Polyphenols Extracted from Chinese Hickory (Carya cathayensis) Promote Apoptosis and Inhibit Proliferation through the p53-Dependent Intrinsic and HIF-1α-VEGF Pathways in Ovarian Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Preparation of CHSP
2.3. Determination of Total Phenolic Content and Total Flavonoid Content
2.4. Assessment of Cell Viability
2.5. Apoptosis Analysis
2.6. Detection of Caspase-3/7 Enzyme Activities
2.7. Western Blot
2.8. ELISA for VEGF
2.9. Transient Transfection and Luciferase Assay
2.10. Statistical Analysis
3. Results
3.1. Determination of the Total Phenolic Content and Total Flavonoid Content of CHSP
3.2. Inhibitory Effect of CHSP on Proliferation of Ovarian Cancer Cells
3.3. CHSP Induces Apoptosis in Ovarian Cancer Cells through the p53-Dependent Intrinsic Pathway
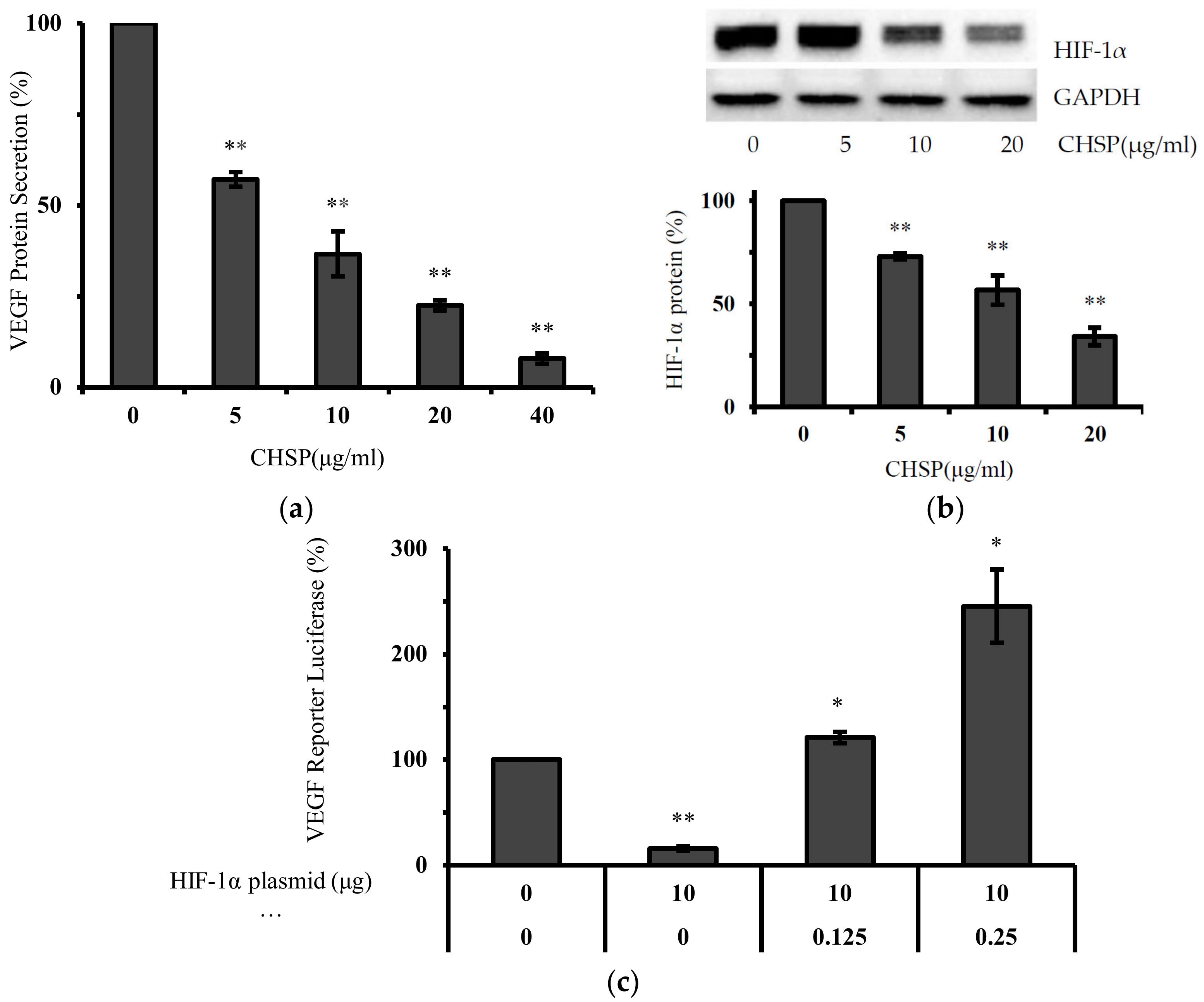
3.4. CHSP Inhibits Ovarian Cancer Cell Proliferation via the HIF-1α/VEGF Pathway
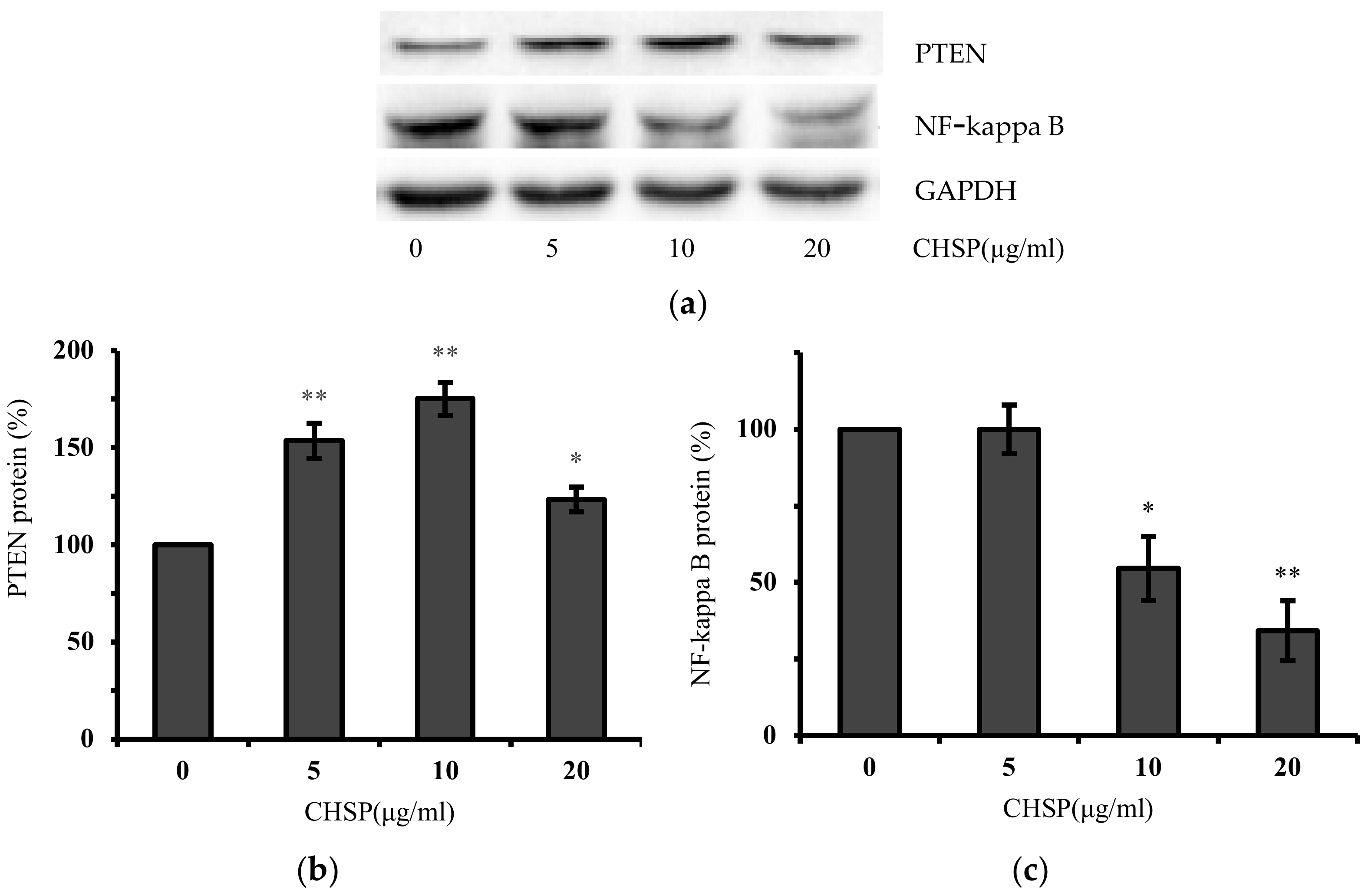
3.5. CHSP Up-Regulates the Expression of PTEN and NF-Kappa B Protein in Ovarian Cancer Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Trinidad, C.V.; Tetlow, A.L.; Bantis, L.E.; Godwin, A.K. Reducing ovarian cancer mortality through early detection: Approaches using circulating biomarkers. Cancer Prev. Res. (Phila.) 2020, 13, 241–252. [Google Scholar] [CrossRef]
- Kim, J.; Park, E.Y.; Kim, O.; Schilder, J.M.; Coffey, D.M.; Cho, C.H.; Bast, R.C., Jr. Cell origins of high-grade serous ovarian cancer. Cancers (Basel) 2018, 10, 433. [Google Scholar] [CrossRef]
- Lavoué, V.; Thédrez, A.; Levêque, J.; Foucher, F.; Henno., S.; Jauffret, V.; Belaud-Rotureau, M.-A.; Catros, V.; Cabillic, F. Immunity of human epithelial ovarian carcinoma: The paradigm of immune suppression in cancer. J. Transl. Med. 2013, 11, 147–158. [Google Scholar]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef]
- Amba, V.; Murphy, G.; Etemadi, A.; Wang, S.; Abnet, C.C.; Hashemian, M. Nut and Peanut Butter Consumption and Mortality in the National Institutes of Health-AARP Diet and Health Study. Nutrients 2019, 11, 1508. [Google Scholar] [CrossRef]
- Hardman, W.E.; Primerano, D.A.; Legenza, M.T.; Morgan, J.; Fan, J.; Denvir, J. Dietary walnut altered gene expressions related to tumor growth, survival, and metastasis in breast cancer patients: A pilot clinical trial. Nutr. Res. 2019, 66, 82–94. [Google Scholar] [CrossRef]
- Gu, H.-F.; Mao, X.-Y.; Du, M. Prevention of breast cancer by dietary polyphenols-role of cancer stem cells. Crit. Rev. Food Sci. Nutr. 2020, 60, 810–825. [Google Scholar] [CrossRef]
- Shin, P.K.; Zoh, Y.; Choi, J.; Kim, M.S.; Kim, Y.; Choi, S.W. Walnut phenolic extracts reduce telomere length and telomerase activity in a colon cancer stem cell model. Nutr. Res. Pract. 2019, 13, 58–63. [Google Scholar] [CrossRef]
- Hilbig, J.; Policarpi, P.d.B.; Alves de Souza Grinevicius, V.M.; Ramos Santos Mota, N.S.; Toaldo, I.M.; Bordignon Luiz, M.T.; Pedrosa, R.C.; Block, J.M. Aqueous extract from pecan nut Carya illinoinensis (Wangenh) C. Koch shell show activity against breast cancer cell line MCF-7 and Ehrlich ascites tumor in Balb-C mice. J. Ethnopharmacol. 2018, 211, 256–266. [Google Scholar] [CrossRef]
- Koutsoulas, A.; Carnecka, M.; Slanina, J.; Toth, J.; Slaninova, I. Characterization of Phenolic Compounds and Antiproliferative Effects of Salvia pomifera and Salvia fruticosa Extracts. Molecules 2019, 24, 2921. [Google Scholar] [CrossRef]
- He, Z.P.; Fu, M.R.; Mao, L.C. Changes of Phenolics, Condensed Tannins and Antioxidant Activity of Chinese Hickory (Carya cathayensis Sarg.) after Different Thermal Processing. Asian J. Chem. 2012, 24, 1685–1688. [Google Scholar]
- Krol, K.; Gantner, M.; Piotrowska, A.; Hallmann, E. Effect of Climate and Roasting on Polyphenols and Tocopherols in the Kernels and Skin of Six Hazelnut Cultivars (Corylus avellana L.). Agriculture 2020, 10, 36. [Google Scholar] [CrossRef]
- Leon-Bejarano, M.; Durmus, Y.; Ovando-Martinez, M.; Simsek, S. Physical, Barrier, Mechanical, and Biodegradability Properties of Modified Starch Films with Nut By-Products Extracts. Foods 2020, 9, 226. [Google Scholar] [CrossRef]
- Tas, N.G.; Gokmen, V. Phenolic compounds in natural and roasted nuts and their skins: A brief review. Curr. Opin. Food Sci. 2017, 14, 103–109. [Google Scholar] [CrossRef]
- Mocanu, M.-M.; Nagy, P.; Szollosi, J. Chemoprevention of Breast Cancer by Dietary Polyphenols. Molecules 2015, 20, 22578–22620. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Z.; Lu, D.; Huang, J.; Liu, J.; Hong, L. Paeonol induces cytoprotective autophagy via blocking the Akt/mTOR pathway in ovarian cancer cells. Cell Death Dis. 2019, 10, 609–621. [Google Scholar] [CrossRef]
- Lee, D.; Sung, K.K.; Lee, S.; Ju, C.E.U.N.; Kim, H.Y. Cytotoxic Effects of Strawberry, Korean Raspberry, and Mulberry Extracts on Human Ovarian Cancer A2780 Cells. Preventive Nutr. Food Sci. 2016, 21, 384–388. [Google Scholar] [CrossRef][Green Version]
- Luo, X.; Li, Z.; Zhao, J.; Deng, Y.; Zhong, Y.; Zhang, M. Fyn gene silencing reduces oligodendrocytes apoptosis through inhibiting ERK1/2 phosphorylation in epilepsy. Artif. Cells Nanomed. Biotechnol. 2020, 48, 298–304. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Qin, Q.; Jiang, Y.; Yang, G.; Rao, K.; Wang, Q.; Xiong, W.; Yuan, J. Mono-2-ethylhexyl phthalate induced loss of mitochondrial membrane potential and activation of Caspase3 in HepG2 cells. Environ. Toxicol. Pharmacol. 2012, 33, 421–430. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Tang, Z.-H.; Jiang, L.; Li, X.-F.; Jiang, Z.-S.; Liu, L.-S. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol. Cell. Biochem. 2012, 359, 347–358. [Google Scholar] [CrossRef]
- Poschner, S.; Maier-Salamon, A.; Thalhammer, T.; Jaeger, W. Resveratrol and other dietary polyphenols are inhibitors of estrogen metabolism in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2019, 190, 11–18. [Google Scholar] [CrossRef]
- Fu, Y.; Ye, X.; Lee, M.; Rankin, G.; Chen, Y.C. Prodelphinidins isolated from Chinese bayberry leaves induces apoptosis via the p53-dependent signaling pathways in OVCAR-3 human ovarian cancer cells. Oncol. Lett. 2017, 13, 3210–3218. [Google Scholar] [CrossRef]
- Pan, H.; Kim, E.; Rankin, G.O.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. Theaflavin-3, 3’-digallate inhibits ovarian cancer stem cells via suppressing Wnt/beta-Catenin signaling pathway. J. Funct. Foods 2018, 50, 1–7. [Google Scholar] [CrossRef]
- Huang, H.; Chen, A.Y.; Ye, X.; Guan, R.; Rankin, G.O.; Chen, Y.C. Galangin, a Flavonoid from Lesser Galangal, Induced Apoptosis via p53-Dependent Pathway in Ovarian Cancer Cells. Molecules 2020, 25, 1579. [Google Scholar] [CrossRef]
- Hefler, L.A.; Mustea, A.; Konsgen, D.; Concin, N.; Tanner, B.; Strick, R.; Heinze, G.; Grimm, C.; Schuster, E.; Tempfer, C. Vascular endothelial growth factor gene polymorphisms are associated with prognosis in ovarian cancer. Clin. Cancer Res. 2007, 13, 898–901. [Google Scholar] [CrossRef]
- Yamamizu, K.; Furuta, S.; Hamada, Y.; Yamashita, A.; Kuzumaki, N.; Narita, M.; Doi, K.; Katayama, S.; Nagase, H.; Yamashita, J.K. kappa Opioids inhibit tumor angiogenesis by suppressing VEGF signaling. Sci. Rep. 2013, 3, 3213–3220. [Google Scholar] [CrossRef]
- Dzhalilova, D.S.; Diatroptov, M.E.; Tsvetkov, I.S.; Makarova, O.V.; Kuznetsov, S.L. Expression of Hif-1 alpha, Nf-kappa b, and Vegf Genes in the Liver and Blood Serum Levels of HIF-1 alpha, Erythropoietin, VEGF, TGF-beta, 8-Isoprostane, and Corticosterone in Wistar Rats with High and Low Resistance to Hypoxia. Bull. Exp. Biol. Med. 2018, 165, 781–785. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.-H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of Vascular Endothelial Growth Factor Gene Transcription by Hypoxia-Inducible Factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Jin, C.E.; Kim, G.J.; Lee, T.H.; Jeonghyun, L. Genipin inhibits hypoxia-induced accumulation of HIF-1α and VEGF expression in human cervical carcinoma HeLa cells. Kosin Med. J. 2019, 34, 106–116. [Google Scholar] [CrossRef][Green Version]
- He, Z.; Chen, A.Y.; Rojanasakul, Y.; Rankin, G.O.; Chen, Y.C. Gallic acid, a phenolic compound, exerts anti-angiogenic effects via the PTEN/AKT/HIF-1alpha/VEGF signaling pathway in ovarian cancer cells. Oncol. Rep. 2016, 35, 291–297. [Google Scholar] [CrossRef]
- Rais, J.; Jafri., A.; Siddiqui., S.; Tripathi., M.; Arshad., M. Phytochemicals in the treatment of ovarian cancer. Front. Biosci. 2017, 9, 67–75. [Google Scholar] [CrossRef]
- Kwon, Y. Food-derived polyphenols inhibit the growth of ovarian cancer cells irrespective of their ability to induce antioxidant responses. Heliyon 2018, 4, e00753. [Google Scholar] [CrossRef]
- He, Z.; Li, B.; Rankin, G.O.; Rojanasakul, Y.; Chen, Y.C. Selecting bioactive phenolic compounds as potential agents to inhibit proliferation and VEGF expression in human ovarian cancer cells. Oncol. Lett. 2015, 9, 1444–1450. [Google Scholar] [CrossRef]
- Varela-Rodriguez, L.; Sanchez-Ramirez, B.; Ivonne Hernandez-Ramirez, V.; Varela-Rodriguez, H.; Daniel Castellanos-Mijangos, R.; Gonzalez-Horta, C.; Chavez-Munguia, B.; Talamas-Rohana, P. Effect of Gallic acid and Myricetin on ovarian cancer models: A possible alternative antitumoral treatment. BMC Complement. Med. Ther. 2020, 20, 660–685. [Google Scholar] [CrossRef]
- Liao, C.-C.; Chen, S.-C.; Huang, H.-P.; Wang, C.-J. Gallic acid inhibits bladder cancer cell proliferation and migration via regulating fatty acid synthase (FAS). J. Food Drug Anal. 2018, 26, 620–627. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, L.; Wu, P.; Li, W.; Li, T.; Gu, R.; Dan, X.; Li, Z.; Fan, X.; Xiao, Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in nonsmall cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol. Rep. 2019, 41, 1779–1788. [Google Scholar]
- Li, M.F.; Guan, H.; Zhang, D.D. Effect of overexpression of PTEN on apoptosis of liver cancer cells. Genet. Mol. Res. 2016, 15, 1–9. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, R.; Yan, Y.; Zhang, Z.; Ye, Y.; Liu, X. miR-142 promotes human ovarian granulosa cell growth by targeting PTEN. Int. J. Clin. Exp. Med. 2020, 13, 2044–2051. [Google Scholar]
- Xue, L.; Huang, J.; Zhang, T.; Wang, X.; Fu, J.; Geng, Z.; Zhao, Y.; Chen, H. PTEN inhibition enhances angiogenesis in an in vitro model of ischemic injury by promoting Akt phosphorylation and subsequent hypoxia inducible factor-1alpha upregulation. Metab. Brain Dis. 2018, 33, 1679–1688. [Google Scholar] [CrossRef]
- Patricia Martinez, G.; Rodney Mijares, M.; Chavez, K.; Isabel Suarez, A.; Santi Compagnone, R.; Chirinos, P.; Bautista De Sanctis, J. Caracasine acid, an ent-3,4-seco-kaurene, promotes apoptosis and cell differentiation through NFkB signal pathway inhibition in leukemia cells. Eur. J. Pharmacol. 2019, 862, 172624. [Google Scholar] [CrossRef]
- Yang, C.; Feng, X.; Li, Z.; He, Q. Plumbagin inhibits tumor angiogenesis of gastric carcinoma in mice by modulating nuclear factor-kappa B pathway. Transl. Cancer Res. 2020, 9, 556–564. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Wu, S.; Lin, J.; Booth, A.; Rankin, G.O.; Martinez, I.; Chen, Y.C. Polyphenols Extracted from Chinese Hickory (Carya cathayensis) Promote Apoptosis and Inhibit Proliferation through the p53-Dependent Intrinsic and HIF-1α-VEGF Pathways in Ovarian Cancer Cells. Appl. Sci. 2020, 10, 8615. https://doi.org/10.3390/app10238615
He Z, Wu S, Lin J, Booth A, Rankin GO, Martinez I, Chen YC. Polyphenols Extracted from Chinese Hickory (Carya cathayensis) Promote Apoptosis and Inhibit Proliferation through the p53-Dependent Intrinsic and HIF-1α-VEGF Pathways in Ovarian Cancer Cells. Applied Sciences. 2020; 10(23):8615. https://doi.org/10.3390/app10238615
Chicago/Turabian StyleHe, Zhiping, Shaozhen Wu, Ju Lin, Ashley Booth, Gary O’Neal Rankin, Ivan Martinez, and Yi Charlie Chen. 2020. "Polyphenols Extracted from Chinese Hickory (Carya cathayensis) Promote Apoptosis and Inhibit Proliferation through the p53-Dependent Intrinsic and HIF-1α-VEGF Pathways in Ovarian Cancer Cells" Applied Sciences 10, no. 23: 8615. https://doi.org/10.3390/app10238615
APA StyleHe, Z., Wu, S., Lin, J., Booth, A., Rankin, G. O., Martinez, I., & Chen, Y. C. (2020). Polyphenols Extracted from Chinese Hickory (Carya cathayensis) Promote Apoptosis and Inhibit Proliferation through the p53-Dependent Intrinsic and HIF-1α-VEGF Pathways in Ovarian Cancer Cells. Applied Sciences, 10(23), 8615. https://doi.org/10.3390/app10238615








