Assessment of Antioxidant Contents and Free Radical-Scavenging Capacity of Chlorella vulgaris Cultivated in Low Cost Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Growth Media and Analysis of Its Nutrients
2.2. Experimental Setup
2.3. Preparation of the Extracts
2.4. Determination of the Extraction Yield
2.5. Determination of Total Phenolic Content
2.6. Determination of Total Flavonoid Content
2.7. Determination of β-Carotene and Lycopene
2.8. Determination of Antiradical Activity against DPPH Radical
2.9. Statistical Analyses
3. Results
3.1. Extraction Yield
3.2. Total Phenolic Content
3.3. Total Flavonoid Content
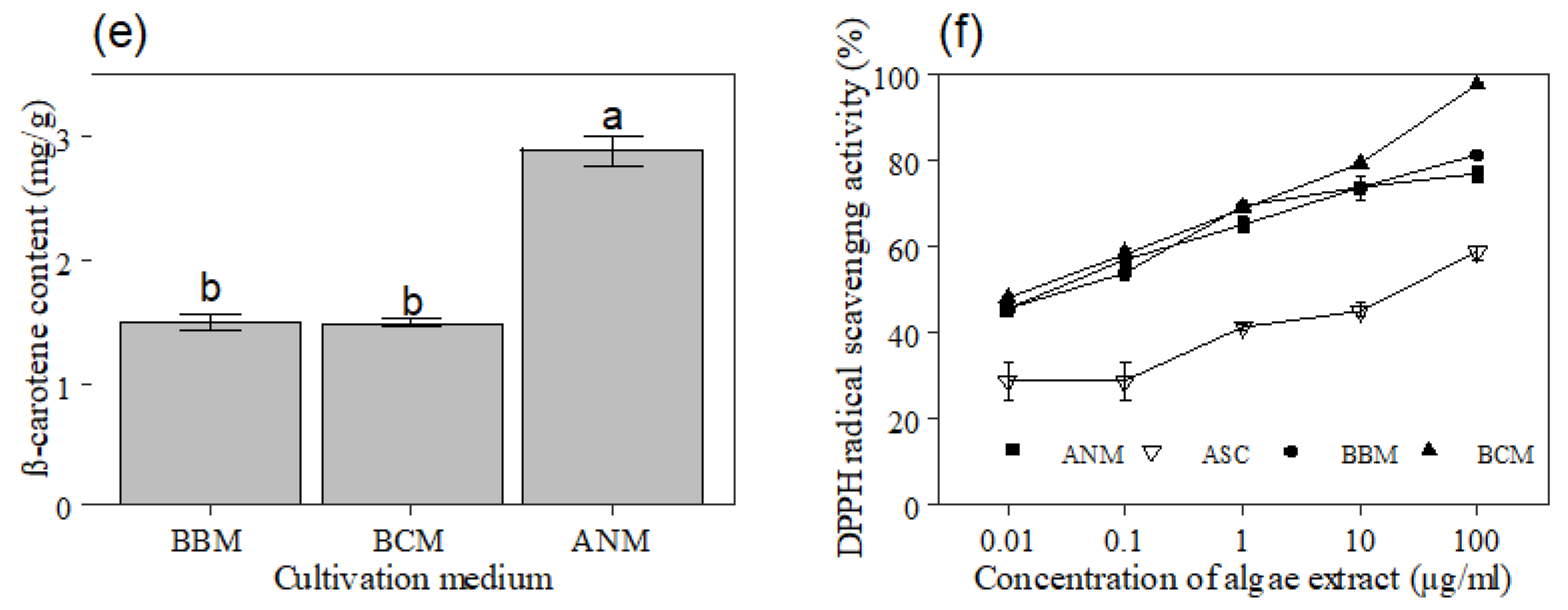
3.4. β-Carotene and Lycopene
3.5. Free Radical Scavenging Using DPPH Assay
4. Discussion
4.1. Chlorella Vulgaris Extraction Yield
4.2. Total Phenolic Contents
4.3. Flavanoid Content
4.4. β-Carotene and Lycopene
4.5. Free Radical Scavenging Using DPPH Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar]
- Lopaczynski, W.; Zeisel, S.H. Antioxidants, programmed cell death, and cancer. Nutr. Res. 2001, 21, 295–307. [Google Scholar]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar]
- Hajhashemi, V.; Vaseghi, G.; Pourfarzam, M.; Abdollahi, A. Are antioxidants helpful for disease prevention? Res. Pharm. Sci. 2010, 5, 5–12. [Google Scholar]
- Zouari, M.; Ben Ahmed, C.; Elloumi, N.; Bellassoued, K.; Delmail, D.; Labrousse, P.; Ben Abdallah, F.; Ben Rouina, B. Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. cv Chemlali exposed to cadmium stress. Ecotoxicol. Environ. Saf. 2016, 128, 195–205. [Google Scholar]
- Bajguz, A. An enhancing effect of exogenous brassinolide on the growth and antioxidant activity in Chlorella vulgaris cultures under heavy metals stress. Environ. Exp. Bot. 2010, 68, 175–179. [Google Scholar]
- Nordberg, J.; Arnér, E.S.J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible flowers—A new promising source of mineral elements in human nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar]
- Chu, W. Potential Applications of Antioxidant. Curr. Top. Nutraceutical Res. 2011, 9, 83–98. [Google Scholar]
- Ambrozova, J.V.; Misurcova, L.; Vicha, R.; Machu, L.; Samek, D.; Baron, M.; Mlcek, J.; Sochor, J.; Jurikova, T. Influence of extractive solvents on lipid and fatty acids content of edible freshwater algal and seaweed products, the green microalga Chlorella kessleri and the cyanobacterium Spirulina platensis. Molecules 2014, 19, 2344–2360. [Google Scholar]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2009, 78, 275–281. [Google Scholar]
- Kay, R.A. Microalgae as Food and Supplement. Crit. Rev. Food Sci. Nutr. 1991, 30, 555–573. [Google Scholar]
- Chen, Y.X.; Liu, X.Y.; Xiao, Z.; Huang, Y.F.; Liu, B. Antioxidant activities of polysaccharides obtained from Chlorella pyrenoidosa via different ethanol concentrations. Int. J. Biol. Macromol. 2016, 91, 505–509. [Google Scholar]
- Wu, L.C.; Ho, J.A.A.; Shieh, M.C.; Lu, I.W. Antioxidant and antiproliferative activities of spirulina and Chlorella water extracts. J. Agric. Food Chem. 2005, 53, 4207–4212. [Google Scholar]
- Rodriguez-Garcia, I.; Guil-Guerrero, J.L. Evaluation of the antioxidant activity of three microalgal species for use as dietary supplements and in the preservation of foods. Food Chem. 2008, 108, 1023–1026. [Google Scholar]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar]
- Renju, G.L.; Kurup, G.M.; Saritha Kumari, C.H. Effect of lycopene from Chlorella marina on high cholesterol-induced oxidative damage and inflammation in rats. Inflammopharmacology 2014, 22, 45–54. [Google Scholar]
- Lee, S.H.; Kang, H.J.; Lee, H.J.; Kang, M.H.; Park, Y.K. Six-week supplementation with Chlorella has favorable impact on antioxidant status in Korean male smokers. Nutrition 2010, 26, 175–183. [Google Scholar]
- Duan, X.J.; Zhang, W.W.; Li, X.M.; Wang, B.G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006, 95, 37–43. [Google Scholar]
- Michael, A.; Kyewalyanga, M.S.; Mtolera, M.S.; Lugomela, C.V. Antioxidants activity of the cyanobacterium, Arthrospira (Spirulina) fusiformis cultivated in a low- cost medium. Afr. J. Food Sci. 2018, 12, 188–195. [Google Scholar]
- Xia, A.; Murphy, J.D. Microalgal Cultivation in Treating Liquid Digestate from Biogas Systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar]
- Ahmed, F.; Fanning, K.; Netzel, M.; Turner, W.; Li, Y.; Schenk, P.M. Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem. 2014, 165, 300–306. [Google Scholar]
- Goiris, K.; Van Colen, W.; Wilches, I.; León-Tamariz, F.; De Cooman, L.; Muylaert, K. Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res. 2015, 7, 51–57. [Google Scholar]
- Maadane, A.; Merghoub, N.; Ainane, T.; El Arroussi, H.; Benhima, R.; Amzazi, S.; Bakri, Y.; Wahby, I. Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J. Biotechnol. 2015, 215, 13–19. [Google Scholar]
- Allen, S. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK, 1989; pp. 46–60. [Google Scholar]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar]
- Serra Bonvehi, J.; Soliva Torrentó, M.; Centelles Lorente, E. Evaluation of polyphenolic and flavonoid compounds in honeybee-collected pollen produced in Spain. J. Agric. Food Chem. 2001, 49, 1848–1853. [Google Scholar]
- Nagata, M.; Yamashita, I. Method Tomato Masayasu * National NAGATA * and Ichiji YAMASHITA * of Vegetables rnamental Plants and Tea, Ministry of Agriculture, Forestry and Fisheries. Forestry 1992, 39, 1–4. [Google Scholar]
- Masuda, T.; Yonemori, S.; Oyama, Y.; Takeda, Y.; Tanaka, T. Evaluation of the Antioxidant Activity of Environmental Plants: Activity of the leaf extracts from seashore plants. J. Agric. Food Chem. 1999, 150, 1749–1754. [Google Scholar]
- Marxen, K.; Vanselow, K.H.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U. Determination of DPPH Radical Oxidation Caused by Methanolic Extracts of Some Microalgal Species by Linear Regression Analysis of Spectrophotometric Measurements. Sensors 2007, 7, 2080–2095. [Google Scholar]
- Hayouni, E.A.; Abedrabba, M.; Bouix, M.; Hamdi, M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 2007, 105, 1126–1134. [Google Scholar]
- López, A.; Rico, M.; Rivero, A.; Suárez de Tangil, M. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar]
- Agregán, R.; Lorenzo, J.M.; Munekata, P.E.S.; Dominguez, R.; Carballo, J.; Franco, D. Assessment of the antioxidant activity of Bifurcaria bifurcata aqueous extract on canola oil. Effect of extract concentration on the oxidation stability and volatile compound generation during oil storage. Food Res. Int. 2017, 99, 1095–1102. [Google Scholar]
- Agregán, R.; Munekata, P.; Franco, D.; Carballo, J.; Barba, F.; Lorenzo, J. Antioxidant Potential of Extracts Obtained from Macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and Micro-Algae (Chlorella vulgaris and Spirulina platensis) Assisted by Ultrasound. Medicines 2018, 5, 33. [Google Scholar]
- Maisuthisakul, P.; Suttajit, M.; Pongsawatmanit, R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007, 100, 1409–1418. [Google Scholar]
- Hajimahmoodi, M.; Faramarzi, M.A.; Mohammadi, N.; Soltani, N.; Oveisi, M.R.; Nafissi-Varcheh, N. Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J. Appl. Phycol. 2010, 22, 43–50. [Google Scholar]
- Zakaria, S.M.; Kamal, S.M.M.; Harun, M.R.; Omar, R.; Siajam, S.I. Subcritical water technology for extraction of phenolic compounds from Chlorella sp. microalgae and assessment on its antioxidant activity. Molecules 2017, 22, 1105. [Google Scholar] [CrossRef] [Green Version]
- Connan, S.; Stengel, D.B. Impacts of ambient salinity and copper on brown algae: 2. Interactive effects on phenolic pool and assessment of metal binding capacity of phlorotannin. Aquat. Toxicol. 2011, 104, 1–13. [Google Scholar]
- Wijesekara, I.; Kim, S.K.; Li, Y.; Li, Y.X. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [PubMed]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [PubMed]
- Li, H.B.; Cheng, K.W.; Wong, C.C.; Fan, K.W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar]
- Tirado, J.O.; Flores, F.; Maldonado, S.; Mihae, R.; Naranjo, B.; Muñoz, D.; Manjunatha, B.; Selvanayagam, M.; Rajeswari, B. Molecular characterization and antioxidant potential of Andean chlorophytes from Ecuador. J. Appl. Pharm. Sci. 2017, 7, 56–60. [Google Scholar]
- Stahl, W.; Sies, H. Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Mol. Biotechnol. 2007, 37, 26–30. [Google Scholar]
- Borowitzka, M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar]
- Amengual, J.; Gouranton, E.; van Helden, Y.G.J.; Hessel, S.; Ribot, J.; Kramer, E.; Kiec-Wilk, B.; Razny, U.; Lietz, G.; Wyss, A.; et al. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS ONE 2011, 6, e20644. [Google Scholar] [CrossRef]
- Von Lintig, J.; Hessel, S.; Isken, A.; Kiefer, C.; Lampert, J.M.; Voolstra, O.; Vogt, K. Towards a better understanding of carotenoid metabolism in animals. Biochim. Biophys. Acta-Mol. Basis Dis. 2005, 1740, 122–131. [Google Scholar]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and Beta-carotene Induce Cell-Cycle Arrest and Apoptosis in Human Breast Cancer Cell Lines. Anticancer Res. 2014, 34, 1377–1386. [Google Scholar]
- Giovannucci, E.; Rimm, E.B.; Wolk, A.; Ascherio, A.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Calcium and fructose intake in relation to risk of prostate cancer. Cancer Res. 1998, 58, 442–447. [Google Scholar] [PubMed]
- Schwarz, S.; Obermüller-Jevic, U.C.; Hellmis, E.; Koch, W.; Jacobi, G.; Biesalski, H.K. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. J. Nutr. 2008, 138, 49–53. [Google Scholar] [PubMed]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar]
- Zhong, K.; Wang, Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr. Polym. 2010, 80, 19–25. [Google Scholar]
- Mohanasundari, L.; Suja, S. Antioxidant and free radical scavenging activity of the mixture of ethanolic extracts of alpinia speciosa and alpinia calcarata rhizome. Int. J. Pharm. Pharm. Sci. 2016, 8, 164–170. [Google Scholar]
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT-Food Sci. Technol. 2008, 41, 1067–1072. [Google Scholar]
- Custódio, L.; Justo, T.; Silvestre, L.; Barradas, A.; Duarte, C.V.; Pereira, H.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem. 2012, 131, 134–140. [Google Scholar]

| Parameter | Concentration (mg/L) | ||
|---|---|---|---|
| BBM | BCM | ANM | |
| NO3− | 45.591 | 5.707 ± 0.007 | 28.757 ± 0.227 |
| NH4+ | - | 2.942 ± 0.026 | 45.722 ± 0.028 |
| P | 13.29 | 9.086 ± 0.043 | 11.465 ± 0.012 |
| K+ | 20.987 | 315 ± 0.732 | 350.6 ± 1.0263 |
| Na+ | 20.579 | 27.5 ± 0.577 | 294.467 ± 6.389 |
| Ca2+ | 1.703 | 154.133 ± 3.287 | 392.333 ± 0.635 |
| Zn2+ | 0.008 | 70.917 ± 2.321 | 51.067 ± 0.133 |
| Mn+ | 0.002 | 15.15 ± 0.465 | 4.487 ± 0.134 |
| Mg2+ | 1.84 | 168.617 ± 2.093 | 270.8 ± 1.155 |
| Cu2+ | 0.002 | 13.317 ± 0.3 | 4.747 ± 0.237 |
| Fe2+ | 0.183 | 42.133 ± 0.765 | 53.333 ± 0.067 |
| Co2+ | 0.0004 | - | - |
| B3+ | 0.199 | - | - |
| Mo4+ | 0.002 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mtaki, K.; Kyewalyanga, M.S.; Mtolera, M.S.P. Assessment of Antioxidant Contents and Free Radical-Scavenging Capacity of Chlorella vulgaris Cultivated in Low Cost Media. Appl. Sci. 2020, 10, 8611. https://doi.org/10.3390/app10238611
Mtaki K, Kyewalyanga MS, Mtolera MSP. Assessment of Antioxidant Contents and Free Radical-Scavenging Capacity of Chlorella vulgaris Cultivated in Low Cost Media. Applied Sciences. 2020; 10(23):8611. https://doi.org/10.3390/app10238611
Chicago/Turabian StyleMtaki, Kulwa, Margareth S. Kyewalyanga, and Matern S. P. Mtolera. 2020. "Assessment of Antioxidant Contents and Free Radical-Scavenging Capacity of Chlorella vulgaris Cultivated in Low Cost Media" Applied Sciences 10, no. 23: 8611. https://doi.org/10.3390/app10238611
APA StyleMtaki, K., Kyewalyanga, M. S., & Mtolera, M. S. P. (2020). Assessment of Antioxidant Contents and Free Radical-Scavenging Capacity of Chlorella vulgaris Cultivated in Low Cost Media. Applied Sciences, 10(23), 8611. https://doi.org/10.3390/app10238611





