Pluripotent Stem Cells for Transgenesis in the Rabbit: A Utopia?
Abstract
:Featured Application
Abstract
1. Introduction
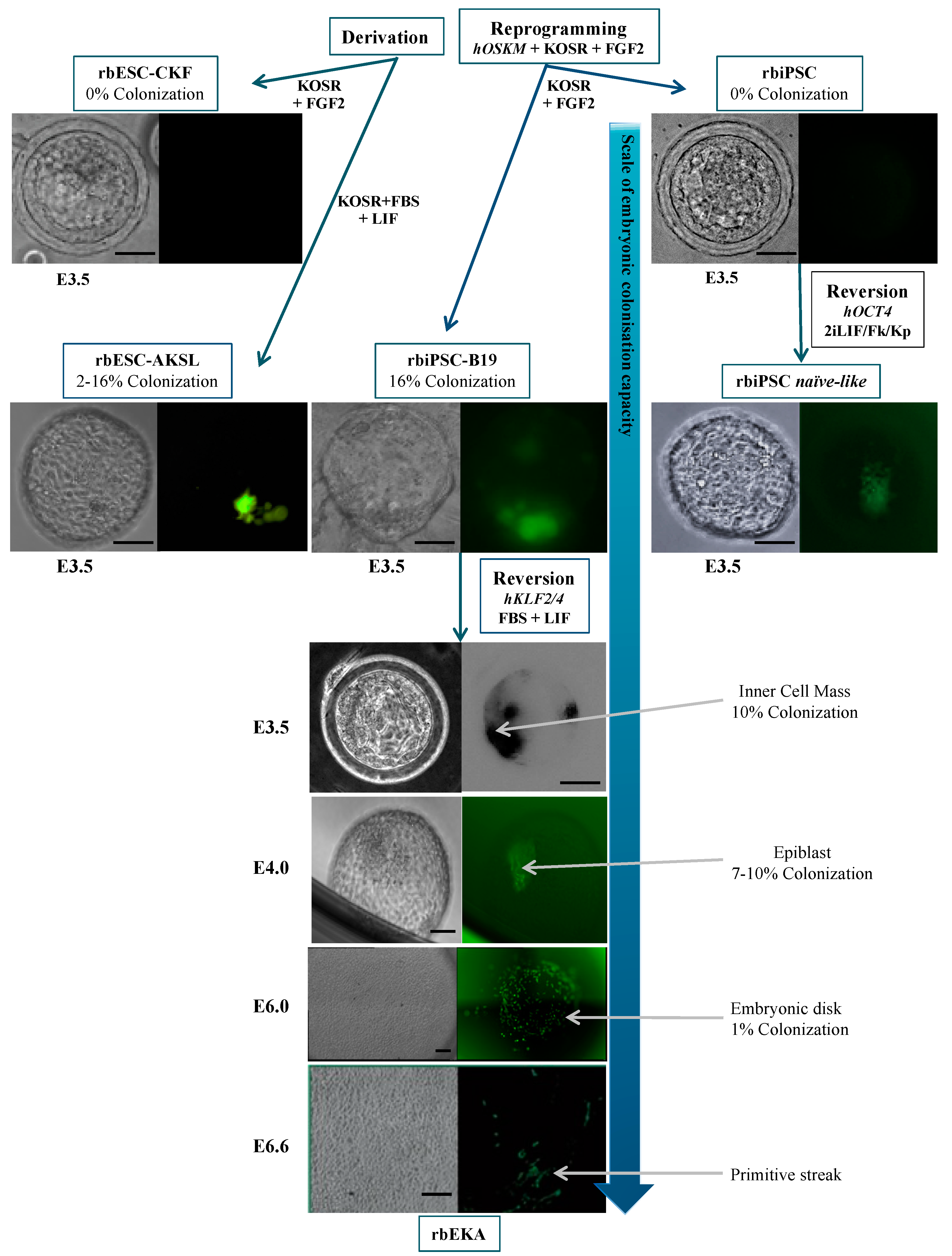
2. Rabbit Pluripotent Stem Cells (rbPSCs)
2.1. Rabbit Embryonic Stem Cells (rbESCs)
2.2. Rabbit-Induced Pluripotent Stem Cells (rbiPSCs)
2.3. Pluripotent State of rbPSCs
2.4. Reprogramming of rbPSCs toward the Naive State of Pluripotency
3. Rabbit Chimeras
3.1. Definition and Benefits of Chimeras
3.2. Techniques Used in Mice
3.3. Chimeras Produced in Rabbit
3.4. Colonization and Chimerism
4. Barriers to Overcome
4.1. The Pluripotency Barrier
4.2. The Developmental Barrier
4.3. The Apoptotic Barrier
4.4. The Cell Division Barrier
4.5. The Epigenetic Barrier
4.6. The Adhesion Barrier
4.7. The Metabolic Barrier
4.8. The Germinal Competency and the Euploid Karyotype
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, A.; Evans, M.; Kaufman, M.H.; Robertson, E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 1984, 309, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Brons, I.G.; Smithers, L.E.; Trotter, M.W.; Rugg-Gunn, P.; Sun, B.; Chuva de Sousa Lopes, S.M.; Howlett, S.K.; Clarkson, A.; Ahrlund-Richter, L.; Pedersen, R.A.; et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 2007, 448, 191–195. [Google Scholar] [CrossRef]
- Tesar, P.J.; Chenoweth, J.G.; Brook, F.A.; Davies, T.J.; Evans, E.P.; Mack, D.L.; Gardner, R.L.; McKay, R.D. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007, 448, 196–199. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Bar-Nur, O.; Brumbaugh, J.; Verheul, C.; Apostolou, E.; Pruteanu-Malinici, I.; Walsh, R.M.; Ramaswamy, S.; Hochedlinger, K. Small molecules facilitate rapid and synchronous iPSC generation. Nat. Methods 2014, 11, 1170–1176. [Google Scholar] [CrossRef] [Green Version]
- Buganim, Y.; Markoulaki, S.; van Wietmarschen, N.; Hoke, H.; Wu, T.; Ganz, K.; Akhtar-Zaidi, B.; He, Y.; Abraham, B.J.; Porubsky, D.; et al. The developmental potential of iPSCs is greatly influenced by reprogramming factor selection. Cell Stem Cell 2014, 15, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Hassani, S.N.; Moradi, S.; Taleahmad, S.; Braun, T.; Baharvand, H. Transition of inner cell mass to embryonic stem cells: Mechanisms, facts, and hypotheses. Cell Mol. Life Sci. 2019, 76, 873–892. [Google Scholar] [CrossRef]
- Soto, D.A.; Ross, P.J. Pluripotent stem cells and livestock genetic engineering. Transgenic Res. 2016, 25, 289–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Zhu, J.; Salman, S.; Tang, Y. The Induced Pluripotent Stem Cells from Farm Animals. J. Anim. Sci. 2020. [Google Scholar] [CrossRef]
- Savatier, P.; Osteil, P.; Tam, P.P. Pluripotency of embryo-derived stem cells from rodents, lagomorphs, and primates: Slippery slope, terrace and cliff. Stem Cell Res. 2017, 19, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yang, M.; Guo, H.; Yang, L.; Wu, J.; Li, R.; Liu, P.; Lian, Y.; Zheng, X.; Yan, J.; et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat. Struct. Mol. Biol. 2013, 20, 1131–1139. [Google Scholar] [CrossRef]
- Nakamura, T.; Yabuta, Y.; Okamoto, I.; Sasaki, K.; Iwatani, C.; Tsuchiya, H.; Saitou, M. Single-cell transcriptome of early embryos and cultured embryonic stem cells of cynomolgus monkeys. Sci. Data 2017, 4, 170067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, R.J.; Narva, E.; Lahesmaa, R. Genetic and epigenetic stability of human pluripotent stem cells. Nat. Rev. Genet. 2012, 13, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.E.; Loring, J.F. Genomic instability in pluripotent stem cells: Implications for clinical applications. J. Biol. Chem. 2014, 289, 4578–4584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.M.; Hochedlinger, K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat. Cell Biol. 2011, 13, 497–505. [Google Scholar] [CrossRef]
- Zeltner, N.; Studer, L. Pluripotent stem cell-based disease modeling: Current hurdles and future promise. Curr. Opin. Cell Biol. 2015, 37, 102–110. [Google Scholar] [CrossRef]
- Guo, G.; Yang, J.; Nichols, J.; Hall, J.S.; Eyres, I.; Mansfield, W.; Smith, A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 2009, 136, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Nichols, J.; Theunissen, T.W.; Guo, G.; van Oosten, A.L.; Barrandon, O.; Wray, J.; Yamanaka, S.; Chambers, I.; Smith, A. Nanog is the gateway to the pluripotent ground state. Cell 2009, 138, 722–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; van Oosten, A.L.; Theunissen, T.W.; Guo, G.; Silva, J.C.; Smith, A. Stat3 activation is limiting for reprogramming to ground state pluripotency. Cell Stem Cell 2010, 7, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, D.; Ye, S.; Ruiz, B.; Zhou, X.; Liu, D.; Zhang, Q.; Ying, Q.L. Klf2 and Tfcp2l1, Two Wnt/beta-Catenin Targets, Act Synergistically to Induce and Maintain Naive Pluripotency. Stem Cell Rep. 2015, 5, 314–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okashita, N.; Suwa, Y.; Nishimura, O.; Sakashita, N.; Kadota, M.; Nagamatsu, G.; Kawaguchi, M.; Kashida, H.; Nakajima, A.; Tachibana, M.; et al. PRDM14 Drives OCT3/4 Recruitment via Active Demethylation in the Transition from Primed to Naive Pluripotency. Stem Cell Rep. 2016, 7, 1072–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukiyama, T.; Ohinata, Y. A modified EpiSC culture condition containing a GSK3 inhibitor can support germline-competent pluripotency in mice. PLoS ONE 2014, 9, e95329. [Google Scholar] [CrossRef] [PubMed]
- Illich, D.J.; Zhang, M.; Ursu, A.; Osorno, R.; Kim, K.P.; Yoon, J.; Arauzo-Bravo, M.J.; Wu, G.; Esch, D.; Sabour, D.; et al. Distinct Signaling Requirements for the Establishment of ESC Pluripotency in Late-Stage EpiSCs. Cell Rep. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, J.; Cheng, A.W.; Saha, K.; Kim, J.; Lengner, C.J.; Soldner, F.; Cassady, J.P.; Muffat, J.; Carey, B.W.; Jaenisch, R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. USA 2010, 107, 9222–9227. [Google Scholar] [CrossRef] [Green Version]
- Theunissen, T.W.; Jaenisch, R. Molecular control of induced pluripotency. Cell Stem Cell 2014, 14, 720–734. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Aksoy, I.; Gonnot, F.; Osteil, P.; Aubry, M.; Hamela, C.; Rognard, C.; Hochard, A.; Voisin, S.; Fontaine, E.; et al. Reinforcement of STAT3 activity reprogrammes human embryonic stem cells to naive-like pluripotency. Nat. Commun. 2015, 6, 7095. [Google Scholar] [CrossRef] [Green Version]
- Guo, G.; von Meyenn, F.; Rostovskaya, M.; Clarke, J.; Dietmann, S.; Baker, D.; Sahakyan, A.; Myers, S.; Bertone, P.; Reik, W.; et al. Epigenetic resetting of human pluripotency. Development 2017, 144, 2748–2763. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Okamura, D.; Li, M.; Suzuki, K.; Luo, C.; Ma, L.; He, Y.; Li, Z.; Benner, C.; Tamura, I.; et al. An alternative pluripotent state confers interspecies chimaeric competency. Nature 2015, 521, 316–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, H.; Kato-Itoh, M.; Takahashi, Y.; Umino, A.; Sato, H.; Ito, K.; Yanagida, A.; Nishimura, T.; Yamaguchi, T.; Hirabayashi, M.; et al. Inhibition of Apoptosis Overcomes Stage-Related Compatibility Barriers to Chimeras Formation in Mouse Embryos. Cell Stem Cell 2016, 19, 587–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afanassieff, M.; Aksoy, I.; Beaujean, N.; Bourillot, P.Y.; Savatier, P. Fifty shades of pluripotency. Med. Sci. (Paris) 2018, 34, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Madeja, Z.E.; Pawlak, P.; Piliszek, A. Beyond the mouse: Non-rodent animal models for study of early mammalian development and biomedical research. Int. J. Dev. Biol. 2019, 63, 187–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leandri, R.D.; Archilla, C.; Bui, L.C.; Peynot, N.; Liu, Z.; Cabau, C.; Chastellier, A.; Renard, J.P.; Duranthon, V. Revealing the dynamics of gene expression during embryonic genome activation and first differentiation in the rabbit embryo with a dedicated array screening. Physiol. Genom. 2009, 36, 98–113. [Google Scholar] [CrossRef]
- Reis Silva, A.R.; Adenot, P.; Daniel, N.; Archilla, C.; Peynot, N.; Lucci, C.M.; Beaujean, N.; Duranthon, V. Dynamics of DNA methylation levels in maternal and paternal rabbit genomes after fertilization. Epigenetics 2011, 6, 987–993. [Google Scholar] [CrossRef] [Green Version]
- Reis e Silva, A.R.; Bruno, C.; Fleurot, R.; Daniel, N.; Archilla, C.; Peynot, N.; Lucci, C.M.; Beaujean, N.; Duranthon, V. Alteration of DNA demethylation dynamics by in vitro culture conditions in rabbit pre-implantation embryos. Epigenetics 2012, 7, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, I.; Patrat, C.; Thepot, D.; Peynot, N.; Fauque, P.; Daniel, N.; Diabangouaya, P.; Wolf, J.P.; Renard, J.P.; Duranthon, V.; et al. Eutherian mammals use diverse strategies to initiate X-chromosome inactivation during development. Nature 2011, 472, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Idkowiak, J.; Weisheit, G.; Plitzner, J.; Viebahn, C. Hypoblast controls mesoderm generation and axial patterning in the gastrulating rabbit embryo. Dev. Genes Evol. 2004, 214, 591–605. [Google Scholar] [CrossRef]
- Nicholls, P.K.; Sun, Z.; Heng, S.; Li, Y.; Wang, J.; Nie, G. Embryo implantation is closely associated with dynamic expression of proprotein convertase 5/6 in the rabbit uterus. Reprod. Biol. Endocrinol. 2011, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Duranthon, V.; Beaujean, N.; Brunner, M.; Odening, K.E.; Santos, A.N.; Kacskovics, I.; Hiripi, L.; Weinstein, E.J.; Bosze, Z. On the emerging role of rabbit as human disease model and the instrumental role of novel transgenic tools. Transgenic Res. 2012, 21, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.R.; Yang, X.; Mark, W.; Foote, R.H. Pluripotency of cultured rabbit inner cell mass cells detected by isozyme analysis and eye pigmentation of fetuses following injection into blastocysts or morulae. Mol. Reprod. Dev. 1993, 36, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Graves, K.H.; Moreadith, R.W. Derivation and characterization of putative pluripotential embryonic stem cells from preimplantation rabbit embryos. Mol. Reprod. Dev. 1993, 36, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, X.; Niu, Y.; Chen, H.; Li, B.; Li, T.; Zhang, X.; Hu, Z.; Zhou, Q.; Ji, W. Generation and characterization of rabbit embryonic stem cells. Stem Cells 2007, 25, 481–489. [Google Scholar] [CrossRef]
- Honda, A.; Hirose, M.; Inoue, K.; Ogonuki, N.; Miki, H.; Shimozawa, N.; Hatori, M.; Shimizu, N.; Murata, T.; Hirose, M.; et al. Stable embryonic stem cell lines in rabbits: Potential small animal models for human research. Reprod. Biomed. Online 2008, 17, 706–715. [Google Scholar] [CrossRef]
- Honda, A.; Hirose, M.; Ogura, A. Basic FGF and Activin/Nodal but not LIF signaling sustain undifferentiated status of rabbit embryonic stem cells. Exp. Cell Res. 2009, 315, 2033–2042. [Google Scholar] [CrossRef]
- Osteil, P.; Tapponnier, Y.; Markossian, S.; Godet, M.; Schmaltz-Panneau, B.; Jouneau, L.; Cabau, C.; Joly, T.; Blachere, T.; Gocza, E.; et al. Induced pluripotent stem cells derived from rabbits exhibit some characteristics of naive pluripotency. Biol. Open 2013, 2, 613–628. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.C.; Intawicha, P.; Lee, K.H.; Chiu, Y.T.; Lo, N.W.; Ju, J.C. LIF and FGF cooperatively support stemness of rabbit embryonic stem cells derived from parthenogenetically activated embryos. Cell Reprogram. 2011, 13, 241–255. [Google Scholar] [CrossRef]
- Lo, N.W.; Intawicha, P.; Chiu, Y.T.; Lee, K.H.; Lu, H.C.; Chen, C.H.; Chang, Y.H.; Chen, C.D.; Ju, J.C. Leukemia inhibitory factor and fibroblast growth factor 2 critically and mutually sustain pluripotency of rabbit embryonic stem cells. Cell Transplant. 2015, 24, 319–338. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Y.; Yuan, X.; Chen, K.; Guo, X.; Chen, Y.; Niu, Y.; Li, J.; Xu, R.H.; Yan, X.; et al. Dissecting signaling pathways that govern self-renewal of rabbit embryonic stem cells. J. Biol. Chem. 2008, 283, 35929–35940. [Google Scholar] [CrossRef] [Green Version]
- Osteil, P.; Moulin, A.; Santamaria, C.; Joly, T.; Jouneau, L.; Aubry, M.; Tapponnier, Y.; Archilla, C.; Schmaltz-Panneau, B.; Lecardonnel, J.; et al. A Panel of Embryonic Stem Cell Lines Reveals the Variety and Dynamic of Pluripotent States in Rabbits. Stem Cell Rep. 2016, 7, 383–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intawicha, P.; Ou, Y.W.; Lo, N.W.; Zhang, S.C.; Chen, Y.Z.; Lin, T.A.; Su, H.L.; Guu, H.F.; Chen, M.J.; Lee, K.H.; et al. Characterization of embryonic stem cell lines derived from New Zealand white rabbit embryos. Cloning Stem Cells 2009, 11, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Catunda, A.P.; Gocza, E.; Carstea, B.V.; Hiripi, L.; Hayes, H.; Rogel-Gaillard, C.; Bertaud, M.; Bosze, Z. Characterization, chromosomal assignment, and role of LIFR in early embryogenesis and stem cell establishment of rabbits. Cloning Stem Cells 2008, 10, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.; Smith, A. The ground state of embryonic stem cell self-renewal. Nature 2008, 453, 519–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bontovics, B.; Maraghechi, P.; Lazar, B.; Anand, M.; Nemeth, K.; Fabian, R.; Vasicek, J.; Makarevich, A.V.; Gocza, E.; Chrenek, P. The effect of dual inhibition of Ras-MEK-ERK and GSK3beta pathways on development of in vitro cultured rabbit embryos. Zygote 2020, 28, 183–190. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Li, J.; Liu, Z.; Liu, Y.; Xue, F.; Yang, L.; An, L.; Chen, C.H.; Presicce, G.A.; et al. Deriving rabbit embryonic stem cells by small molecule inhibitors. Am. J. Transl. Res. 2019, 11, 5122–5133. [Google Scholar] [PubMed]
- Bai, Q.; Ramirez, J.M.; Becker, F.; Pantesco, V.; Lavabre-Bertrand, T.; Hovatta, O.; Lemaitre, J.M.; Pellestor, F.; De Vos, J. Temporal analysis of genome alterations induced by single-cell passaging in human embryonic stem cells. Stem Cells Dev. 2015, 24, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Ohgushi, M.; Sasai, Y. Lonely death dance of human pluripotent stem cells: ROCKing between metastable cell states. Trends Cell Biol. 2011, 21, 274–282. [Google Scholar] [CrossRef]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.; Muguruma, K.; et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef]
- Schmaltz-Panneau, B.; Jouneau, L.; Osteil, P.; Tapponnier, Y.; Afanassieff, M.; Moroldo, M.; Jouneau, A.; Daniel, N.; Archilla, C.; Savatier, P.; et al. Contrasting transcriptome landscapes of rabbit pluripotent stem cells in vitro and in vivo. Anim. Reprod. Sci. 2014, 149, 67–79. [Google Scholar] [CrossRef]
- Honda, A.; Hirose, M.; Hatori, M.; Matoba, S.; Miyoshi, H.; Inoue, K.; Ogura, A. Generation of induced pluripotent stem cells in rabbits: Potential experimental models for human regenerative medicine. J. Biol. Chem. 2010, 285, 31362–31369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tancos, Z.; Nemes, C.; Varga, E.; Bock, I.; Rungarunlert, S.; Tharasanit, T.; Techakumphu, M.; Kobolak, J.; Dinnyes, A. Establishment of a rabbit induced pluripotent stem cell (RbiPSC) line using lentiviral delivery of human pluripotency factors. Stem Cell Res. 2017, 21, 16–18. [Google Scholar] [CrossRef]
- Phakdeedindan, P.; Setthawong, P.; Tiptanavattana, N.; Rungarunlert, S.; Ingrungruanglert, P.; Israsena, N.; Techakumphu, M.; Tharasanit, T. Rabbit induced pluripotent stem cells retain capability of in vitro cardiac differentiation. Exp. Anim. 2019, 68, 35–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Kou, Z.; Wu, T.; An, W.; Zhou, R.; Wang, H.; Gao, Y.; Gao, S. Xist deficiency and disorders of X-inactivation in rabbit embryonic stem cells can be rescued by transcription-factor-mediated conversion. Stem Cells Dev. 2014, 23, 2283–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapponnier, Y.; Afanassieff, M.; Aksoy, I.; Aubry, M.; Moulin, A.; Medjani, L.; Bouchereau, W.; Mayere, C.; Osteil, P.; Nurse-Francis, J.; et al. Reprogramming of rabbit induced pluripotent stem cells toward epiblast and chimeric competency using Kruppel-like factors. Stem Cell Res. 2017, 24, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Sokol, S.Y. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development 2011, 138, 4341–4350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz-Descalzo, S.; Hadjantonakis, A.K.; Arias, A.M. Wnt/ss-catenin signalling and the dynamics of fate decisions in early mouse embryos and embryonic stem (ES) cells. Semin. Cell Dev. Biol. 2015, 47–48, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Jin, Y. Signaling networks in the control of pluripotency. Curr. Opin. Genet. Dev. 2017, 46, 141–148. [Google Scholar] [CrossRef]
- Osman, A.M.; van Dartel, D.A.; Zwart, E.; Blokland, M.; Pennings, J.L.; Piersma, A.H. Proteome profiling of mouse embryonic stem cells to define markers for cell differentiation and embryotoxicity. Reprod. Toxicol. 2010, 30, 322–332. [Google Scholar] [CrossRef]
- Frohlich, T.; Kosters, M.; Graf, A.; Wolf, E.; Kobolak, J.; Brochard, V.; Dinnyes, A.; Jouneau, A.; Arnold, G.J. iTRAQ proteome analysis reflects a progressed differentiation state of epiblast derived versus inner cell mass derived murine embryonic stem cells. J. Proteomics 2013, 90, 38–51. [Google Scholar] [CrossRef]
- Afanassieff, M.; Perold, F.; Bouchereau, W.; Cadiou, A.; Beaujean, N. Embryo-derived and induced pluripotent stem cells: Towards naive pluripotency and chimeric competency in rabbits. Exp. Cell Res. 2020, 389, 111908. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Hatori, M.; Hirose, M.; Honda, C.; Izu, H.; Inoue, K.; Hirasawa, R.; Matoba, S.; Togayachi, S.; Miyoshi, H.; et al. Naive-like conversion overcomes the limited differentiation capacity of induced pluripotent stem cells. J. Biol. Chem. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honsho, K.; Hirose, M.; Hatori, M.; Yasmin, L.; Izu, H.; Matoba, S.; Togayachi, S.; Miyoshi, H.; Sankai, T.; Ogura, A.; et al. Naive-like conversion enhances the difference in innate in vitro differentiation capacity between rabbit ES cells and iPS cells. J. Reprod. Dev. 2015, 61, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankenberg, S.R. Different Species Choose Their Own Paths to Pluripotency. Dev. Cell 2015, 35, 267–268. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.A.; Wert, K.J.; Goldmann, J.; Markoulaki, S.; Buganim, Y.; Fu, D.; Jaenisch, R. Human neural crest cells contribute to coat pigmentation in interspecies chimerass after in utero injection into mouse embryos. Proc. Natl. Acad. Sci. USA 2016, 113, 1570–1575. [Google Scholar] [CrossRef] [Green Version]
- Masaki, H.; Nakauchi, H. Interspecies chimerass for human stem cell research. Development 2017, 144, 2544–2547. [Google Scholar] [CrossRef] [Green Version]
- Kraus, P.; Leong, G.; Tan, V.; Xing, X.; Goh, J.W.; Yap, S.P.; Lufkin, T. A more cost effective and rapid high percentage germ-line transmitting chimeric mouse generation procedure via microinjection of 2-cell, 4-cell, and 8-cell embryos with ES and iPS cells. Genesis 2010, 48, 394–399. [Google Scholar] [CrossRef]
- Nagy, A.; Gocza, E.; Diaz, E.M.; Prideaux, V.R.; Ivanyi, E.; Markkula, M.; Rossant, J. Embryonic stem cells alone are able to support fetal development in the mouse. Development 1990, 110, 815–821. [Google Scholar]
- Eggan, K.; Jaenisch, R. Differentiation of F1 embryonic stem cells into viable male and female mice by tetraploid embryo complementation. Methods Enzymol. 2003, 365, 25–39. [Google Scholar]
- Li, T.D.; Feng, G.H.; Li, Y.F.; Wang, M.; Mao, J.J.; Wang, J.Q.; Li, X.; Wang, X.P.; Qu, B.; Wang, L.Y.; et al. Rat embryonic stem cells produce fertile offspring through tetraploid complementation. Proc. Natl. Acad. Sci. USA 2017, 114, 11974–11979. [Google Scholar] [CrossRef] [Green Version]
- Poueymirou, W.T.; Auerbach, W.; Frendewey, D.; Hickey, J.F.; Escaravage, J.M.; Esau, L.; Dore, A.T.; Stevens, S.; Adams, N.C.; Dominguez, M.G.; et al. F0 generation mice fully derived from gene-targeted embryonic stem cells allowing immediate phenotypic analyses. Nat. Biotechnol. 2007, 25, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Deng, K.; Wu, H.; Liu, Z.; Chen, Z.; Cao, S.; Zhou, L.; Ye, X.; Keefe, D.L.; Liu, L. Efficient production of mice from embryonic stem cells injected into four- or eight-cell embryos by piezo micromanipulation. Stem Cells 2008, 26, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Li, W.; Lv, Z.; Liu, L.; Tong, M.; Hai, T.; Hao, J.; Guo, C.L.; Ma, Q.W.; Wang, L.; et al. iPS cells produce viable mice through tetraploid complementation. Nature 2009, 461, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Osorno, R.; Tsakiridis, A.; Wilson, V. In Vivo differentiation potential of epiblast stem cells revealed by chimeric embryo formation. Cell Rep. 2012, 2, 1571–1578. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Niu, Y.; Li, Y.; Ai, Z.; Kang, Y.; Shi, H.; Xiang, Z.; Yang, Z.; Tan, T.; Si, W.; et al. Generation of Cynomolgus Monkey Chimeric Fetuses using Embryonic Stem Cells. Cell Stem Cell 2015, 17, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Tancos, Z.; Nemes, C.; Polgar, Z.; Gocza, E.; Daniel, N.; Stout, T.A.; Maraghechi, P.; Pirity, M.K.; Osteil, P.; Tapponnier, Y.; et al. Generation of rabbit pluripotent stem cell lines. Theriogenology 2012, 78, 1774–1786. [Google Scholar] [CrossRef]
- Gardner, R.L.; Munro, A.J. Successful construction of chimaeric rabbit. Nature 1974, 250, 146–147. [Google Scholar] [CrossRef]
- Yang, X.Z.; Foote, R.H. Production of chimeric rabbits from morulae by a simple procedure. Gamete Res. 1988, 21, 345–351. [Google Scholar] [CrossRef]
- Chrenek, P.; Makarevich, A.V. Production of rabbit chimeric embryos by aggregation of zona-free nuclear transfer blastomeres. Zygote 2005, 13, 39–44. [Google Scholar] [CrossRef]
- Bodo, S.; Gocza, E.; Revay, T.; Hiripi, L.; Carstea, B.; Kovacs, A.; Bodrogi, L.; Bosze, Z. Production of transgenic chimeric rabbits and transmission of the transgene through the germline. Mol. Reprod. Dev. 2004, 68, 435–440. [Google Scholar] [CrossRef]
- Moens, A.; Betteridge, K.J.; Brunet, A.; Renard, J.P. Low levels of chimerism in rabbit fetuses produced from preimplantation embryos microinjected with fetal gonadal cells. Mol. Reprod. Dev. 1996, 43, 38–46. [Google Scholar] [CrossRef]
- Schoonjans, L.; Albright, G.M.; Li, J.L.; Collen, D.; Moreadith, R.W. Pluripotential rabbit embryonic stem (ES) cells are capable of forming overt coat color chimerass following injection into blastocysts. Mol. Reprod. Dev. 1996, 45, 439–443. [Google Scholar] [CrossRef]
- Fang, Z.F.; Gai, H.; Huang, Y.Z.; Li, S.G.; Chen, X.J.; Shi, J.J.; Wu, L.; Liu, A.; Xu, P.; Sheng, H.Z. Rabbit embryonic stem cell lines derived from fertilized, parthenogenetic or somatic cell nuclear transfer embryos. Exp. Cell Res. 2006, 312, 3669–3682. [Google Scholar] [CrossRef] [PubMed]
- Tam, P.P. Human Stem Cells Can Differentiate in Post-implantation Mouse Embryos. Cell Stem Cell 2016, 18, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, B.; Xu, J.; Wang, J.; Wu, J.; Shi, C.; Xu, Y.; Dong, J.; Wang, C.; Lai, W.; et al. Derivation of Pluripotent Stem Cells with In Vivo Embryonic and Extraembryonic Potency. Cell 2017, 169, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.; Liu, K.; Zhao, Y.; Li, H.; Zhu, D.; Du, Y.; Xiang, C.; Li, X.; Liu, H.; Miao, Z.; et al. Generation of naive induced pluripotent stem cells from rhesus monkey fibroblasts. Cell Stem Cell 2014, 15, 488–497. [Google Scholar] [CrossRef] [Green Version]
- Gafni, O.; Weinberger, L.; Mansour, A.A.; Manor, Y.S.; Chomsky, E.; Ben-Yosef, D.; Kalma, Y.; Viukov, S.; Maza, I.; Zviran, A.; et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013, 504, 282–286. [Google Scholar] [CrossRef]
- Wu, J.; Platero-Luengo, A.; Sakurai, M.; Sugawara, A.; Gil, M.A.; Yamauchi, T.; Suzuki, K.; Bogliotti, Y.S.; Cuello, C.; Morales Valencia, M.; et al. Interspecies Chimerism with Mammalian Pluripotent Stem Cells. Cell 2017, 168, 473–486. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, M.; Sasaki, H. Cell competition controls differentiation in mouse embryos and stem cells. Curr. Opin. Cell Biol. 2020, 67, 1–8. [Google Scholar] [CrossRef]
- Merino, M.M.; Levayer, R.; Moreno, E. Survival of the Fittest: Essential Roles of Cell Competition in Development, Aging, and Cancer. Trends Cell Biol. 2016, 26, 776–788. [Google Scholar] [CrossRef]
- Bowling, S.; Lawlor, K.; Rodriguez, T.A. Cell competition: The winners and losers of fitness selection. Development 2019, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancho, M.; Di-Gregorio, A.; George, N.; Pozzi, S.; Sanchez, J.M.; Pernaute, B.; Rodriguez, T.A. Competitive interactions eliminate unfit embryonic stem cells at the onset of differentiation. Dev. Cell 2013, 26, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macfarlan, T.S.; Gifford, W.D.; Driscoll, S.; Lettieri, K.; Rowe, H.M.; Bonanomi, D.; Firth, A.; Singer, O.; Trono, D.; Pfaff, S.L. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 2012, 487, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgani, S.M.; Canham, M.A.; Nichols, J.; Sharov, A.A.; Migueles, R.P.; Ko, M.S.; Brickman, J.M. Totipotent embryonic stem cells arise in ground-state culture conditions. Cell Rep. 2013, 3, 1945–1957. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Ryan, D.J.; Wang, W.; Tsang, J.C.; Lan, G.; Masaki, H.; Gao, X.; Antunes, L.; Yu, Y.; Zhu, Z.; et al. Establishment of mouse expanded potential stem cells. Nature 2017, 550, 393–397. [Google Scholar] [CrossRef] [Green Version]
- Baker, C.L.; Pera, M.F. Capturing Totipotent Stem Cells. Cell Stem Cell 2018, 22, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.A.; Markoulaki, S.; Jaenisch, R. Matched Developmental Timing of Donor Cells with the Host Is Crucial for Chimeras Formation. Stem Cell Rep. 2018, 10, 1445–1452. [Google Scholar] [CrossRef] [Green Version]
- Mascetti, V.L.; Pedersen, R.A. Human-Mouse Chimerism Validates Human Stem Cell Pluripotency. Cell Stem Cell 2016, 18, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Stanger, B.Z.; Tanaka, A.J.; Melton, D.A. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 2007, 445, 886–891. [Google Scholar] [CrossRef]
- Xiang, J.; Cao, S.; Zhong, L.; Wang, H.; Pei, Y.; Wei, Q.; Wen, B.; Mu, H.; Zhang, S.; Yue, L.; et al. Pluripotent stem cells secrete Activin A to improve their epiblast competency after injection into recipient embryos. Protein Cell 2018, 9, 717–728. [Google Scholar] [CrossRef] [Green Version]
- Dupont, C.; Loos, F.; Kong, A.S.J.; Gribnau, J. FGF treatment of host embryos injected with ES cells increases rates of chimaerism. Transgenic Res. 2017, 26, 237–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandrova, S.; Kalkan, T.; Humphreys, P.; Riddell, A.; Scognamiglio, R.; Trumpp, A.; Nichols, J. Selection and dynamics of embryonic stem cell integration into early mouse embryos. Development 2016, 143, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, T.; Cui, T.; Yu, D.; Liu, C.; Jiang, L.; Feng, G.; Wang, L.; Fu, R.; Zhang, X.; et al. Human embryonic stem cells contribute to embryonic and extraembryonic lineages in mouse embryos upon inhibition of apoptosis. Cell Res. 2017. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Ke, Z.; Tombline, G.; Macoretta, N.; Hayes, K.; Tian, X.; Lv, R.; Ablaeva, J.; Gilbert, M.; Bhanu, N.V.; et al. Naked Mole Rat Cells Have a Stable Epigenome that Resists iPSC Reprogramming. Stem Cell Rep. 2017, 9, 1721–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.G.; Mikhalchenko, A.E.; Yim, S.H.; Lobanov, A.V.; Park, J.K.; Choi, K.H.; Bronson, R.T.; Lee, C.K.; Park, T.J.; Gladyshev, V.N. Naked Mole Rat Induced Pluripotent Stem Cells and Their Contribution to Interspecific Chimeras. Stem Cell Rep. 2017, 9, 1706–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halacheva, V.; Fuchs, M.; Donitz, J.; Reupke, T.; Puschel, B.; Viebahn, C. Planar cell movements and oriented cell division during early primitive streak formation in the mammalian embryo. Dev. Dyn. 2011, 240, 1905–1916. [Google Scholar] [CrossRef]
- Kojima, Y.; Tam, O.H.; Tam, P.P. Timing of developmental events in the early mouse embryo. Semin. Cell Dev. Biol. 2014, 34, 65–75. [Google Scholar] [CrossRef]
- Boward, B.; Wu, T.; Dalton, S. Concise Review: Control of Cell Fate Through Cell Cycle and Pluripotency Networks. Stem Cells 2016, 34, 1427–1436. [Google Scholar] [CrossRef] [Green Version]
- Dalton, S. Linking the Cell Cycle to Cell Fate Decisions. Trends Cell Biol. 2015, 25, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Gonnot, F.; Langer, D.; Bourillot, P.Y.; Doerflinger, N.; Savatier, P. Regulation of Cyclin E by transcription factors of the naive pluripotency network in mouse embryonic stem cells. Cell Cycle 2019, 18, 2697–2712. [Google Scholar] [CrossRef] [Green Version]
- Cartwright, P.; McLean, C.; Sheppard, A.; Rivett, D.; Jones, K.; Dalton, S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 2005, 132, 885–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.N.; Singh, A.M.; Dalton, S. Myc represses primitive endoderm differentiation in pluripotent stem cells. Cell Stem Cell 2010, 7, 343–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savatier, P.; Lapillonne, H.; van Grunsven, L.A.; Rudkin, B.B.; Samarut, J. Withdrawal of differentiation inhibitory activity/leukemia inhibitory factor up-regulates D-type cyclins and cyclin-dependent kinase inhibitors in mouse embryonic stem cells. Oncogene 1996, 12, 309–322. [Google Scholar] [PubMed]
- Coronado, D.; Godet, M.; Bourillot, P.Y.; Tapponnier, Y.; Bernat, A.; Petit, M.; Afanassieff, M.; Markossian, S.; Malashicheva, A.; Iacone, R.; et al. A short G1 phase is an intrinsic determinant of naive embryonic stem cell pluripotency. Stem Cell Res 2013, 10, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Michowski, W.; Inuzuka, H.; Shimizu, K.; Nihira, N.T.; Chick, J.M.; Li, N.; Geng, Y.; Meng, A.Y.; Ordureau, A.; et al. G1 cyclins link proliferation, pluripotency and differentiation of embryonic stem cells. Nat. Cell Biol. 2017, 19, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Claveria, C.; Giovinazzo, G.; Sierra, R.; Torres, M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature 2013, 500, 39–44. [Google Scholar] [CrossRef]
- Diaz-Diaz, C.; Fernandez de Manuel, L.; Jimenez-Carretero, D.; Montoya, M.C.; Claveria, C.; Torres, M. Pluripotency Surveillance by Myc-Driven Competitive Elimination of Differentiating Cells. Dev. Cell 2017, 42, 585–599. [Google Scholar] [CrossRef] [Green Version]
- Biechele, S.; Lin, C.J.; Rinaudo, P.F.; Ramalho-Santos, M. Unwind and transcribe: Chromatin reprogramming in the early mammalian embryo. Curr. Opin. Genet. Dev. 2015, 34, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.X.; Liu, Z.; Fleurot, R.; Adenot, P.; Duranthon, V.; Vignon, X.; Zhou, Q.; Renard, J.P.; Beaujean, N. Heterochromatin reprogramming in rabbit embryos after fertilization, intra-, and inter-species SCNT correlates with preimplantation development. Reproduction 2013, 145, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda-Rincon, L.P.; Solanas Edel, L.; Serrano-Revuelta, E.; Ruddick, L.; Maalouf, W.E.; Beaujean, N. Early epigenetic reprogramming in fertilized, cloned, and parthenogenetic embryos. Theriogenology 2016, 86, 91–98. [Google Scholar] [CrossRef]
- Geng, T.; Zhang, D.; Jiang, W. Epigenetic Regulation of Transition Among Different Pluripotent States: Concise Review. Stem Cells 2019, 37, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Tang, W.W.; Wu, B.; Kim, S.; Li, J.; Li, L.; Kobayashi, T.; Lee, C.; Chen, Y.; Wei, M.; et al. Derivation of hypermethylated pluripotent embryonic stem cells with high potency. Cell Res. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojdyla, K.; Collier, A.J.; Fabian, C.; Nisi, P.S.; Biggins, L.; Oxley, D.; Rugg-Gunn, P.J. Cell-Surface Proteomics Identifies Differences in Signaling and Adhesion Protein Expression between Naive and Primed Human Pluripotent Stem Cells. Stem Cell Rep. 2020, 14, 972–988. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, S.; Nishikawa-Torikai, S.; Niwa, H. E-cadherin promotes incorporation of mouse epiblast stem cells into normal development. PLoS ONE 2012, 7, e45220. [Google Scholar] [CrossRef]
- Hawkins, K.; Keramari, M.; Soncin, F.; Segal, J.M.; Mohamet, L.; Miazga, N.; Ritson, S.; Bobola, N.; Merry, C.L.; Ward, C.M. Novel cell lines isolated from mouse embryonic stem cells exhibiting de novo methylation of the E-cadherin promoter. Stem Cells 2014, 32, 2869–2879. [Google Scholar] [CrossRef]
- Lees, J.G.; Gardner, D.K.; Harvey, A.J. Pluripotent Stem Cell Metabolism and Mitochondria: Beyond ATP. Stem Cells Int. 2017, 2017, 2874283. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, J.; Dahan, P.; Lu, V.; Zhang, C.; Li, H.; Teitell, M.A. Metabolism in Pluripotent Stem Cells and Early Mammalian Development. Cell Metab. 2018, 27, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Gardner, D.K.; Harvey, A.J. Blastocyst metabolism. Reprod. Fertil. Dev. 2015, 27, 638–654. [Google Scholar] [CrossRef]
- Zhang, H.; Badur, M.G.; Divakaruni, A.S.; Parker, S.J.; Jager, C.; Hiller, K.; Murphy, A.N.; Metallo, C.M. Distinct Metabolic States Can Support Self-Renewal and Lipogenesis in Human Pluripotent Stem Cells under Different Culture Conditions. Cell Rep. 2016, 16, 1536–1547. [Google Scholar] [CrossRef] [Green Version]
- Perestrelo, T.; Correia, M.; Ramalho-Santos, J.; Wirtz, D. Metabolic and Mechanical Cues Regulating Pluripotent Stem Cell Fate. Trends Cell Biol. 2018, 28, 1014–1029. [Google Scholar] [CrossRef]
- Cliff, T.S.; Dalton, S. Metabolic switching and cell fate decisions: Implications for pluripotency, reprogramming and development. Curr. Opin. Genet. Dev. 2017, 46, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Tsogtbaatar, E.; Landin, C.; Minter-Dykhouse, K.; Folmes, C.D.L. Energy Metabolism Regulates Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A. Formative pluripotency: The executive phase in a developmental continuum. Development 2017, 144, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neagu, A.; van Genderen, E.; Escudero, I.; Verwegen, L.; Kurek, D.; Lehmann, J.; Stel, J.; Dirks, R.A.M.; van Mierlo, G.; Maas, A.; et al. In vitro capture and characterization of embryonic rosette-stage pluripotency between naive and primed states. Nat. Cell Biol. 2020, 22, 534–545. [Google Scholar] [CrossRef]
- Kinoshita, M.; Barber, M.; Mansfield, W.; Cui, Y.; Spindlow, D.; Stirparo, G.G.; Dietmann, S.; Nichols, J.; Smith, A. Capture of mouse and human stem cells with features of formative pluripotency. BioRxiv 2020, 1–48. [Google Scholar] [CrossRef]
- Hayashi, K.; Saitou, M. Generation of eggs from mouse embryonic stem cells and induced pluripotent stem cells. Nat. Protoc. 2013, 8, 1513–1524. [Google Scholar] [CrossRef]
- Cai, H.; Xia, X.; Wang, L.; Liu, Y.; He, Z.; Guo, Q.; Xu, C. In vitro and in vivo differentiation of induced pluripotent stem cells into male germ cells. Biochem. Biophys. Res. Commun. 2013, 433, 286–291. [Google Scholar] [CrossRef]
- Kou, Z.; Kang, L.; Yuan, Y.; Tao, Y.; Zhang, Y.; Wu, T.; He, J.; Wang, J.; Liu, Z.; Gao, S. Mice cloned from induced pluripotent stem cells (iPSCs). Biol. Reprod. 2010, 83, 238–243. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Ding, C.; Zhao, X.; Wang, E.; Dai, X.; Liu, L.; Li, W.; Liu, Z.; Wan, H.; Feng, C.; et al. Successful generation of cloned mice using nuclear transfer from induced pluripotent stem cells. Cell Res. 2010, 20, 850–853. [Google Scholar] [CrossRef]
- Chesne, P.; Adenot, P.G.; Viglietta, C.; Baratte, M.; Boulanger, L.; Renard, J.P. Cloned rabbits produced by nuclear transfer from adult somatic cells. Nat. Biotechnol. 2002, 20, 366–369. [Google Scholar] [CrossRef]
- Gavin-Plagne, L.; Perold, F.; Osteil, P.; Voisin, S.; Moreira, S.C.; Combourieu, Q.; Saidou, V.; Mure, M.; Louis, G.; Baudot, A.; et al. Insights into Species Preservation: Cryobanking of Rabbit Somatic and Pluripotent Stem Cells. Int. J. Mol. Sci. 2020, 21, 7285. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.X.; Pek, N.M.Q.; Soh, B.S. Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2018, 19, 936. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samruan, W.; Beaujean, N.; Afanassieff, M. Pluripotent Stem Cells for Transgenesis in the Rabbit: A Utopia? Appl. Sci. 2020, 10, 8861. https://doi.org/10.3390/app10248861
Samruan W, Beaujean N, Afanassieff M. Pluripotent Stem Cells for Transgenesis in the Rabbit: A Utopia? Applied Sciences. 2020; 10(24):8861. https://doi.org/10.3390/app10248861
Chicago/Turabian StyleSamruan, Worawalan, Nathalie Beaujean, and Marielle Afanassieff. 2020. "Pluripotent Stem Cells for Transgenesis in the Rabbit: A Utopia?" Applied Sciences 10, no. 24: 8861. https://doi.org/10.3390/app10248861




