Combustion and Emission Characteristics of a Biodiesel-Hydrogen Dual-Fuel Engine
Abstract
:1. Introduction
2. Experimental Setup and Procedure
2.1. Research Engine and Apparatus
- -
- Pressure sensor Kistler 6061, range 0–25 MPa, linearity < ±0.5% FS,
- -
- Charge amplifier Kistler 511, range ±10…±999,000 pC for 10 V FS, error < ±3%, linearity < ±0.05% FS,
- -
- Data acquisition module, Measurement Computing USB-1608HS-16 bits resolution, sampling frequency 20 kHz with software [23],
- -
- Air rotor flowmeter Common CGR-01 G40 DN50, measuring range 0.65–65 m3/h, accuracy class 1,
- -
- H2 rotor flowmeter Common CGR-01 G10 DN50, measuring range 0.25–25 m3/h, accuracy class 1,
- -
- Exhaust gas analyzer: THC, CO, CO2, O2-Bosch BEA 350 (THC: range 0–9999 ppm vol. accuracy: 12 ppm vol.; NOx: range 0–5000 ppm accuracy: 10 ppm.; CO: range 0–10 %vol. accuracy: 0.06 %vol.; CO2: range 0–18 %vol. accuracy: 0.4 %vol.; O2: range 0–22 %vol. accuracy: 0.1 %vol.; λ: range 0.5–9.999 accuracy: 0.01),
- -
- AVL Smoke Meter: measurement range 0–10 FSN, detection limit: 0.002 FSN or 0.02 mg/m³, standard deviation 1σ ≤ ±(0.005 FSN + 3%).
2.2. Methodology
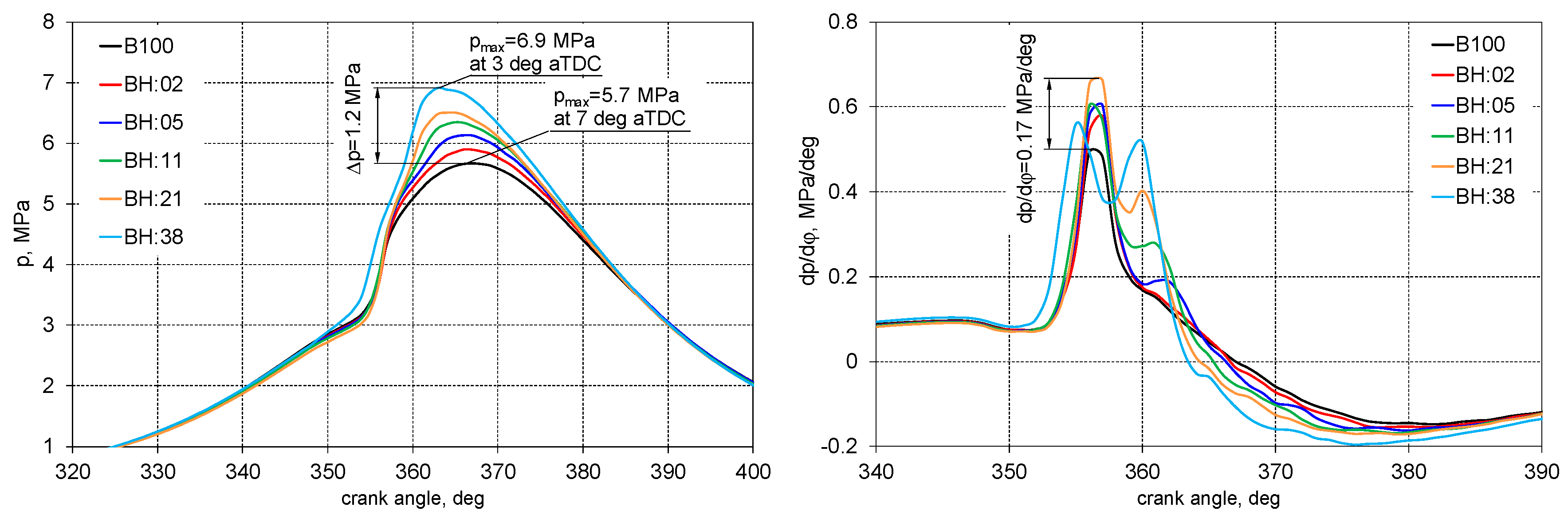
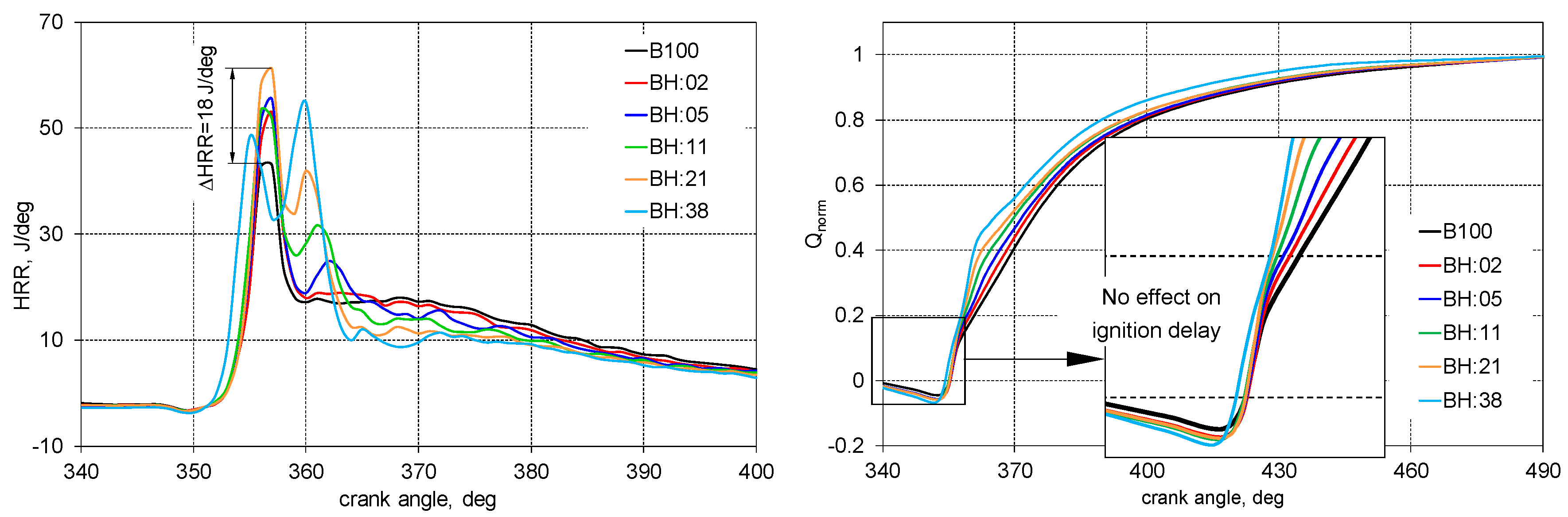
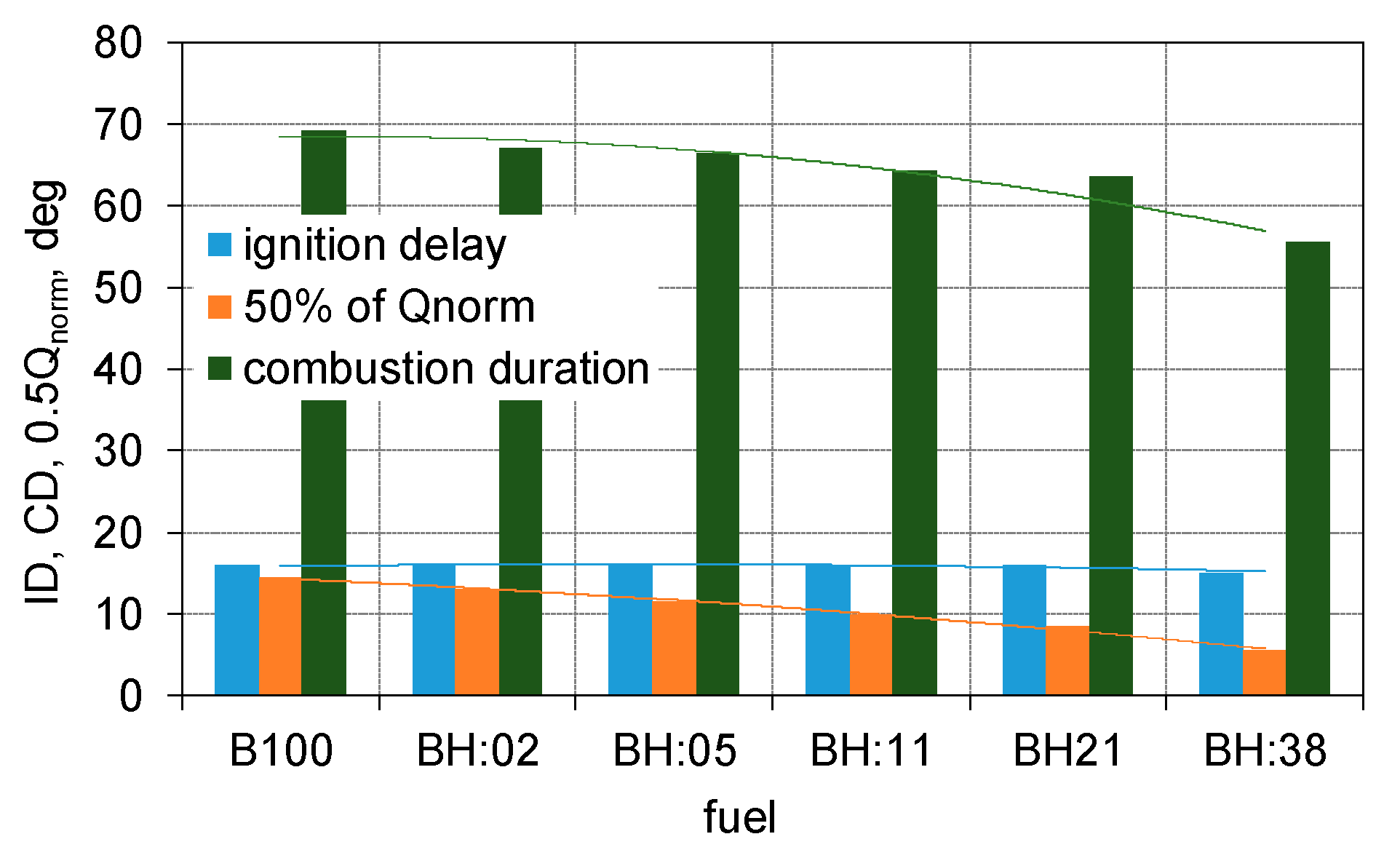
3. Results
4. Conclusions
- −
- An increase in the share of hydrogen causes an increase in the maximum combustion pressure value;
- −
- Increase in H2 energetic share cause a reduction in the diffusion combustion phase in favor of an increase in the kinetic phase;
- −
- For the 38% hydrogen share, the second peak on the HRR curve was higher than the first peak of combustion which is typical for engines;
- −
- The addition of hydrogen moves the combustion process closer to the isochoric one and this brings the engine closer to the spark ignition engine combustion;
- −
- The share of hydrogen practically did not affect the engine ignition delay time, while the duration of combustion decreases. With 38% hydrogen, the duration of combustion decreased by 25% compared to the biodiesel engine;
- −
- The addition of hydrogen improving the thermal efficiency of the engine, the maximum value was obtained for a 21% hydrogen share;
- −
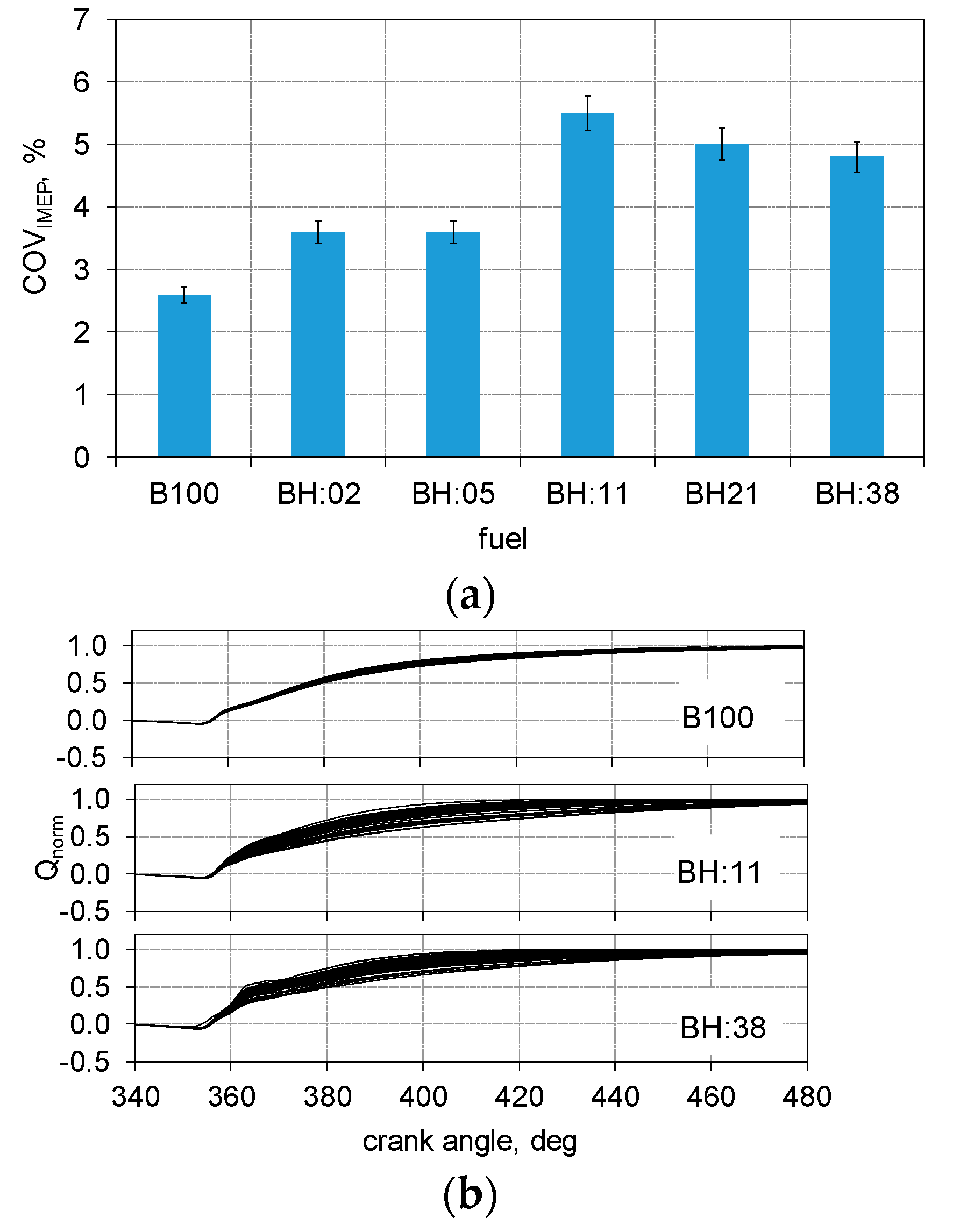
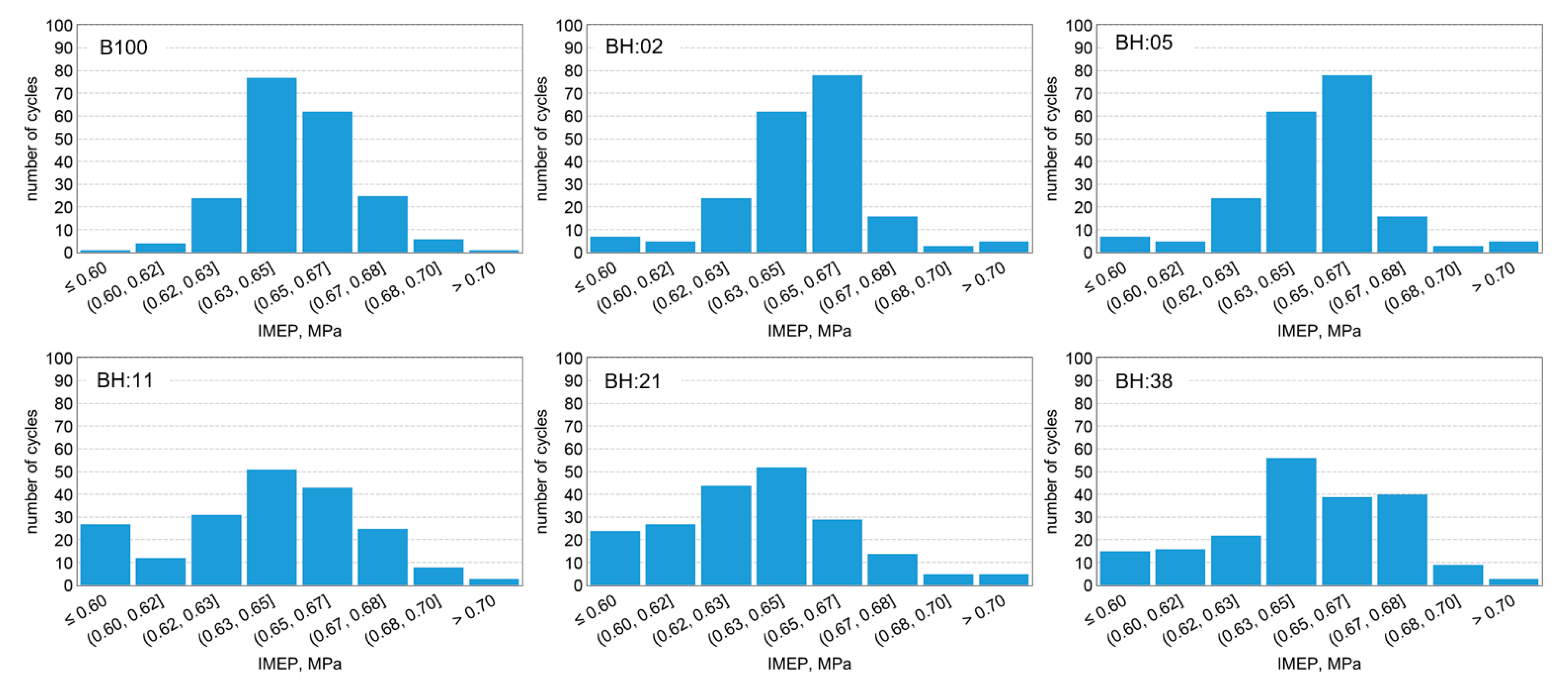
- Instability of biodiesel and hydrogen fueled engine operation is within the allowable range for industrial engines (COVIMEP < 5%).
- −
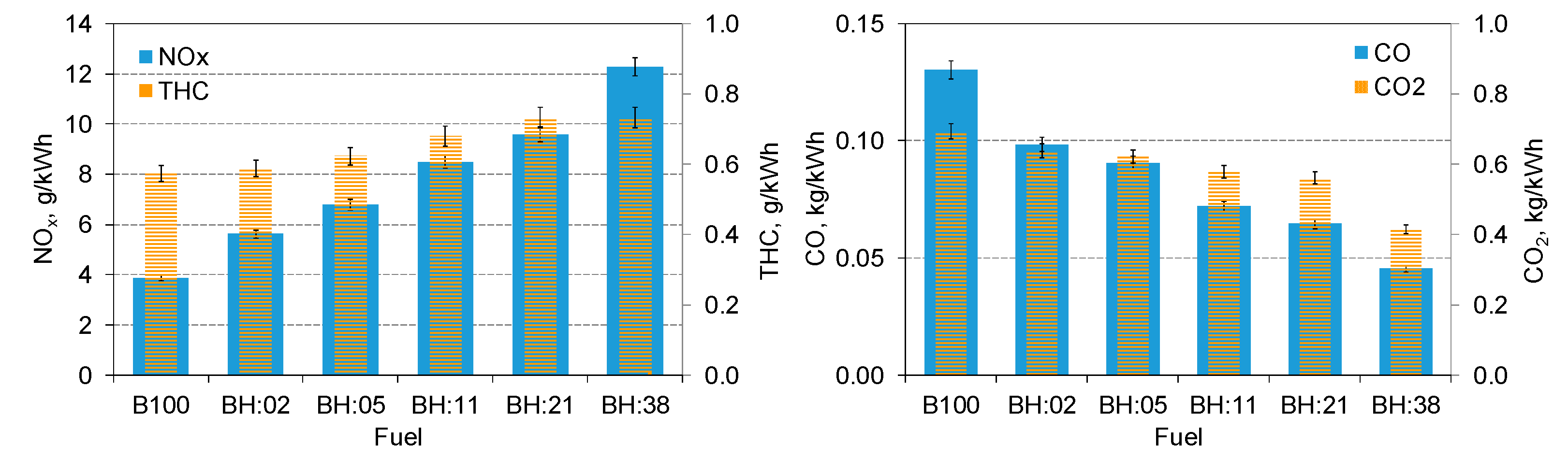
- With the increase of hydrogen energy share, there was an increase in specific THC emission. The highest increase in THC emissions was for 38% H2 and it was higher than that obtained for biodiesel fueled engine by 26%;
- −
- H2 contributed to an increase in nitrogen oxide emissions in the entire range of its share, for 38% hydrogen share an increase in specific NOx emission of over 2.5 times was noted;
- −
- The share of hydrogen caused a decrease in CO and CO2 emissions, which is normal, for a 38% share of hydrogen there was a 2.4 times decrease in specific CO emissions;
- −
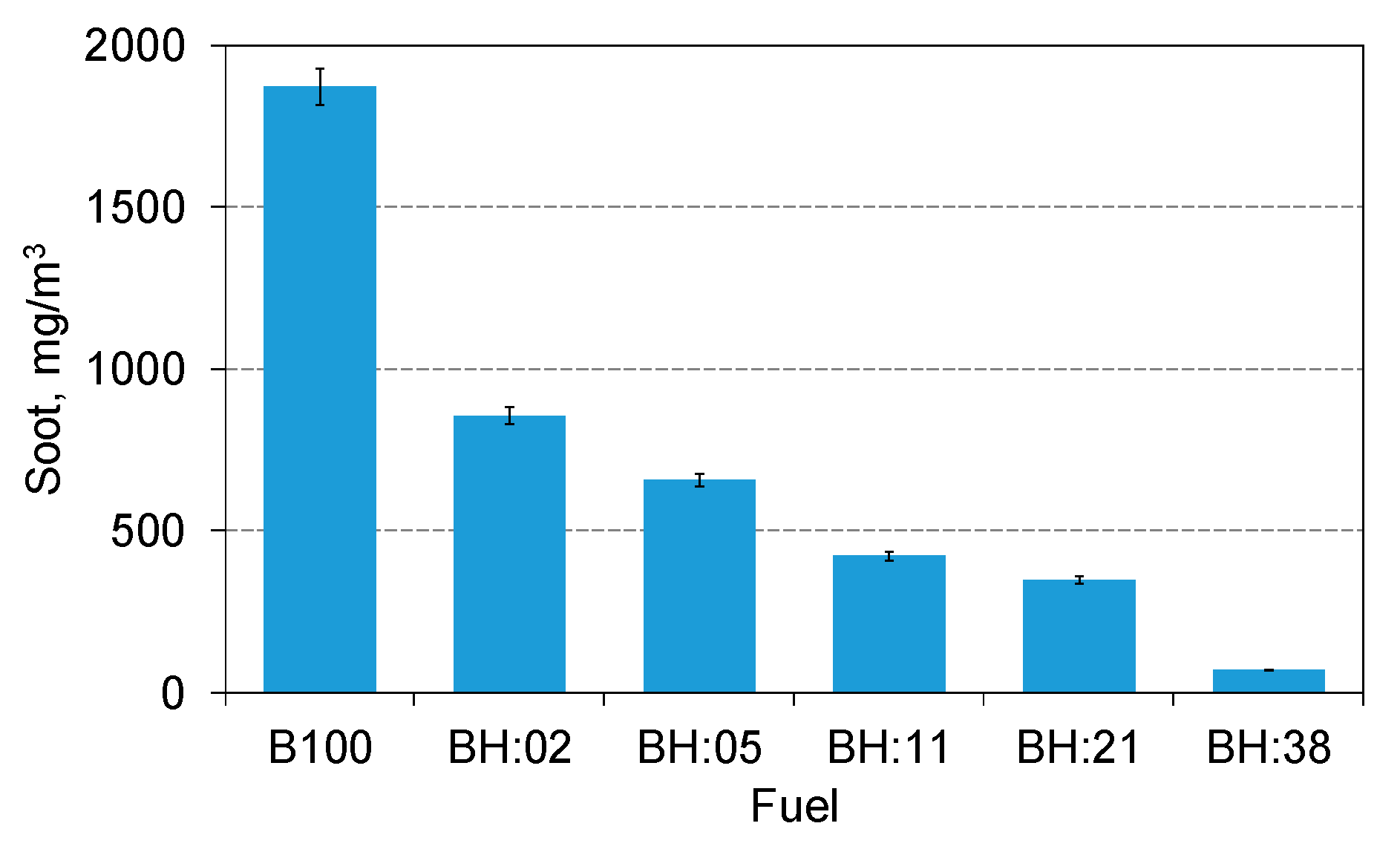
- The share of hydrogen has a very beneficial effect on soot emission because its 2% energy share has caused a reduction of its emission by over two times. With 38% hydrogen content, soot emission was only 70 mg/m3 and was over 25 times lower than when biodiesel was combusted.
Author Contributions
Funding
Conflicts of Interest
References
- Merkisz, J.; Fuć, P.; Lijewski, P.; Pielecha, J. Actual Emissions from Urban Buses Powered with Diesel and Gas Engines. Transp. Res. Procedia 2016, 14, 3070–3078. [Google Scholar] [CrossRef] [Green Version]
- Zöldy, M.; Török, Á. Road transport liquid fuel today and tomorrow: Literature overview. Period. Polytech. Transp. Eng. 2015, 43, 172–176. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Bereczky, Á.; Lukács, K. Effects of injection timing of diesel fuel on performance and emission of dual fuel diesel engine powered by diesel/E85 fuels. Transprt 2018, 33, 633–646. [Google Scholar] [CrossRef]
- Jamrozik, A.; Tutak, W.; Grab-Rogaliński, K. An experimental study on the performance and emission of the diesel/CNG dual-fuel combustion mode in a stationary CI engine. Energies 2019, 12, 3857. [Google Scholar] [CrossRef] [Green Version]
- Jamrozik, A.; Tutak, W.; Gnatowska, R.; Nowak, Ł. Comparative analysis of the combustion stability of diesel-methanol and diesel-ethanol in a dual fuel engine. Energies 2019, 12, 971. [Google Scholar] [CrossRef] [Green Version]
- Kuszewski, H. Experimental investigation of the autoignition properties of ethanol–biodiesel fuel blends. Fuel 2019, 235, 1301–1308. [Google Scholar] [CrossRef]
- Jamrozik, A.; Tutak, W.; Pyrc, M.; Sobiepański, M. Experimental investigations on combustion, performance and emission characteristics of stationary CI engine fuelled with diesel-methanol and biodiesel-methanol blends. Environ. Prog. Sustain. Energy 2017, 36, 1151–1163. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Gnatowska, R. Combustion of different reactivity fuel mixture in a dual fuel engine. Therm. Sci. 2018, 22, 1191–1203. [Google Scholar] [CrossRef] [Green Version]
- Barik, D.; Murugan, S. Simultaneous reduction of NOx and smoke in a dual fuel DI diesel engine. Energy Convers. Manag. 2014, 84, 217–226. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, M.; Zhang, D. Effect of a homogeneous combustion catalyst on the characteristics of diesel soot emitted from a compression ignition engine. Appl. Energy 2014, 113, 751–757. [Google Scholar] [CrossRef]
- Adu-Mensah, D.; Mei, D.; Zuo, L.; Zhang, Q.; Wang, J. A review on partial hydrogenation of biodiesel and its influence on fuel properties. Fuel 2019, 251, 660–668. [Google Scholar] [CrossRef]
- Lilik, G.K.; Zhang, H.; Herreros, J.M.; Haworth, D.C.; Boehman, A.L. Hydrogen assisted diesel combustion. Int. J. Hydrog. Energy 2010, 35, 4382–4398. [Google Scholar] [CrossRef]
- Szwaja, S.; Grab-Rogaliński, K. Hydrogen combustion in a compression ignitron diesel engine. Int. J. Hydrog. Energy 2009, 34, 4413–4421. [Google Scholar] [CrossRef]
- Tuccar, G.; Uludamar, E. Emission and engine performance analysis of a diesel engine using hydrogen enriched pomegranate seed oil biodiesel. Int. J. Hydrog. Energy 2018, 43, 18014–18019. [Google Scholar] [CrossRef]
- Serin, H.; Yıldızhan, S. Hydrogen addition to tea seed oil biodiesel: Performance and emission characteristics. Int. J. Hydrog. Energy 2018, 43, 18020–18027. [Google Scholar] [CrossRef]
- Akar, M.A.; Kekilli, E.; Bas, O.; Yildizhan, S.; Serin, H.; Ozcanli, M. Hydrogen enriched waste oil biodiesel usage in compression ignition engine. Int. J. Hydrog. Energy 2018, 43, 18046–18052. [Google Scholar] [CrossRef]
- Rajasekar, E.; Selvi, S. Review of combustion characteristics of CI engines fueled with biodiesel. Renew. Sustain. Energy Rev. 2014, 35, 390–399. [Google Scholar] [CrossRef]
- Dimitriou, P.; Kumar, M.; Tsujimura, T.; Suzuki, Y. Combustion and emission characteristics of a hydrogen-diesel dual-fuel engine. Int. J. Hydrog. Energy 2018, 43, 13605–13617. [Google Scholar] [CrossRef]
- Dimitriou, P.; Tsujimura, T.; Suzuki, Y. Adopting biodiesel as an indirect way to reduce the NOx emission of a hydrogen fumigated dual-fuel engine. Fuel 2019, 244, 324–334. [Google Scholar] [CrossRef]
- An, H.; Yang, W.M.; Maghbouli, A.; Li, J.; Chou, S.K.; Chua, K.J.; Wang, J.X.; Li, L. Numerical investigation on the combustion and emission characteristics of a hydrogen assisted biodiesel combustion in a diesel engine. Fuel 2014, 120, 186–194. [Google Scholar] [CrossRef]
- Çalık, A. Determination of vibration characteristics of a compression ignition engine operated by hydrogen enriched diesel and biodiesel fuels. Fuel 2018, 230, 355–358. [Google Scholar] [CrossRef]
- Dimitriou, P.; Tsujimura, T. A fully renewable and efficient backup power system with a hydrogen-biodiesel-fueled IC engine. Energy Procedia 2019, 157, 1305–1319. [Google Scholar] [CrossRef]
- Gruca, M. Software for Acquisition of Internal Combustion Engine data. J. Kones 2004, 11, 205–211. [Google Scholar]
- Jamrozik, A.; Kociszewski, A.; Tutak, W. Indication errors of engine with two stage combustion system. J. Kones 2009, 16, 179–193. [Google Scholar]
- Heywood, J.B. Internal Combustion Engine Fundamentals; Mc Graw Hill: New York, NY, USA, 2018. [Google Scholar]
- Koten, H. Hydrogen effects on the diesel engine performance and emissions. Int. J. Hydrog. Energy 2018, 43, 10511–10519. [Google Scholar] [CrossRef]
- Castro, N.; Toledo, M.; Amador, G. An experimental investigation of the performance and emissions of a hydrogen-diesel dual fuel compression ignition internal combustion engine. Appl. Therm. Eng. 2019, 156, 660–667. [Google Scholar] [CrossRef]
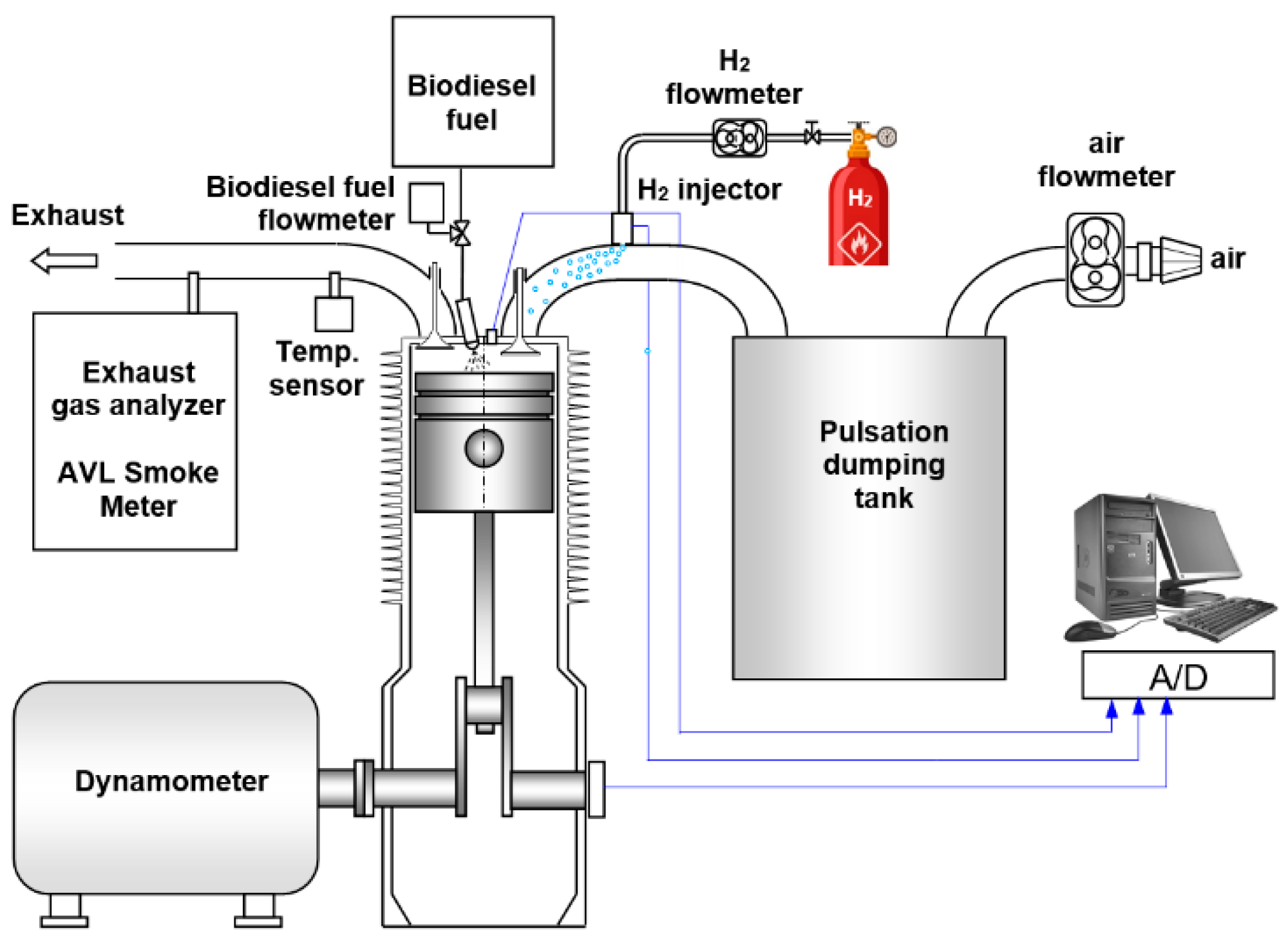

| Parameter | Value |
|---|---|
| Engine | 1CA90 Andoria |
| Type of engine | Four stroke compression ignition |
| Number of cylinders | 1 |
| Bore | 90 mm |
| Stroke | 90 mm |
| Displaced volume | 573 cm3 |
| Number of valves | 2 |
| Compression ratio | 17 |
| Engine speed | 1500 rpm |
| Diesel injection | Direct injection |
| Hydrogen injection | Port injection |
| Diesel injection pressure | 21 MPa |
| Diesel injection timing | 343 deg |
| Parameter | Biodiesel | Hydrogen |
|---|---|---|
| Molecular formula | CH3(CH2)nCOOH3 | H2 |
| Cetane number | 56 | 5–10 |
| Density at 1 atm and 15 °C (kg/m3) | 855 | 0.085 |
| Lower heating value (MJ/kg) | 37.1 | 119.81 |
| Heat of evaporation (kJ/kg) | 250 | - |
| Auto-ignition temperature (°C) | >101 | 585 |
| Flame speed, m/s | - | 2.65–3.25 |
| Stoichiometric air-fuel ratio | 12.5 | 34.3 |
| Viscosity at 40 °C (mPa·s) | 4.51 | - |
| Boiling point (°C) | 180–360 | −2529 |
| Carbon content (%) | 85 | 0 |
| Oxygen content, (%) | 10.8 | 0 |
| Hydrogen content, (%) | 12.1 | 100 |
| Fuel | B100 | BH:02 | BH:05 | BH:11 | BH:21 | BH:38 |
|---|---|---|---|---|---|---|
| Biodiesel, J/cycle | 1102.2 | 1000.1 | 896.8 | 884.4 | 763.4 | 543.1 |
| H2, J/cycle | 0 | 21.9 | 44.0 | 97.6 | 205.3 | 325.9 |
| Energy, J/cycle | 1102 | 1022 | 940.8 | 882 | 968.7 | 869 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutak, W.; Grab-Rogaliński, K.; Jamrozik, A. Combustion and Emission Characteristics of a Biodiesel-Hydrogen Dual-Fuel Engine. Appl. Sci. 2020, 10, 1082. https://doi.org/10.3390/app10031082
Tutak W, Grab-Rogaliński K, Jamrozik A. Combustion and Emission Characteristics of a Biodiesel-Hydrogen Dual-Fuel Engine. Applied Sciences. 2020; 10(3):1082. https://doi.org/10.3390/app10031082
Chicago/Turabian StyleTutak, Wojciech, Karol Grab-Rogaliński, and Arkadiusz Jamrozik. 2020. "Combustion and Emission Characteristics of a Biodiesel-Hydrogen Dual-Fuel Engine" Applied Sciences 10, no. 3: 1082. https://doi.org/10.3390/app10031082
APA StyleTutak, W., Grab-Rogaliński, K., & Jamrozik, A. (2020). Combustion and Emission Characteristics of a Biodiesel-Hydrogen Dual-Fuel Engine. Applied Sciences, 10(3), 1082. https://doi.org/10.3390/app10031082





