 10.25 |
| Total length (cm/fish) | 15.87 ± 0.7 | 13.38 ± 1.98 | 16.04 ± 1.18 | 16.2 ± 1.63 |
| Liver weight (g/fish) | 1.57 ± 0.05 | 1.20 ± 0.33 | 1.49 ± 0.24 | 1.39 ± 0.30 |
| Gonad weight (g/fish) | 0.64 ± 0.03 | 0.20 ± 0.15 | 0.40 ± 0.23 | 0.73 ± 0.31 |
| HSI | 2.68 ± 0.21 | 2.03 ± 0.25 | 2.32 ± 0.21 | 2.12 ± 0.21 |
| GSI | 1.86 ± 0.03 | 0.55 ± 0.39 | 0.62 ± 0.22 | 1.11 ± 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, S.; Mitroi, D.N.; Stan, M.S.; Staicu, C.A.; Cicirma, M.; Lucaciu, I.E.; Nita-Lazar, M.; Dinischiotu, A. Evaluation of Sub-Lethal Toxicity of Benzethonium Chloride in Cyprinus carpio Liver. Appl. Sci. 2020, 10, 8485. https://doi.org/10.3390/app10238485
Gheorghe S, Mitroi DN, Stan MS, Staicu CA, Cicirma M, Lucaciu IE, Nita-Lazar M, Dinischiotu A. Evaluation of Sub-Lethal Toxicity of Benzethonium Chloride in Cyprinus carpio Liver. Applied Sciences. 2020; 10(23):8485. https://doi.org/10.3390/app10238485
Chicago/Turabian StyleGheorghe, Stefania, Daniel N. Mitroi, Miruna S. Stan, Cristina A. Staicu, Marius Cicirma, Irina E. Lucaciu, Mihai Nita-Lazar, and Anca Dinischiotu. 2020. "Evaluation of Sub-Lethal Toxicity of Benzethonium Chloride in Cyprinus carpio Liver" Applied Sciences 10, no. 23: 8485. https://doi.org/10.3390/app10238485
APA StyleGheorghe, S., Mitroi, D. N., Stan, M. S., Staicu, C. A., Cicirma, M., Lucaciu, I. E., Nita-Lazar, M., & Dinischiotu, A. (2020). Evaluation of Sub-Lethal Toxicity of Benzethonium Chloride in Cyprinus carpio Liver. Applied Sciences, 10(23), 8485. https://doi.org/10.3390/app10238485









