Featured Application
This is a contribution for enlarging the thermodynamic database of organic compounds and ketone derivatives. These data are very relevant to controlling experimental investigations to produce value-added chemical products from biomass. Moreover, the knowledge from such data can be used in the development of schemes for the prediction of homologous properties of related compounds, in particular, modeling studies for the prediction of bio-oil composition.
Abstract
The energetic study of 6-hydroxy-1-indanone and 7-hydroxy-1-indanone was performed using experimental techniques and computational calculations. The enthalpies of combustion and sublimation of the two compounds were determined and allowed to derive the corresponding gas-phase standard molar enthalpies of formation. For this purpose, static-bomb combustion calorimetry and drop-method Calvet microcalorimetry were the experimental techniques used. Further, the enthalpy of fusion of each compound was obtained from scanning differential calorimetry measurements. Additionally, the gas-phase standard molar enthalpies of formation of these compounds were calculated through high-level ab initio calculations. The computational study of the molecular structures of the indanones was carried out and two possible conformers were observed for 6-hydroxy-1-indanone. Furthermore, the energetic effects associated with the presence of one hydroxyl group as a substituent on the benzenic ring of 1-indanone were also evaluated. Both experimental and theoretical methods show that 7-hydroxy-1-indanone is thermodynamically more stable than the 6-isomer in the gaseous phase and these results provide evidence for the existence of a strong intramolecular H-bond in 7-hydroxy-1-indanone. Finally, the intramolecular proton transfer in 7-hydroxy-1-indanone has been evaluated and as expected, it is not energetically favorable.
1. Introduction
Accurate data about thermochemical and thermophysical properties of biomass-derived compounds are essential for the evaluation of the corresponding chemical behavior. These data are very relevant when controlling experimental investigations to produce value-added chemical products from biomass. Moreover, data can be used in the development of schemes for the prediction of the homologous properties of related compounds [1,2], in particular, modeling studies for the prediction of bio-oil composition [3]. In this context, our research group has been involved in an extensive experimental and theoretical thermodynamic study on key biomass-derived compounds, namely, vanillyl alcohol [1], levoglucosan [4], cellulose allomorphs [5], α-D-xylose [6], and more recently, cyclopentenones [7], indanones [2], and other classes of relevant compounds extracted from biomass.
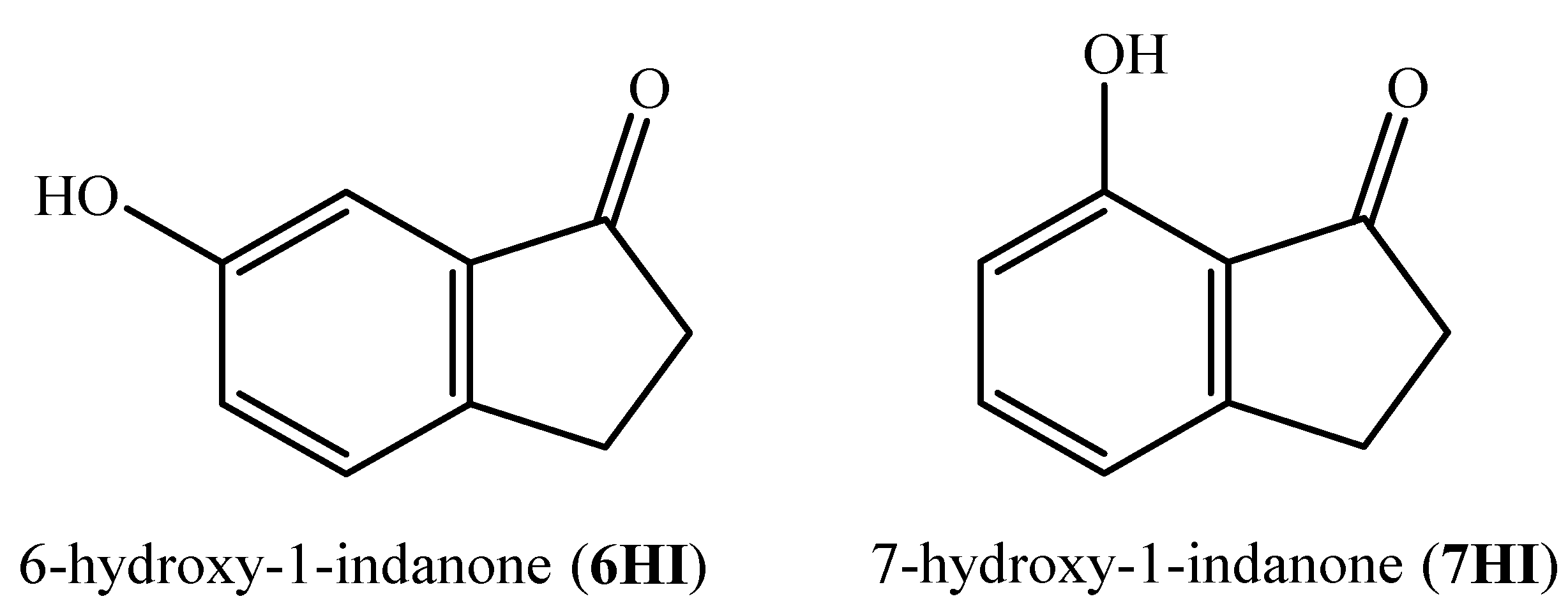
The purpose of this study is to contribute to enlarging the thermodynamic database of this class of compounds, as well as to establish some energy-structure relationships for the prediction of the same properties and to evaluate their reactivity. Thus, we present a detailed experimental and computational study on the energetics of the 6- and 7-hydroxy-1-indanone, whose structural formulae are depicted in Figure 1. The experimental data are determined from calorimetric measurements focusing on the determination of the standard molar enthalpy of formation in the gaseous phase, an essential thermodynamic parameter associated with molecular energy. This property was also obtained by G3(MP2)//B3LYP calculations, using several working reactions. This composite method was also used to perform a conformational analysis of the studied compounds. In addition, we addressed the energetic effects associated with the presence of a hydroxyl group on the core of the benzenic ring of 1-indanone, and the hydrogen bond network was also evaluated.
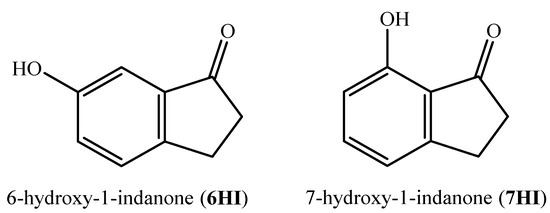
Figure 1.
Structural formulae of the compounds studied.
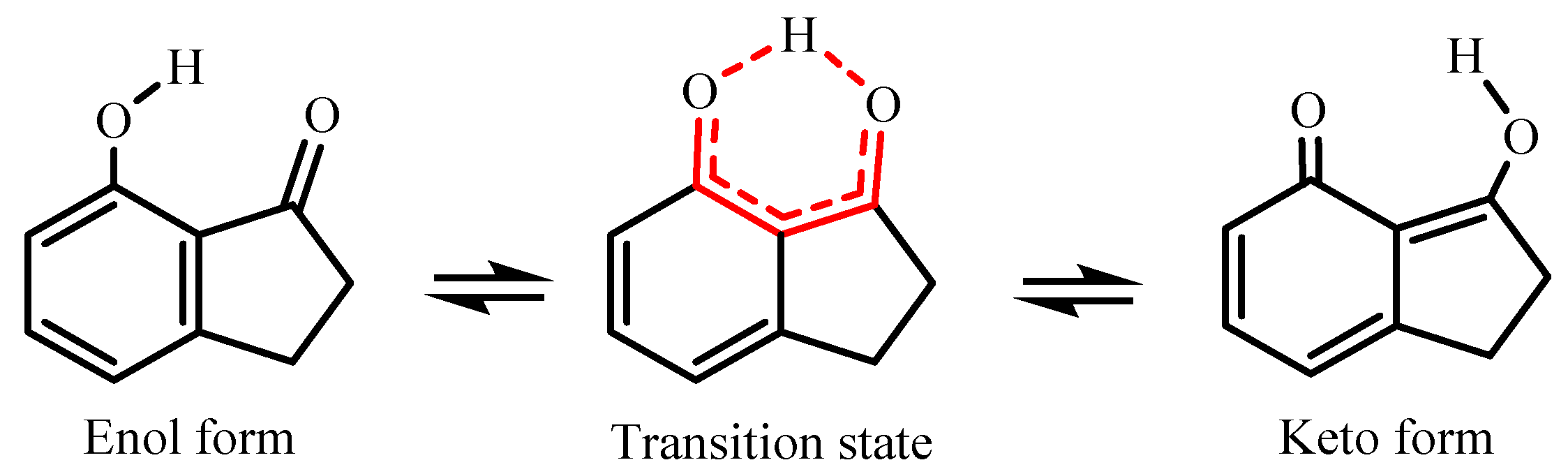
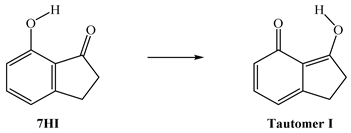
In the literature, several studies have been conducted involving 7-hydroxi-1-indanone [8,9,10], since it is a prototype in the excited-state intramolecular proton transfer process (ESIPT, Figure 2). These important characteristics of 7-hydroxi-1-indanone and its derivatives confer several applications, namely, as laser dyes, probe sensors, and molecular devices [11,12]. Thus, in this study, the gas-phase intramolecular proton transfer process of 7-hydroxy-1-indanone was evaluated.
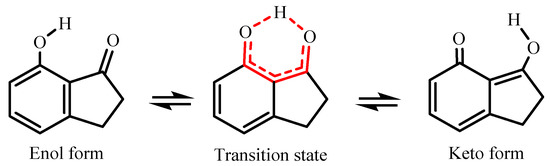
Figure 2.
Diagram for the structures of 7HI and the excited-state intramolecular proton transfer (ESIPT) process between the normal form and its tautomer.
2. Materials and Methods
2.1. Materials
6-Hydroxy-1-indanone (6HI, CAS No. 62803-47-8) and 7-hydroxy-1-indanone (7HI, CAS No. 6968-35-0) were obtained commercially. The samples were analyzed by a gas chromatography-flame ionization detector (Agilent 4890D) with a capillary column HP-5. For 7HI, no purification process was applied due to the high purity of the commercial sample. For 6HI, sublimation under reduced pressure was the purification method used in this study. The results obtained after the combustion experiments, particularly the carbon dioxide recovery ratios, were consistent with the high purity of the samples (Tables S1 and S2, Supplementary Materials).
2.2. Methods
2.2.1. Differential Scanning Calorimetry
The heat-flux DSC instrument (differential scanning calorimeter, Hitachi-DSC7020) was used to study the thermal behavior of each compound. It determined the respective onset temperature and enthalpy of fusion.
The samples (1 to 4 mg) were hermetically sealed in aluminum crucibles. Five independent runs in a flowing nitrogen atmosphere were performed, using a heating rate of 0.033 K s−1, from T ≈ 298 K to a temperature approximately 20 K above the temperature of fusion.
The temperature and power scales of the apparatus were calibrated under the same conditions as the experimental determinations by using the onset and the area, respectively, of the fusion peaks of the recommended standard materials [13]: indium (Sigma-Aldrich, mass fraction > 0.99999), tin (Sigma-Aldrich, mass fraction > 0.99999), and benzoic acid (NIST SRM 39j).
2.2.2. Combustion Calorimetry
The standard (p° = 0.1 MPa) massic energies of combustion of the compounds studied were measured using a static-bomb combustion calorimeter. The twin-valve bomb (type 1108, Parr Instrument Company, Moline, IL, USA) with an internal volume of 0.342 dm3 was used. The apparatus and technique have been previously described [14,15].
For each compound, the standard massic energy of combustion, , was calculated by a similar procedure to that developed by Hubbard et al. [16,17]. Further details about the experimental procedure are provided in Supplementary Materials.
2.2.3. Calvet Microcalorimetry
The enthalpy of sublimation of the two indanone derivatives was measured on a high-temperature Calvet microcalorimeter (Setaram HT 1000), using the “vacuum sublimation” drop method described by Skinner et al. [18]. The details of the apparatus and the technique were previously reported [19]. Samples of ~5 mg were dropped, simultaneously with the corresponding blank tube, at a known room temperature (T~298.15 K), into the reaction vessel of the microcalorimeter (at T = 417 K for 6HI and T = 375 K for 7HI). After thermal equilibrium was achieved, the sample was removed from the cell by vacuum sublimation. A correction, k, to the internal calibration constant of the calorimeter was obtained as the average of six independent experiments with anthracene, at T = 417 K, k = (1.044 ± 0.005). Six calibration experiments were made with naphthalene, at T = 375 K, obtaining k = (1.020 ± 0.005).
2.2.4. Computational Approach
The standard ab initio molecular orbital calculations for the compounds studied were performed using the composite G3(MP2)//B3LYP method [20] and the Gaussian 03 series of programs [21]. The geometry optimization of molecules is obtained from B3LYP density functional theory (B3LYP/6-31G(d)). Further information related with this method is reported in literature [20].
3. Results and Discussion
3.1. Temperatures and Enthalpies of Fusion
The mean values of the temperature and enthalpy of fusion obtained from the DSC study for the solids 6HI and 7HI are presented in Table 1, as well as the available values from the literature with respect to the melting temperature of the compounds studied. The assigned uncertainties are twice the standard error of the mean of five independent runs and include the standard uncertainties allocated to the calibration results. No phase transitions were detected in the crystalline phase, between T = 298 K and the temperature of fusion of each compound.

Table 1.
Mean values of the temperature and enthalpy of fusion obtained from the DSC study for the indanones studied.
3.2. Enthalpies of Formation in the Crystalline Phase
Results of all the combustion experiments and the individual values of the massic energy of combustion, , with the mean value and the corresponding standard deviation of the mean, for the compounds studied, are given in Supplementary Materials (Tables S1 and S2).
The internal energy for the isothermal bomb process, ∆U(IBP), was calculated through Equation (1), where Δm(H2O) is the deviation of the mass of water added to the calorimeter from 3119.6 g, ∆Tad is the corrected temperature rise obtained in each experiment, εf is the energy equivalent of contents in the final state, and ∆U(ign) is the electrical energy for ignition.
ΔU(IBP) = −{εcal + Cp(H2O, l) ··· Δm(H2O) + εf} ΔTad + ∆U(ign)
The standard massic energies of combustion, , for hydroxyl-indanones refer to the quantitative combustion reaction (2).
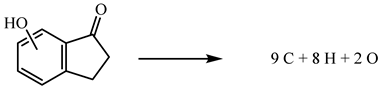
C9H8O2 (cr) + 10O2(g) → 9CO2(g) + 4H2O(l)
The mean value of the standard (p° = 0.1 MPa) massic energy of combustion of each compound, as well as the derived standard molar internal energy of combustion, , and enthalpy of combustion, , and the standard molar enthalpy of formation in the crystalline phase, , at T = 298.15 K, are reported in Table 2. The uncertainties associated with the standard molar energy and enthalpy of combustion are twice the overall standard deviation of the mean and include the uncertainties in calibration with benzoic acid [22,23]. To derive from , the standard molar enthalpies of formation, at T = 298.15 K, were used for H2O(l), −(285.830 ± 0.040) kJ⋅mol−1 [24] and CO2(g), −(393.51 ± 0.13) kJ⋅mol−1 [24].

Table 2.
Standard (p° = 0.1 MPa) massic energy of combustion, , molar energy of combustion, enthalpy of combustion, and enthalpy of formation, for the compounds studied, at T = 298.15 K a.
3.3. Enthalpies of Sublimation
The standard molar enthalpies of sublimation, , of indanones were determined by Calvet microcalorimetry. Table 3 presents the results of these experiments. The observed enthalpies of sublimation at the experimental temperature T, , were converted to T = 298.15 K, through Equation (3), using the enthalpic term . This enthalpic term is calculated from the gas-phase molar heat capacities, (g), whose values are derived from statistical thermodynamics using the vibrational frequencies obtained from DFT calculations with the B3LYP functional and the 6-31G(d) basis set (scaled by a factor of 0.9613 [25]). These computed (g) values in the range of 200–600 K are collected in Tables S3 and S4.

Table 3.
Standard (p° = 0.1 MPa) molar enthalpies of sublimation, , at T = 298.15 K for the compounds studied, as determined by Calvet microcalorimetry.
3.4. Conformational Analysis
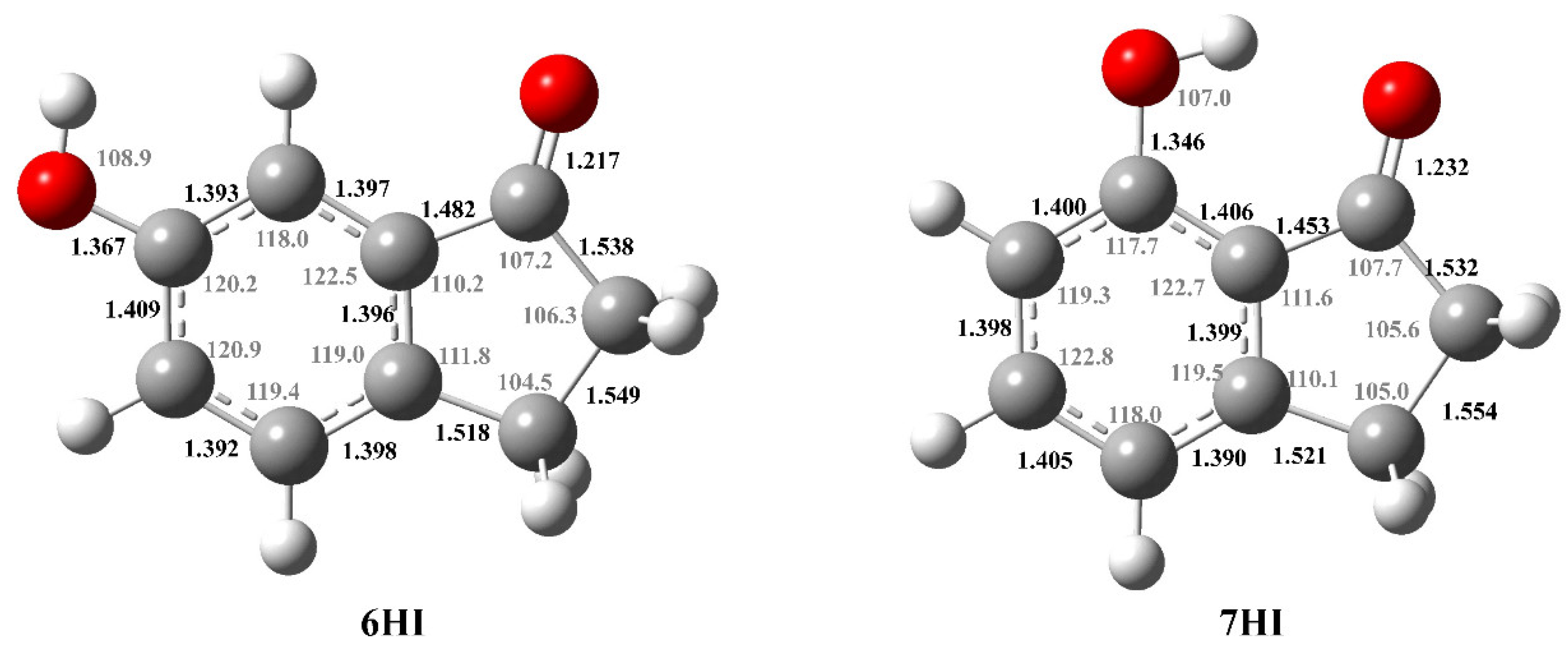
The optimized structures of the most stable conformation of each compound are presented in Figure 3. The geometry optimization was performed at the B3LYP/6-31G(d) level of theory. All the conformations are planar existing, and in the case of 6HI, two possible conformations associated with geometrical changes of the hydroxyl group exist, while for 7HI, there is only one stable conformer that corresponds to the global minimum energy. The conformational analysis of the hydroxy-1-indanones studied and their corresponding total electronic energy, plus the internal thermal energy, are presented in Table S5.
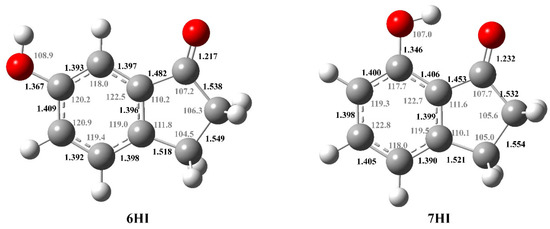
Figure 3.
Molecular structures of the most stable conformation of 6HI and 7HI in the gaseous phase, optimized by B3LYP/6-31G(d) level of theory, with the corresponding calculated bond distances (10−10 m) and angles (°).
The conformational composition of 6HI in the gas phase, at T = 298 K, can be calculated through Equation (4), where is the relative Gibbs energy of conformer i to the lowest energy conformer I and can be calculated from Equation (5).
The values calculated at the G3(MP2)//B3LYP level are collected in Table S6, as well as the Boltzmann weighted populations derived from Gibbs energies. The lowest-energy conformer of 6HI accounts for more than 73% of the composition in the gas phase.
Using Equation (6), the final value for the absolute enthalpy of 6HI is calculated at the G3(MP2)//B3LYP level. Consequently, these values were finally applied for the theoretical gaseous enthalpy of formation calculation, at T = 298.15 K. As discussed above, the conformer I of 7HI accounts for more than 99.9% of the composition in the gas phase. Therefore, conformer II was not considered in the present study.
3.5. Experimental and Computational Enthalpies of Formation in the Gaseous Phase
The experimental gas-phase standard molar enthalpy of formation, , at T = 298.15 K, of each compound was derived from the combination of the standard molar enthalpy of formation in the crystalline phase with its standard molar enthalpy of sublimation. These data are summarized in Table 4.

Table 4.
Experimental derived standard (p° = 0.1 MPa) molar enthalpies of formation, in the gaseous phase, at T = 298.15 K, for the compounds studied.
The computational gas-phase enthalpies of formation of the two indanone derivatives were calculated at the G3(MP2)//B3LYP level of theory, using the atomization reaction and the isodesmic reactions (8) to (13) presented in Table 5. The working reaction (13) was applied to 7-hydroxy-α-tetralone and also to three more isomers, namely, 5-hydroxy-α-tetralone, 6-hydroxy-α-tetralone, and 8-hydroxy-α-tetralone [26]. In this study, we present results only for 7-hydroxy-α-tetralone, since for the other isomers, there is a large discrepancy between the experimental value and the results obtained, higher than 8 kJ·mol−1 for 6HI or 7HI molecules. Despite this, as can be seen in Table 6, there is an excellent agreement between the values of the gaseous enthalpy of formation of both compounds derived from the experimental data and the mean value obtained by the composite method. Data related to the G3(MP2)//B3LYP absolute enthalpies and literature values of , at T = 298.15 K, for all the atoms and molecules involved herein are given in SI (Table S7).

Table 5.
Working reactions and computed enthalpies of reaction, , and formation, , in the gaseous state, of hydroxy-1-indanones, at T = 298.15 K a.

Table 6.
Comparison between the calculated and the experimental gas-phase enthalpies of formation of the compounds studied, at T = 298.15 K, through atomization and isodesmic reactions (8)–(13); all values are in kJ·mol−1.
To the best of our knowledge, there are no experimental or computational data available to compare with those obtained in this study. However, the consistency of the results described here gives us the confidence to use an identical methodology to estimate the values of the corresponding properties for related compounds.
3.6. Calculated Enthalpic Increments (H → OH) and Analysis of the Intramolecular H-Bond Interaction
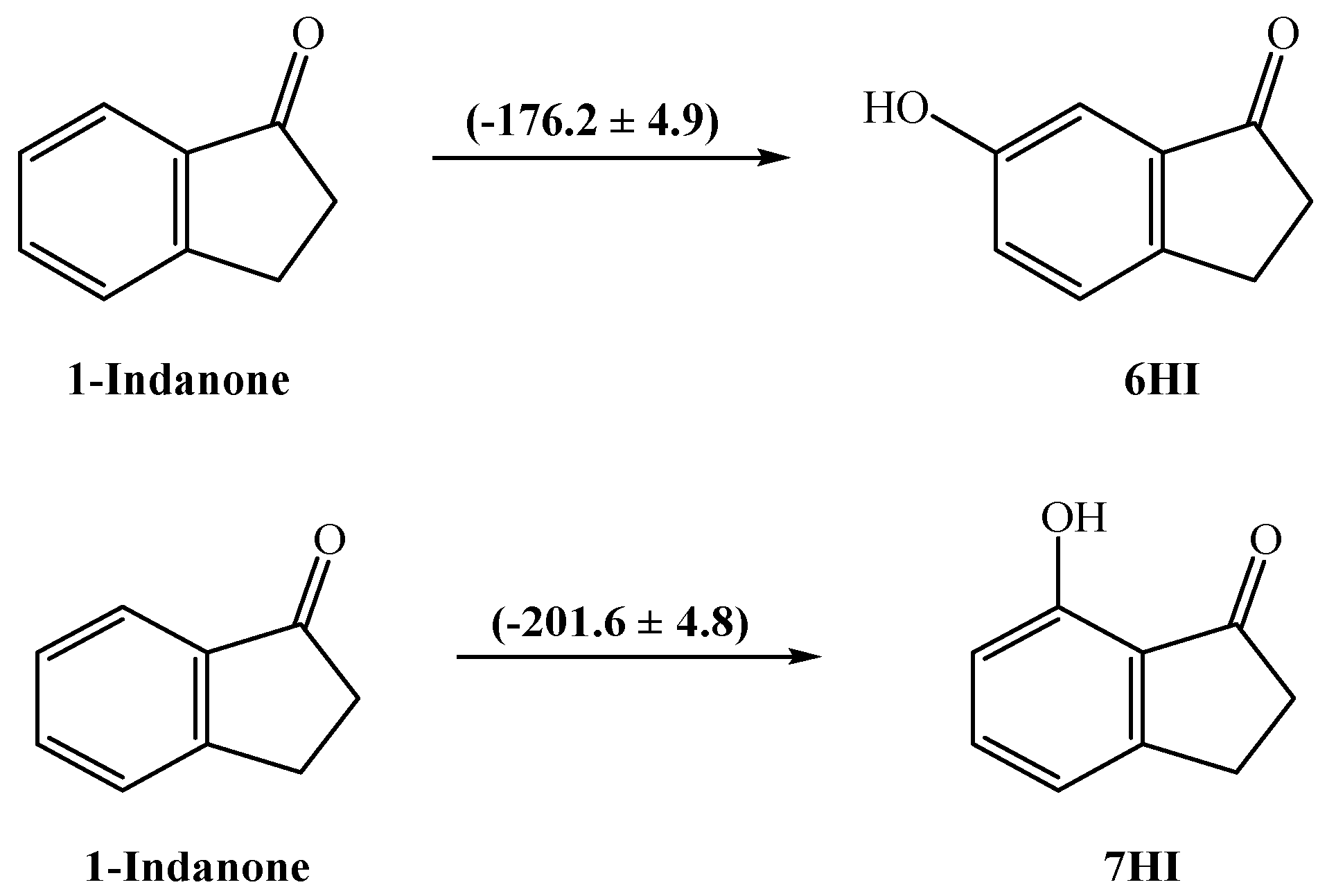
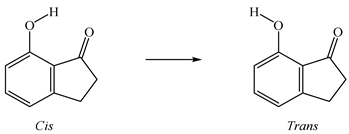
The energetic effect involving the presence of a hydroxyl group in position 6 or 7 of 1-indanone was also analyzed by calculating the respective enthalpic increment (Figure 4). The enthalpic increment related to the hydroxyl group in position 6 is −(176.2 ± 4.9) kJ⋅mol−1, instead of −(201.6 ± 4.8) kJ⋅mol−1 obtained for position 7. As a comparison, the corresponding substitution in benzene gives −(179.0 ± 1.1) kJ⋅mol−1 (for this calculation, the literature values for benzene, (82.6 ± 0.7) kJ⋅mol−1 [27] and for phenol, −(96.4 ± 0.9) kJ⋅mol−1 [27] were used). In the case of 7HI, there is a difference of ~25 kJ⋅mol−1 between the observed magnitude of the enthalpic increments, probably due to the existence of a strong intramolecular interaction O−H O. Following our results, we have studied the intramolecular H-bond in this molecule using the cis-trans method [28], which compares energetically the H–bonded system and the homologous system with the O–H fragment rotated around the C–O bond toward the trans conformation, so that the H atom points away from the acceptor oxygen from the carbonyl group (Equation (7)). To obtain the intramolecular hydrogen bond energy, ΔEintra-HB, we used the energy values obtained at the G3(MP2)//B3LYP level (see Table S5). The ΔEintra-HB value calculated with the cis-trans method is 33.8 kJ⋅mol−1. This result is expected to be slightly overestimated, as seen with other studied compounds [29], in which the results with the same method provided overestimated values. Comparable results were also found by Estácio et al. [30] and Korth et al. [31], which could be associated with the additional steric or electronic interactions, or both.
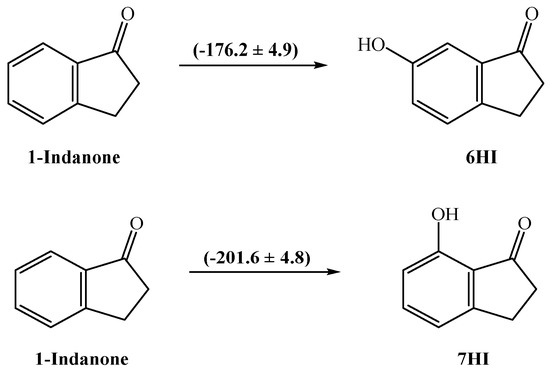
Figure 4.
Enthalpic increments (values in kJ⋅mol−1) for the presence of one −OH group in position 6 or 7 of 1-indanone.
Finally, the intramolecular proton transfer process of 7HI was evaluated (Equation (8)), using its G3(MP2)//B3LYP calculated tautomerization enthalpy (absolute enthalpies of 7HI and its tautomer are calculated as −497.486145 hartrees and −497.462180 hartrees, respectively). The G3(MP2)//B3LYP calculated tautomerization enthalpy of 62.9 kJ⋅mol−1 clearly shows that the tautomeric equilibrium is not energetically favorable for its tautomer conversion.


4. Conclusions
The gas-phase enthalpies of formation of 6HI and 7HI, at T = 298.15 K, were derived from experimental data of the enthalpies of formation of the crystalline compounds and of sublimation, as determined by combustion calorimetry and Calvet microcalorimetry, respectively. The phase behavior of the two hydroxy-1-indanones was also studied.
Computational calculations at the G3(MP2)//B3LYP level were carried out, and the estimated gas-phase enthalpies of formation of the two referred compounds compared very well with the experimental data. This methodology can be used to estimate the values of the corresponding property for related compounds.
The energetic effect associated with the insertion of a hydroxyl group in position 6 or 7 of 1-indanone was analyzed, and this effect was compared with the same effect in the benzene molecule.
In this study, it was confirmed that the 7HI molecule presents a strong H-bond interaction, being thermodynamically more stable than the 6-isomer in the gaseous phase. In addition, the intramolecular proton transfer in 7HI structure was evaluated and was not energetically favorable.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/23/8512/s1, Data of combustion calorimetry experiments of 6HI and 7HI (Tables S1 and S2); the values of standard molar heat capacities in the gaseous phase for the compounds studied (Table S3 and S4); Conformational analysis of the hydroxy-1-indanones studied and their corresponding total electronic energy plus the internal thermal energy (Table S5); G3(MP2)//B3LYP results for 6HI and 7HI conformers (Table S6); G3(MP2)//B3LYP calculated absolute enthalpies, at T = 298.15 K, and experimental gas-phase values for all the atoms/molecules used (Table S7).
Author Contributions
Conceptualization, A.L.R.d.S. and M.D.M.C.R.d.S.; methodology, A.L.R.d.S. and M.D.M.C.R.d.S.; software, A.L.R.d.S. and M.D.M.C.R.d.S.; investigation, A.L.R.d.S.; writing—original draft preparation, A.L.R.d.S.; writing—review and editing, M.D.M.C.R.d.S.; supervision, M.D.M.C.R.d.S.; funding acquisition, A.L.R.d.S. and M.D.M.C.R.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was developed under the funding of the projects UID/QUI/00081/2019 and UIDB/00081/2020, awarded to CIQUP, financed by Fundação para a Ciência e Tecnologia (FCT), Lisbon, Portugal, and co-financed in the framework of Operational Programme for Competitiveness and Internationalisation, COMPETE, with community funds (FEDER) and national funds of MEC.
Acknowledgments
Ana L.R. Silva thanks FCT/MCTES for her contract under Stimulus of Scientific Employment 2017 (CEECIND/01161/2017).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Freitas, V.L.S.; Lima, A.C.M.O.; Sapei, E.; Ribeiro da Silva, M.D.M.C. Comprehensive thermophysical and thermochemical studies of vanillyl alcohol. J. Chem. Thermodyn. 2016, 102, 287–292. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Lima, A.C.M.O.; Ribeiro da Silva, M.D.M.C. Energetic characterization of indanone derivatives involved in biomass degradation. J. Therm. Anal. Calorim. 2018, 134, 1267–1276. [Google Scholar] [CrossRef]
- Kohl, T.; Sapei, E.; Rocha, I.M.; Galvão, T.L.P.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Modeling of fast pyrolysis of wood for prediction of bio-oil composition. In Proceedings of the Asia Pacific Confederation of Chemical Engineering Congress 2015: APCChE 2015, Incorporating CHEMECA, Melbourne, Australia, 26 September–1 October 2015; Engineers Australia: Melbourne, Australia; p. 2410. [Google Scholar]
- Rocha, I.M.; Galvão, T.L.P.; Sapei, E.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V. Levoglucosan: A calorimetric, thermodynamic, spectroscopic, and computational investigation. J. Chem. Eng. Data. 2013, 58, 1813–1821. [Google Scholar] [CrossRef]
- Goldberg, R.N.; Schliesser, J.; Mittal, A.; Decker, S.R.; Santos, A.F.L.O.M.; Freitas, V.L.S.; Urbas, A.; Lang, B.E.; Heiss, C.; Ribeiro da Silva, M.D.M.C. A thermodynamic investigation of the cellulose allomorphs: Cellulose(am), cellulose Ib(cr), cellulose II(cr), and cellulose III(cr). J. Chem. Thermodyn. 2015, 81, 184–226. [Google Scholar] [CrossRef]
- Goldberg, R.N.; Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Ferreira, A.I.L.; Shi, Q.; Woodfield, B.F. Thermochemistry of α-D-xylose(cr). J. Chem. Thermodyn. 2013, 58, 20–28. [Google Scholar]
- Silva, A.L.R.; Moura, C.; Ribeiro da Silva, M.D.M.C. (2019) Energetic vs structural study of two biomass degradation derivatives: 2-cyclopentenone and 3-methyl-2-cyclopentenone. J. Chem. Thermodyn. 2019, 132, 390–396. [Google Scholar] [CrossRef]
- Chou, P.-T.; Martinez, M.L.; Shannon, L. Studer Role of Triplet States in the Reverse Proton Transfer Mechanism of 7-Hydroxy-1-indanone. J. Phys. Chem. 1991, 95, 10306–10310. [Google Scholar] [CrossRef]
- Graña, A.M.; Rios, M.A.; Rodriguez, J. Ab initio study of the structure and tautomerism in 7-hydroxy-14ndanone. J. Mol. Struct. (Theochem) 1991, 226, 303–306. [Google Scholar] [CrossRef]
- Sobolewski, A.L.; Domcke, W. Ab initio potential-energy functions for excited state intramolecular proton transfer: A comparative study of o-hydroxybenzaldehyde, salicylic acid and 7-hydroxy-1-indanone. Phys. Chem. Chem. Phys. 1999, 1, 3065–3072. [Google Scholar] [CrossRef]
- Tang, K.C.; Chang, M.J.; Lin, T.Y.; Pan, H.A.; Fang, T.C.; Chen, K.Y.; Chou, P.T. Fine tuning the energetics of excited-state intramolecular proton transfer (ESIPT): White light generation in a single ESIPT system. J. Am. Chem. Soc. 2011, 133, 17738–17745. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.; Zhang, H.; Wang, Y.; Zhang, H.; Yamaguchi, S. ESIPT-active organic compounds with white luminescence based on crystallization-induced keto emission (CIKE). Chem. Comm. 2017, 53, 7832–7835. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, R.; Xu-wu, A.; Chickos, J.S.; Leitão, M.L.P.; Roux, M.V.; Torres, L.A. Reference materials for calorimetry and differential thermal analysis. Thermochim. Acta 1999, 331, 93–204. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Pilcher, G. The construction, calibration and use of a new high-precision static-bomb calorimeter. Rev. Port. Quím. 1984, 26, 163–172. [Google Scholar]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Pilcher, G. Enthalpies of combustion of 1, 2-dihydroxybenzene and of six alkylsubstituted 1, 2-dihydroxybenzenes. J. Chem. Thermodyn. 1984, 16, 1149–1155. [Google Scholar] [CrossRef]
- Hubbard, W.N.; Scott, D.W.; Waddington, G. Standard states and corrections for combustions in a bomb at constant volume. In Experimental Thermochemistry; Rossini, F.D., Ed.; Interscience: New York, NY, USA, 1956; Volume 1, pp. 75–128. [Google Scholar]
- Paulechka, E.; Riccardi, D. NIST Standard Reference Database 206: Combustion Calorimetry Tool; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020. Available online: https://trc.nist.gov/cctool/ (accessed on 1 June 2020).
- Adedeji, F.A.; Brown, D.L.S.; Connor, J.A.; Leung, W.L.; Paz-Andrade, I.M.; Skinner, H.A. Thermochemistry of arene chromium tricarbonyls and the strenghts of arene-chromium bonds. J. Organomet. Chem. 1975, 97, 221–228. [Google Scholar] [CrossRef]
- Santos, L.M.N.B.F.; Schröder, B.; Fernandes, O.O.P.; Ribeiro da Silva, M.A.V. Measurement of enthalpies of sublimation by drop method in a Calvet type calorimeter: Design and test of a new system. Thermochim. Acta 2004, 415, 15–20. [Google Scholar] [CrossRef]
- Baboul, A.G.; Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-3 theory using density functional geometries and zero-point energies. J. Chem. Phys. 1999, 110, 7650–7657. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.J.; Stratmann, R.E.; Burant, J.C. Gaussian03, revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Rossini, F.D. (Ed.) Assignment of uncertainties to thermochemical data. In Experimental Thermochemistry; Interscience: New York, NY, USA, 1956; Volume 1, pp. 297–325. [Google Scholar]
- Olofson, G. Assignment of uncertainties. In Combustion Calorimetry; Sunner, S., Mansson, M., Eds.; Pergamon Press: Oxford, UK, 1979; Volume 1, pp. 137–159. [Google Scholar]
- Cox, J.D.; Wagman, D.D.; Medvedev, V.A. CODATA Key Values for Thermodynamics; Hemisphere: New York, NY, USA, 1979. [Google Scholar]
- Merrick, J.P.; Moran, D.; Radom, L. An evaluation of harmonic vibrational frequency scale factors. J. Phys. Chem. A 2007, 111, 11683–11700. [Google Scholar] [CrossRef]
- Matos, M.A.R.; Sousa, C.C.S.; Morais, V.M.F. Energetics of Hydroxytetralones: A Calorimetric and Computational Thermochemical Study. J. Chem. Eng. Data 2009, 54, 2189–2194. [Google Scholar] [CrossRef]
- Pedley, J.P. Thermochemical Data and Structures of Organic Compounds; Thermodynamics Research Centre: College Station, TX, USA, 1994.
- Kovács, A.; Szabó, A.; Hargittai, I. Structural Characteristics of Intramolecular Hydrogen Bonding in Benzene Derivatives. Acc. Chem. Res. 2002, 35, 887–894. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Matos, M.A.R.; Morais, V.M.F.; Ribeiro da Silva, M.D.M.C. Thermochemical and conformational study of optical active phenylbenzazole derivatives. J. Chem. Thermodyn. 2018, 116, 7–20. [Google Scholar] [CrossRef]
- Estácio, S.G.; Couto, P.C.; Cabral, B.J.C.; Minas da Piedade, M.E.; Simões, M.J.A. Energetics of Intramolecular Hydrogen Bonding in Di-substituted Benzenes by the ortho-para Method. J. Phys. Chem. A 2004, 108, 10834–10843. [Google Scholar] [CrossRef]
- Korth, H.G.; Heer, M.I.; Mulder, P. A DFT study on intramolecular hydrogen bonding in 2-substituted phenols: Conformations, enthalpies, and correlation with solute parameters. J. Phys. Chem. A 2002, 106, 8779–8789. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).