Modeling Analysis of Thermal Lesion Characteristics of Unipolar/Bipolar Ablation Using Circumferential Multipolar Catheter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Governing Equation
2.2. Construction of Ablation Model
2.3. Material Properties and Boundary Conditions
2.4. Validation of Simulation Method
3. Results
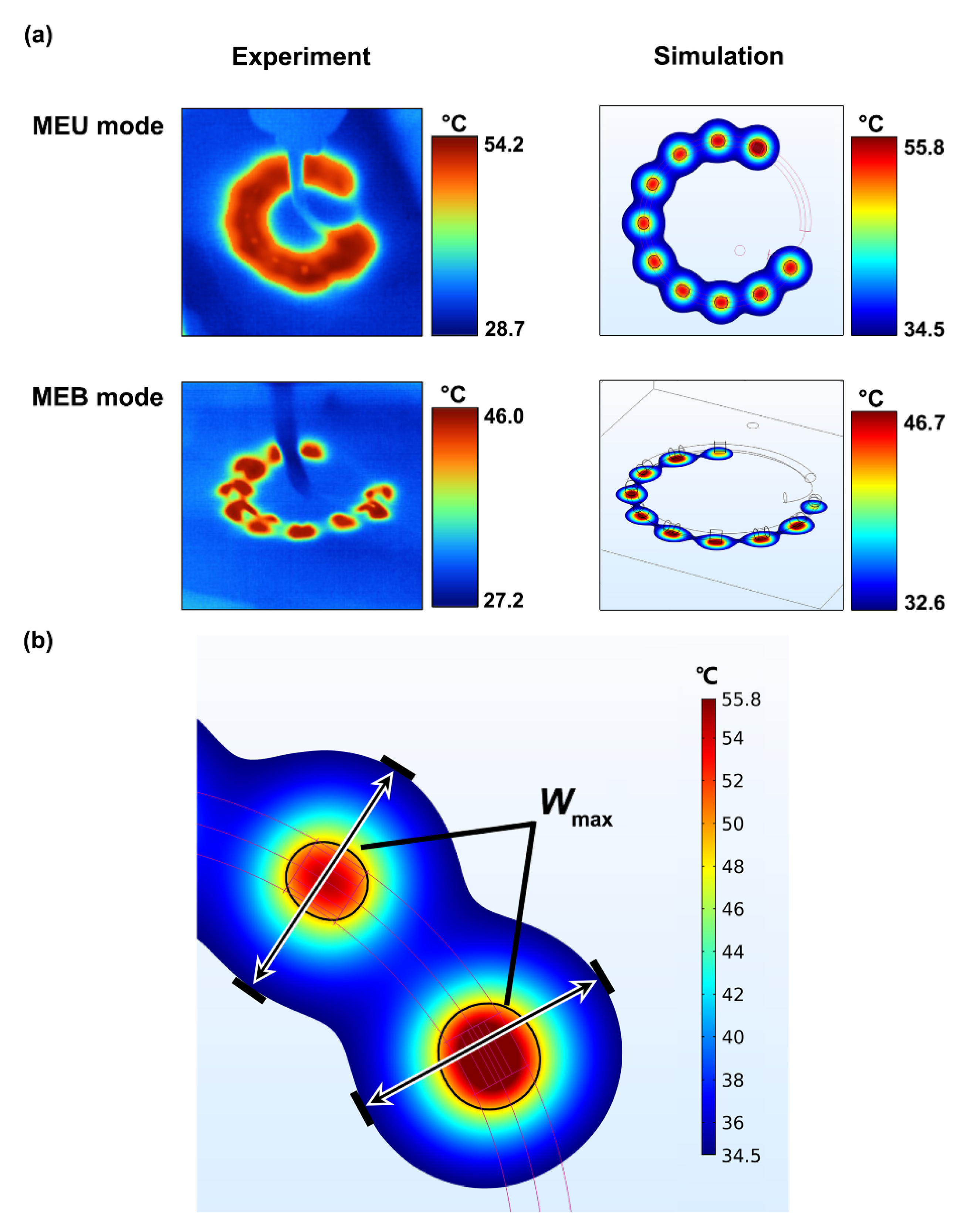
3.1. Experimental Validation
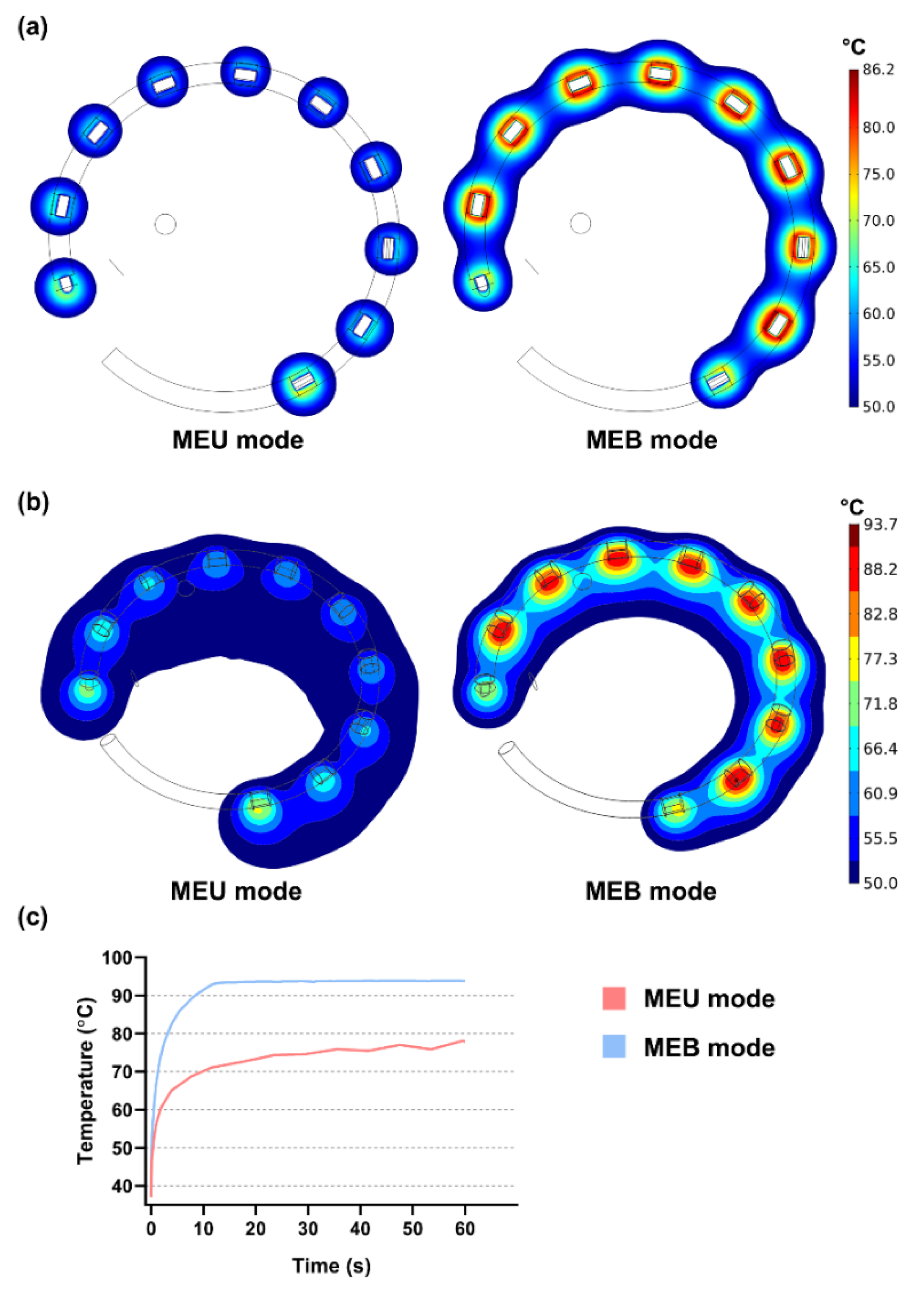
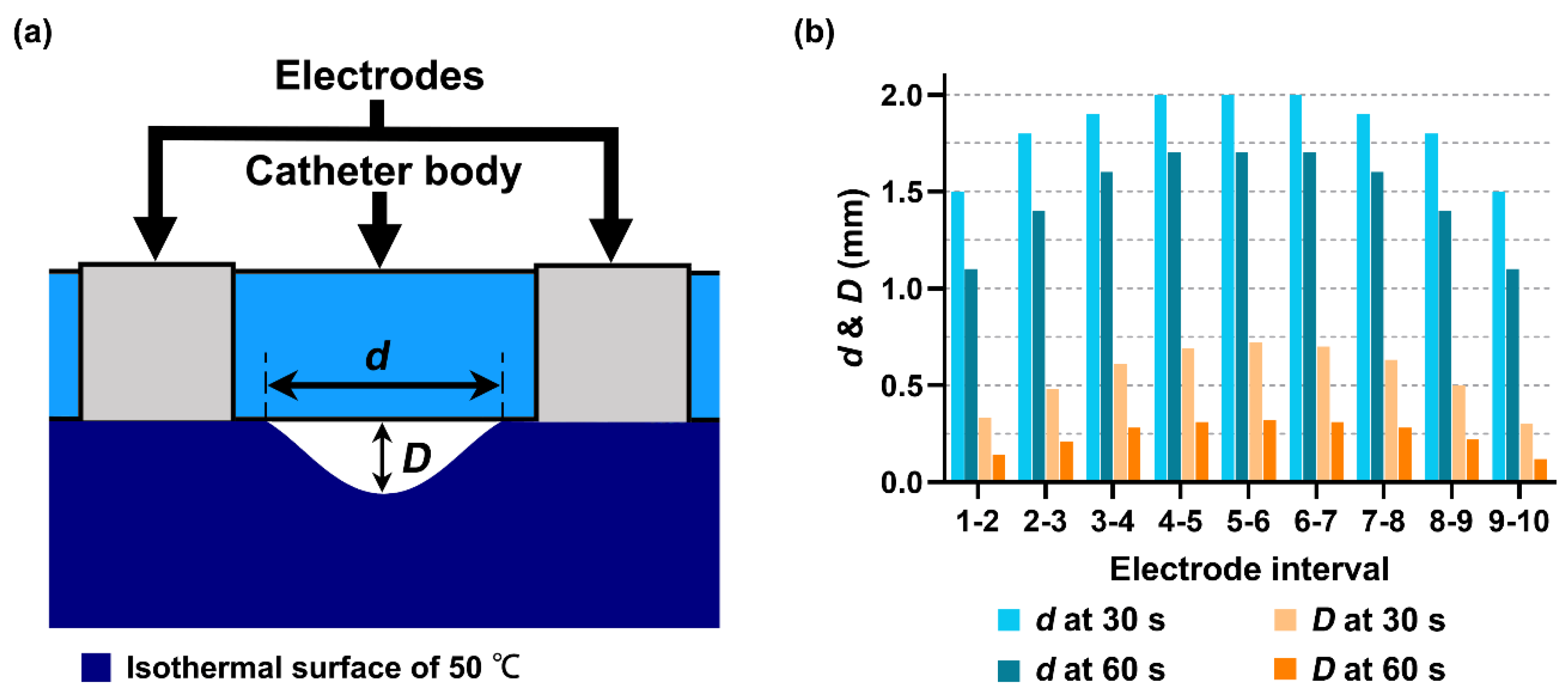
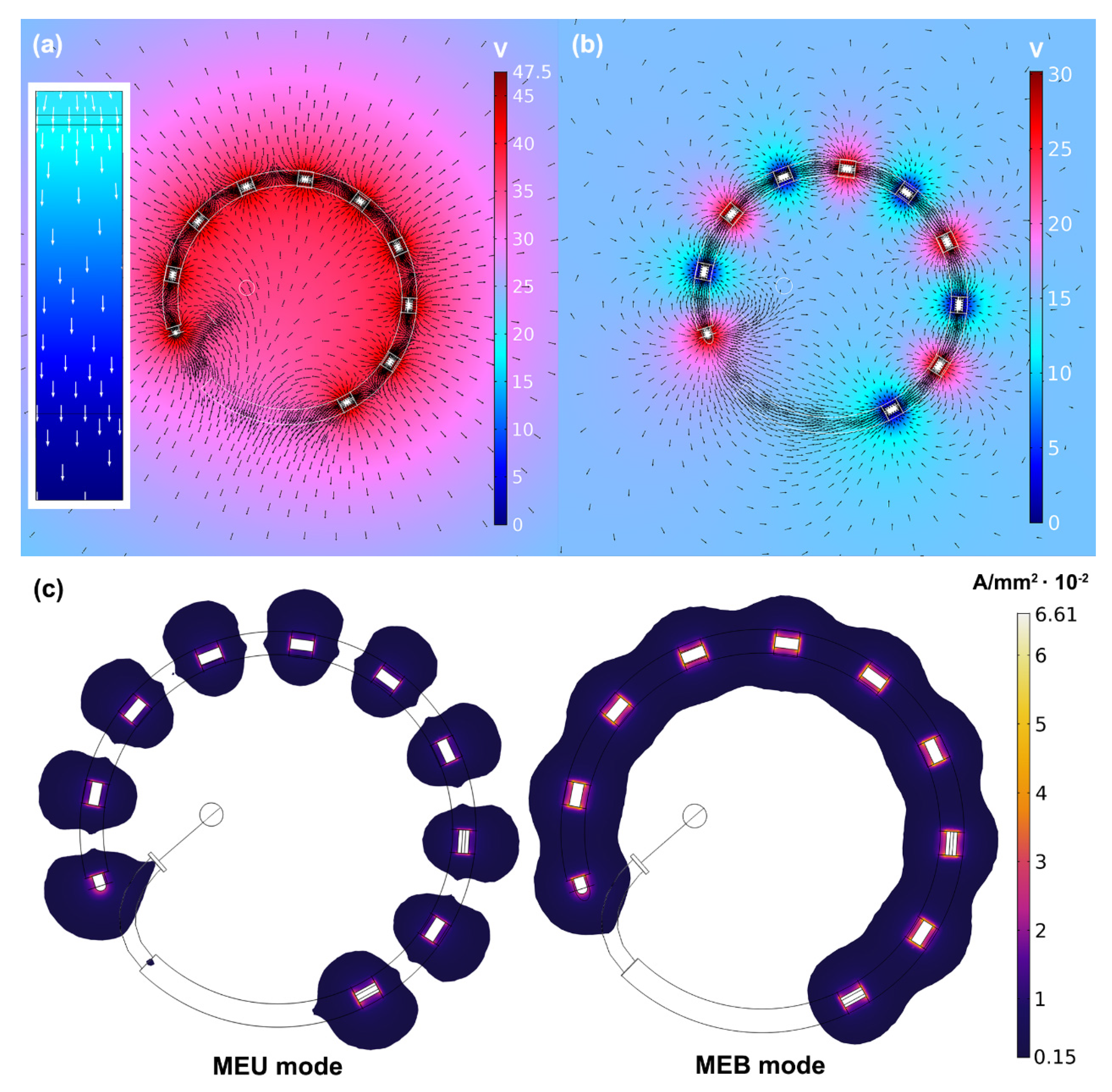
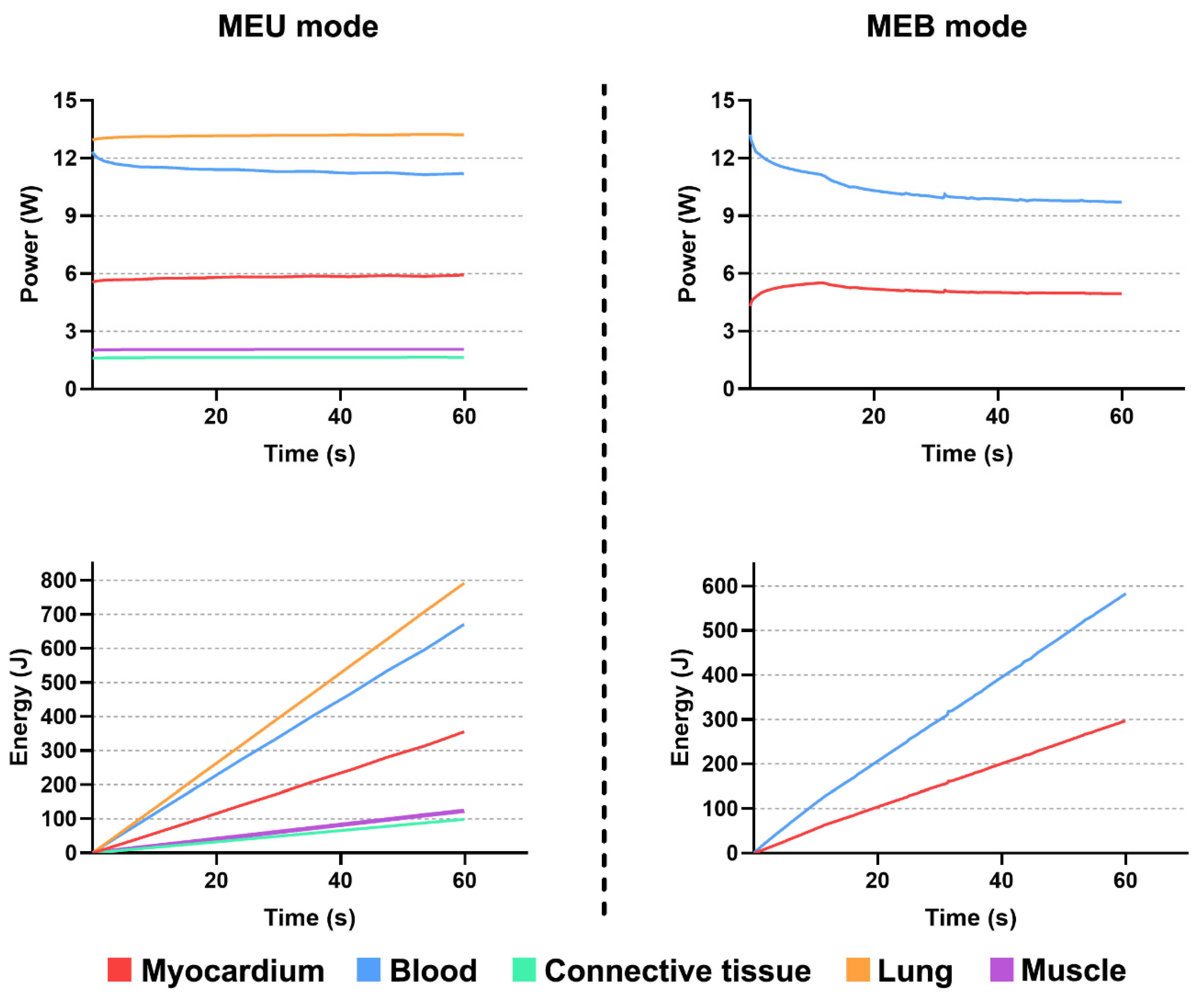
3.2. Performances of Multielectrode Unipolar (MEU) and Multielectrode Bipolar (MEB) Modes
4. Discussion
4.1. Main Findings
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Morillo, C.A.; Banerjee, A.; Perel, P.; Wood, D.; Jouven, X. Atrial fibrillation: The current epidemic. J. Geriatr. Cardiol. 2017, 14, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.; Magnuson, A.; Bryngelsson, I.L.; Frøbert, O.; Henriksson, K.M.; Edvardsson, N.; Poçi, D. All-cause mortality in 272 186 patients hospitalized with incident atrial fibrillation 1995–2008: A Swedish nationwide long-term case-control study. Eur. Heart J. 2013, 34, 1061–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wattigney, W.A.; Mensah, G.A.; Croft, J.B. Increased atrial fibrillation mortality: United States, 1980–1998. Am. J. Epidemiol. 2002, 155, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.C.; Cinquegrani, M.P.; Wright, J. Atrial fibrillation in acute coronary syndrome. J. Atr. Fibrillation 2012, 2, 35–42. [Google Scholar] [CrossRef]
- Safaei, N.; Montazerghaem, H.; Azarfarin, R.; Alizadehasl, A.; Alikhah, H. Radiofrequency ablation for treatment of atrial fibrillation. BioImpacts 2011, 1, 171–177. [Google Scholar] [CrossRef]
- Joseph, J.P.; Rajappan, K. Radiofrequency ablation of cardiac arrhythmias: Past, present and future. QJM 2012, 105, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Calkins, H.; Kuck, K.H.; Cappato, R.; Brugada, J.; Camm, A.J.; Chen, S.-A.; Crijns, H.J.G.; Damiano, R.J., Jr.; Davies, D.W.; DiMarco, J.; et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J. Interv. Card. Electrophysiol. 2012, 33, 171–257. [Google Scholar] [CrossRef]
- European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery; Camm, A.J.; Kirchhof, P.; Lip, G.Y.H.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; Al-Attar, N.; et al. Guidelines for the management of atrial fibrillation The Task Force for the Management of Atrial Fibrillation of the Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar] [CrossRef]
- Calkins, H.; Brugada, J.; Packer, D.L.; Cappato, R.; Chen, S.-A.; Crijns, H.J.G.; Damiano, R.J., Jr.; Davies, D.W.; Haines, D.E.; Haissaguerre, M.; et al. EHRA Scientific Statement HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Personnel, Policy, Procedures and Follow-up: A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2007, 4, 816–861. [Google Scholar] [CrossRef]
- Barnett, A.S.; Bahnson, T.D.; Piccini, J.P. Recent Advances in Lesion Formation for Catheter Ablation of Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2016, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Boersma, L.V.A.; Wijffels, M.C.E.F.; Oral, H.; Wever, E.F.D.; Morady, F. Pulmonary vein isolation by duty-cycled bipolar and unipolar radiofrequency energy with a multielectrode ablation catheter. Heart Rhythm. 2008, 5, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Deneke, T.; Schade, A.; Müller, P.; Schmitt, R.; Christopoulos, G.; Krug, J.; Szöllösi, G.; Mügge, A.; Kerber, S.; Nentwich, K. Acute safety and efficacy of a novel multipolar irrigated radiofrequency ablation catheter for pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2013, 25, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Fredersdorf, S.; Fenzl, C.; Jungbauer, C.; Weber, S.; von Bary, C.; Dietl, A.; Seegers, J.; Maier, L.S.; Ücer, E. Long-term outcomes and predictors of recurrence after pulmonary vein isolation with multielectrode ablation catheter in patients with atrial fibrillation. J. Cardiovasc. Med. 2018, 19, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Nardi, S.; Argenziano, L.; Cappato, R.; Martino, G.D.; Esposito, C.; Scaglione, M.; Borrello, F.; Maglia, G. Ablation of paroxysmal and persistent atrial fibrillation with multielectrode phased radiofrequency duty-cycled catheters: Long-term results from a large cohort of patients. J. Cardiovasc. Med. 2013, 14, 879–885. [Google Scholar] [CrossRef]
- Mulder, A.A.W.; Wijffels, M.C.E.F.; Wever, E.F.D.; Boersma, L.V. Early recurrence of atrial fibrillation as a predictor for 1-year efficacy after successful phased RF pulmonary vein isolation: Evaluation of complaints and multiple Holter recording. Int. J. Cardiol. 2013, 165, 56–60. [Google Scholar] [CrossRef]
- Mulder, A.A.W.; Wijffels, M.C.E.F.; Wever, E.F.D.; Boersma, L.V. Pulmonary vein isolation and left atrial complex- fractionated atrial electrograms ablation for persistent atrial fibrillation with phased radio frequency energy and multi-electrode catheters: Efficacy and safety during 12 months follow-up. Europace 2011, 13, 1695–1702. [Google Scholar] [CrossRef]
- Mulder, A.A.W.; Balt, J.C.; Wijffels, M.C.E.F.; Wever, E.F.D.; Boersma, L.V. Safety of pulmonary vein isolation and left atrial complex fractionated atrial electrograms ablation for atrial fibrillation with phased radiofrequency energy and multi-electrode catheters. Europace 2012, 14, 1433–1440. [Google Scholar] [CrossRef] [Green Version]
- Berjano, E.J. Theoretical modeling for radiofrequency ablation: State-of-the-art and challenges for the future. Biomed. Eng. Online 2006, 5, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Wu, X.; Wang, W. A simulation study to compare the phase-shift angle radiofrequency ablation mode with bipolar and unipolar modes in creating linear lesions for atrial fibrillation ablation. Int. J. Hyperth. 2016, 32, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Wu, X.; Wang, W. Theoretical and experimental analysis of amplitude control ablation and bipolar ablation in creating linear lesion and discrete lesions for treating atrial fibrillation. Int. J. Hyperth. 2017, 33, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Schutt, D.; Berjano, E.J.; Haemmerich, D. Effect of electrode thermal conductivity in cardiac radiofrequency catheter ablation: A computational modeling study. Int. J. Hyperth. 2009, 25, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Berjano, E.J.; Hornero, F. A cooled intraesophageal balloon to prevent thermal injury during endocardial surgical radiofrequency ablation of the left atrium: A finite element study. Phys. Med. Biol. 2005, 50, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Rezaeieh, S.A.; Abbosh, A.; Mustafa, S. Defining optimum frequency range for heart failure detection system considering thickness variations in human body tissues. In Proceedings of the 2013 International Conference on Electromagnetics in Advanced Applications, ICEAA, Torino, Italy, 9–13 September 2013. [Google Scholar]
- Perez, J.J.; D’Avila, A.; Aryana, A.; Berjano, E. Electrical and thermal effects of esophageal temperature probes on radiofrequency catheter ablation of atrial fibrillation: Results from a computational modeling study. J. Cardiovasc. Electrophysiol. 2015, 26, 556–564. [Google Scholar] [CrossRef] [PubMed]
- González-Suárez, A.; Trujillo, M.; Koruth, J.; d’Avila, A.; Berjano, E. Radiofrequency cardiac ablation with catheters placed on opposing sides of the ventricular wall: Computer modelling comparing bipolar and unipolar modes. Int. J. Hyperth. 2014, 30, 372–384. [Google Scholar] [CrossRef]
- Singh, S.; Melnik, R. Domain heterogeneity in radiofrequency therapies for pain relief: A computational study with coupled models. Bioengineering 2020, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- González-Suárez, A.; Pérez, J.J.; Berjano, E. Should fluid dynamics be included in computer models of RF cardiac ablation by irrigated-tip electrodes? Biomed. Eng. Online 2018, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ditter, T.A. Catheter with Variable Arcuate Distal Section. European Patent No. 2985002A1, 17 February 2016. [Google Scholar]
- Wissner, E.; Metzner, A. Biophysics of Modern Ablation Techniques and Their Limitations. In Cardiac Arrhythmias, 1st ed.; Kibos, A., Knight, B., Essebag, V., Fishberger, S., Slevin, M., Țintoiu, I., Eds.; Springer: London, UK, 2014; pp. 361–367. [Google Scholar]
- Haines, D.E.; Watson, D.D. Tissue heating during radiofrequency catheter ablation: A thermodynamic model and observations in isolated perfused and superfused canine right ventricular free wall. Pacing Clin. Electrophysiol. 1989, 12, 962–976. [Google Scholar] [CrossRef]
- Andreas, R.; Lin, T.; Burchard, A.; Kamioka, M.; Heeger, C.; Makimoto, H.; Metzner, A.; Wissner, E.; Wohlmuth, P.; Ouyang, F.; et al. Modified energy settings are mandatory to minimize oesophageal injury using the novel multipolar irrigated radiofrequency ablation catheter for pulmonary vein isolation. Europace 2015, 17, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Ullah, W.; Hunter, R.J.; Baker, V.; Dhinoja, M.B.; Sporton, S.; Earley, M.J.; Schilling, R.J. Target Indices for Clinical Ablation in Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2014, 7, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, S.; Mam, C.; Choe, W.; Aleong, R.; Reddy, K.; Bunch, J.T. Atrial fibrillation ablation performed in the developing world: A description of the first atrial fibrillation ablation performed in Cambodia. HeartRhythm Case Rep. 2015, 1, 360–362. [Google Scholar] [CrossRef] [Green Version]
- Metzner, I.; Wissner, E.; Tilz, R.R.; Rillig, A.; Mathew, S.; Schmidt, B.; Chun, J.; Wohlmuth, P.; Deiss, S.; Lemes, C.; et al. Ablation of atrial fibrillation in patients ≥ 75 years: Long-term clinical outcome and safety. Europace 2016, 18, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Johannes, S.; Silver, M.; Reza, W. Pulmonary vein isolation with the multipolar nMARQTM ablation catheter: Efficacy and safety in acute and long-term follow up. J. Atr. Fibrillation 2017, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wijffels, M.; Oosterhout, M.F.M.; Boersma, L.; Werneth, R.; Kunis, C.; Hu, B.; BEEKMAN, J.; Vos, M. Characterization of In Vitro and In Vivo Lesions Made by a Novel Multichannel Ablation Generator and a Circumlinear Decapolar Ablation Catheter. J. Cardiovasc. Electrophysiol. 2009, 20, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Demolin, J.M.; Eick, O.J.; Muench, K.; Koullick, E.; Nakagawa, H.; Wittkampf, F.H.M. Soft thrombus formation in radiofrequency catheter ablation pacing. Clin. Electrophysiol. 2002, 25, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Sándorfi, G.; Nagy-Baló, E.; Martirosyan, M.; Csanadi, Z. Phased RF ablation: Results and concerns. J. Atr. Fibrillation 2015, 8, 45–53. [Google Scholar] [CrossRef]



| References | |||||
|---|---|---|---|---|---|
| Electrode | 21,500 | 132 | 71 | 4.6 × 106 | [24,25] |
| Catheter body | 70 | 1045 | 0.026 | 1 × 10−5 | [24,25] |
| Blood | 1000 | 4180 | 0.541 | 0.667 | [27] |
| Myocardium | 1060 | 3212 | 0.531 | 0.541 | [24,25] |
| Connective tissue | 1000 | 3200 | 0.400 | 0.090 | [22] |
| Lung | 600 | 1280 | 0.350 | 0.250 | [22] |
| Muscle | 1090 | 3421 | 0.490 | 0.446 | [26] |
| MEU | MEB | |||
|---|---|---|---|---|
| Experiment | Simulation | Experiment | Simulation | |
| Mean SD (mm) | 4.90 ± 0.9 | 4.65 ± 0.1 | 3.3 ± 0.3 | 2.8 ± 0.3 |
| Mean error (%) | 16.2 | 11.1 | ||
| Maximum error (%) | 27.5 | 24.1 | ||
| Mode | Surface Area (mm2) | Maximum Sectional Lesion Area (mm2) | Corresponding Depth (mm) | |||
|---|---|---|---|---|---|---|
| 30 s | 60 s | 30 s | 60 s | 30 s | 60 s | |
| MEU | 40.66 | 52.51 | 186.28 | 206.24 | 1.30 | 2.37 |
| MEB | 104.30 | 109.62 | 152.92 | 171.88 | 1.04 | 1.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, K.; Wang, Y.; Yan, S.; Wu, X. Modeling Analysis of Thermal Lesion Characteristics of Unipolar/Bipolar Ablation Using Circumferential Multipolar Catheter. Appl. Sci. 2020, 10, 9081. https://doi.org/10.3390/app10249081
Gu K, Wang Y, Yan S, Wu X. Modeling Analysis of Thermal Lesion Characteristics of Unipolar/Bipolar Ablation Using Circumferential Multipolar Catheter. Applied Sciences. 2020; 10(24):9081. https://doi.org/10.3390/app10249081
Chicago/Turabian StyleGu, Kaihao, Yiheng Wang, Shengjie Yan, and Xiaomei Wu. 2020. "Modeling Analysis of Thermal Lesion Characteristics of Unipolar/Bipolar Ablation Using Circumferential Multipolar Catheter" Applied Sciences 10, no. 24: 9081. https://doi.org/10.3390/app10249081




