Development of Dual-Scale Fluorescence Endoscopy for In Vivo Bacteria Imaging in an Orthotopic Mouse Colon Tumor Model
Abstract
1. Introduction
2. Materials and Method
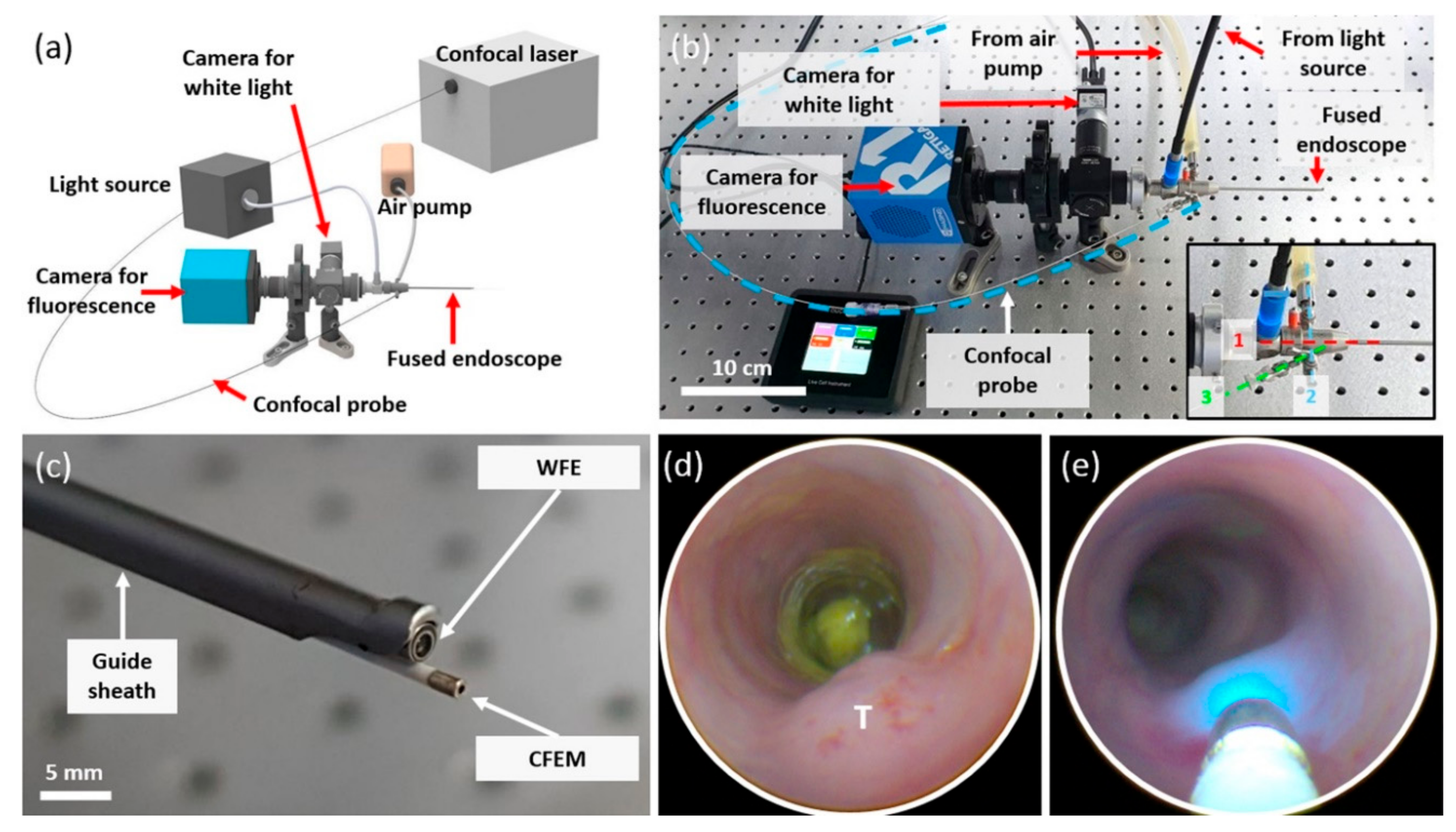
2.1. Development of Dual-Scale Fluorescence Endoscopy System
2.2. Phantom Study for DSFE
2.3. Cell Culture
2.4. Development of Attenuated Bacteria Expressing GFP by Eletroporation
2.5. Development of Orthotopic Colon Tumor Model
2.6. Administration of Attenuated, GFP-Expressing Salmonella in Orthotopic Mouse Model
2.7. In Vivo Bacterial Imaging with the DSFE System
2.8. Ex Vivo Bacterial Imaging with Confocal Endoscopic System
2.9. Bacterial Viable Counting
3. Results
3.1. Development of DSFE System and Phantom Study
3.2. Generation of Orthotopic Colon Tumor Model
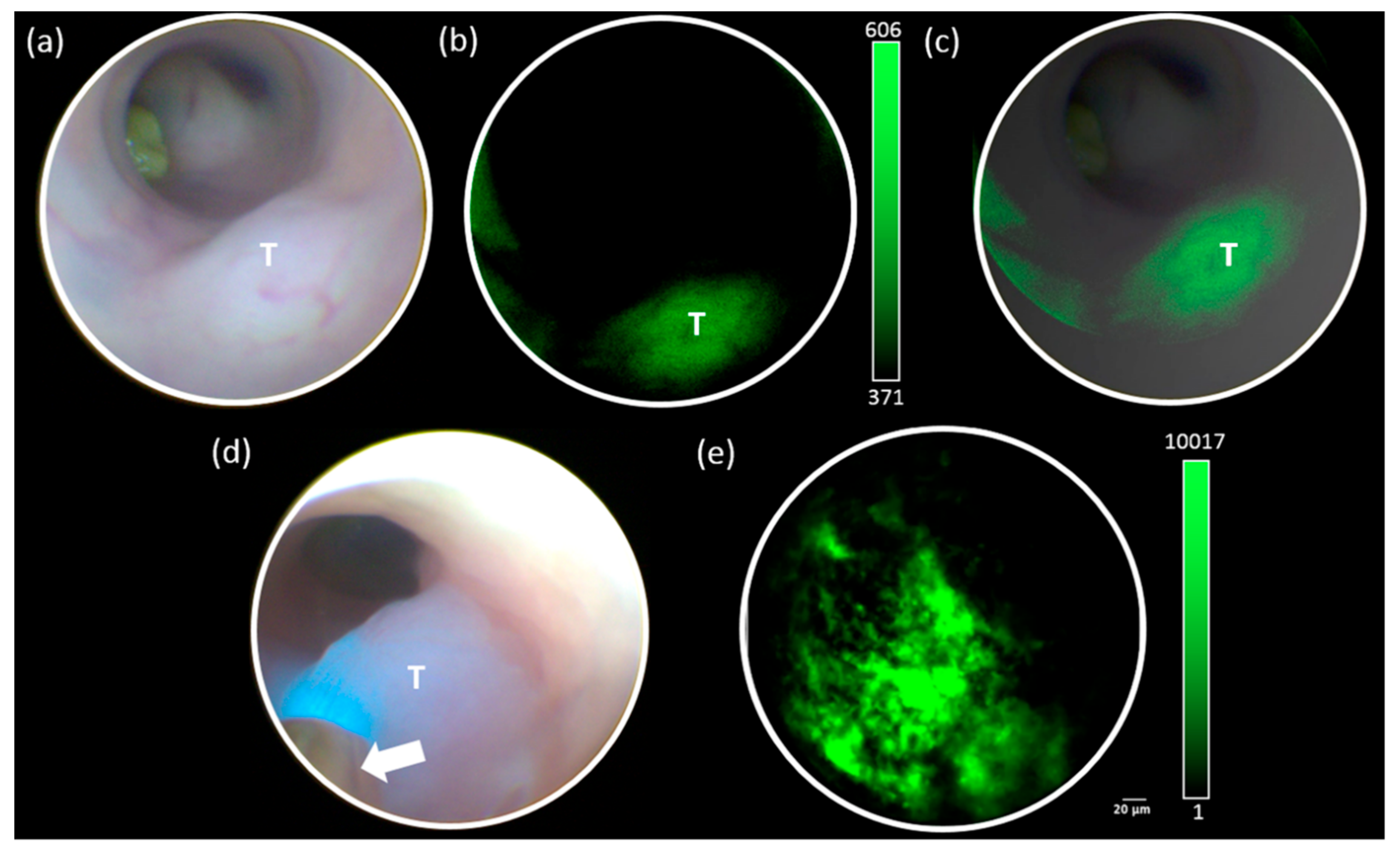
3.3. In Vivo Bacterial Imaging with the DSFE System
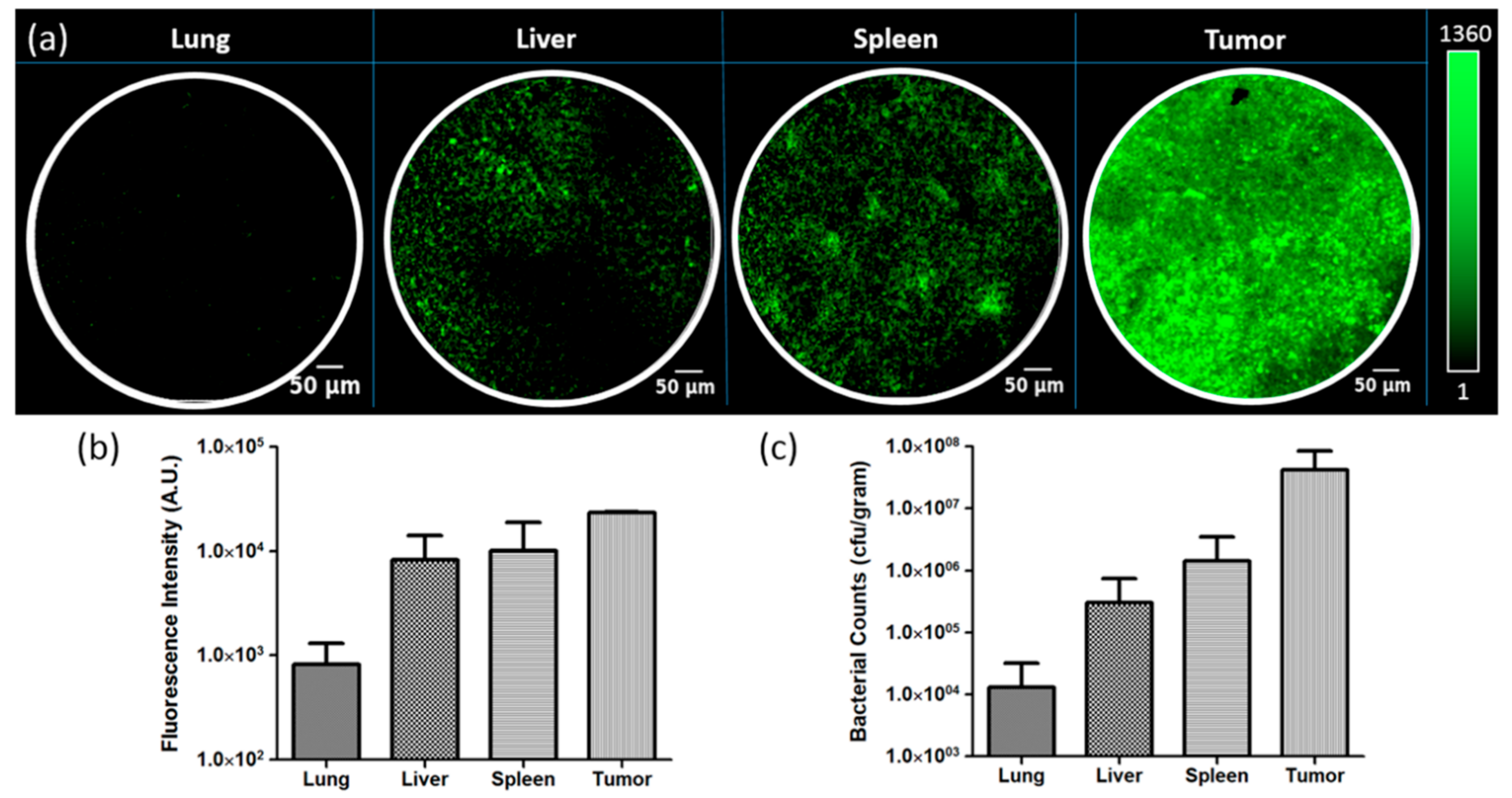
3.4. Ex Vivo Quantification of Fluorescent Bacteria
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Guren, T.K.; Thomsen, M.; Kure, E.H.; Sorbye, H.; Glimelius, B.; Pfeiffer, P.; Osterlund, P.; Sigurdsson, F.; Lothe, I.M.B.; Dalsgaard, A.M.; et al. Cetuximab in treatment of metastatic colorectal cancer: Final survival analyses and extended RAS data from the NORDIC-VII study. Br. J. Cancer 2017, 116, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouche, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Idrees, M.; Tejani, M. Current Treatment Strategies for Elderly Patients with Metastatic Colon Cancer. Cureus 2019, 11, e4713. [Google Scholar] [CrossRef]
- Datta, N.R.; Ordonez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef]
- Forbes, N.S.; Coffin, R.S.; Deng, L.; Evgin, L.; Fiering, S.; Giacalone, M.; Gravekamp, C.; Gulley, J.L.; Gunn, H.; Hoffman, R.M.; et al. White paper on microbial anti-cancer therapy and prevention. J. Immunother Cancer 2018, 6, 78. [Google Scholar] [CrossRef]
- Song, S.; Vuai, M.S.; Zhong, M. The role of bacteria in cancer therapy-enemies in the past, but allies at present. Infect. Agent Cancer 2018, 13, 9. [Google Scholar] [CrossRef]
- Roberts, N.J.; Zhang, L.; Janku, F.; Collins, A.; Bai, R.Y.; Staedtke, V.; Rusk, A.W.; Tung, D.; Miller, M.; Roix, J.; et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med. 2014, 6, 249ra111. [Google Scholar] [CrossRef]
- Agrawal, N.; Bettegowda, C.; Cheong, I.; Geschwind, J.F.; Drake, C.G.; Hipkiss, E.L.; Tatsumi, M.; Dang, L.H.; Diaz, L.A., Jr.; Pomper, M.; et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc. Natl. Acad. Sci. USA 2004, 101, 15172–15177. [Google Scholar] [CrossRef]
- Thamm, D.H.; Kurzman, I.D.; King, I.; Li, Z.; Sznol, M.; Dubielzig, R.R.; Vail, D.M.; MacEwen, E.G. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: Phase I evaluation. Clin. Cancer Res. 2005, 11, 4827–4834. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.W.; Lee, C.H. Salmonella as an innovative therapeutic antitumor agent. Int. J. Mol. Sci. 2014, 15, 14546–14554. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Phan, T.X.; Nguyen, V.H.; Dinh-Vu, H.V.; Zheng, J.H.; Yun, M.; Park, S.G.; Hong, Y.; Choy, H.E.; Szardenings, M.; et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1beta. Theranostics 2015, 5, 1328–1342. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Liu, Q.; Li, P.; Luo, H.; Wang, H.; Kong, Q. Genetically engineered Salmonella Typhimurium: Recent advances in cancer therapy. Cancer Lett. 2019, 448, 168–181. [Google Scholar] [CrossRef]
- Zheng, J.H.; Nguyen, V.H.; Jiang, S.N.; Park, S.H.; Tan, W.; Hong, S.H.; Shin, M.G.; Chung, I.J.; Hong, Y.; Bom, H.S.; et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017, 9, eaak9537. [Google Scholar] [CrossRef]
- Sedighi, M.; Zahedi Bialvaei, A.; Hamblin, M.R.; Ohadi, E.; Asadi, A.; Halajzadeh, M.; Lohrasbi, V.; Mohammadzadeh, N.; Amiriani, T.; Krutova, M.; et al. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 2019, 8, 3167–3181. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Kim, H.S.; Ha, J.M.; Hong, Y.; Choy, H.E.; Min, J.J. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res. 2010, 70, 18–23. [Google Scholar] [CrossRef]
- Heimann, D.M.; Rosenberg, S.A. Continuous intravenous administration of live genetically modified salmonella typhimurium in patients with metastatic melanoma. J. Immunother. 2003, 26, 179–180. [Google Scholar] [CrossRef]
- Toso, J.F.; Gill, V.J.; Hwu, P.; Marincola, F.M.; Restifo, N.P.; Schwartzentruber, D.J.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Stock, F.; et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 2002, 20, 142–152. [Google Scholar] [CrossRef]
- Cunningham, C.; Nemunaitis, J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version: April 9, 2001. Hum. Gene Ther. 2001, 12, 1594–1596. [Google Scholar]
- Min, J.J.; Nguyen, V.H.; Kim, H.J.; Hong, Y.; Choy, H.E. Quantitative bioluminescence imaging of tumor-targeting bacteria in living animals. Nat. Protoc. 2008, 3, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.N.; Park, S.H.; Lee, H.J.; Zheng, J.H.; Kim, H.S.; Bom, H.S.; Hong, Y.; Szardenings, M.; Shin, M.G.; Kim, S.C.; et al. Engineering of bacteria for the visualization of targeted delivery of a cytolytic anticancer agent. Mol. Ther. 2013, 21, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.; Nguyen, V.H.; D’Alincourt, M.S.; Manuel, E.R.; Kaltcheva, T.; Tsai, W.; Blazar, B.R.; Diamond, D.J.; Melstrom, L.G. Salmonella-mediated therapy targeting indoleamine 2, 3-dioxygenase 1 (IDO) activates innate immunity and mitigates colorectal cancer growth. Cancer Gene Ther. 2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, S.; Piao, L.; Yuan, F. Radiotherapy combined with an engineered Salmonella typhimurium inhibits tumor growth in a mouse model of colon cancer. Exp. Anim. 2016, 65, 413–418. [Google Scholar] [CrossRef]
- Bibby, M.C. Orthotopic models of cancer for preclinical drug evaluation: Advantages and disadvantages. Eur. J. Cancer 2004, 40, 852–857. [Google Scholar] [CrossRef]
- Hoffman, R.M. Orthotopic is orthodox: Why are orthotopic-transplant metastatic models different from all other models? J. Cell Biochem. 1994, 56, 1–3. [Google Scholar] [CrossRef]
- Oh, G.; Yoo, S.W.; Jung, Y.; Ryu, Y.M.; Park, Y.; Kim, S.Y.; Kim, K.H.; Kim, S.; Myung, S.J.; Chung, E. Intravital imaging of mouse colonic adenoma using MMP-based molecular probes with multi-channel fluorescence endoscopy. Biomed. Opt. Express 2014, 5, 1677–1689. [Google Scholar] [CrossRef][Green Version]
- Gounaris, E.; Ishihara, Y.; Shrivastrav, M.; Bentrem, D.; Barrett, T.A. Near-Infrared Fluorescence Endoscopy to Detect Dysplastic Lesions in the Mouse Colon. Methods Mol Biol 2016, 1422, 137–147. [Google Scholar]
- Bae, S.M.; Bae, D.J.; Do, E.J.; Oh, G.; Yoo, S.W.; Lee, G.J.; Chae, J.S.; Yun, Y.; Kim, S.; Kim, K.H.; et al. Multi-Spectral Fluorescence Imaging of Colon Dysplasia InVivo Using a Multi-Spectral Endoscopy System. Transl. Oncol. 2019, 12, 226–235. [Google Scholar] [CrossRef]
- Dunbar, K.; Canto, M. Confocal endomicroscopy. Curr. Opin. Gastroenterol. 2008, 24, 631–637. [Google Scholar] [CrossRef]
- Mielke, L.; Preaudet, A.; Belz, G.; Putoczki, T. Confocal laser endomicroscopy to monitor the colonic mucosa of mice. J. Immunol. Methods 2015, 421, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Waldner, M.J.; Wirtz, S.; Neufert, C.; Becker, C.; Neurath, M.F. Confocal laser endomicroscopy and narrow-band imaging-aided endoscopy for in vivo imaging of colitis and colon cancer in mice. Nat. Protoc. 2011, 6, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, F.; Siciliano, S.; Maione, F.; Esposito, D.; Masone, S.; Persico, M.; De Palma, G.D. Confocal Laser Endomicroscopy in the Study of Colonic Mucosa in IBD Patients: A Review. Gastroenterol. Res. Pract. 2012, 2012, 525098. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Fang, Y.; Shen, Z.; Gong, W.; Liu, T.; Wen, J.; Zhang, W.; Zhu, X.; Zhong, H.; Wang, T.; et al. Use of confocal laser endomicroscopy with a fluorescently labeled fatty acid to diagnose colorectal neoplasms. Oncotarget 2017, 8, 58934–58947. [Google Scholar] [CrossRef]
- Gheonea, D.I.; Saftoiu, A.; Ciurea, T.; Popescu, C.; Georgescu, C.V.; Malos, A. Confocal laser endomicroscopy of the colon. J. Gastrointestin. Liver Dis. 2010, 19, 207–211. [Google Scholar]
- Song, M.; Kim, H.J.; Kim, E.Y.; Shin, M.; Lee, H.C.; Hong, Y.; Rhee, J.H.; Yoon, H.; Ryu, S.; Lim, S.; et al. ppGpp-dependent stationary phase induction of genes on Salmonella pathogenicity island 1. J. Biol. Chem. 2004, 279, 34183–34190. [Google Scholar] [CrossRef]
- Yoo, S.W.; Oh, G.; Safi, A.M.; Hwang, S.; Seo, Y.S.; Lee, K.H.; Kim, Y.L.; Chung, E. Endoscopic non-ablative fractional laser therapy in an orthotopic colon tumour model. Sci. Rep. 2018, 8, 1673. [Google Scholar] [CrossRef]
- Becker, C.; Fantini, M.C.; Neurath, M.F. High resolution colonoscopy in live mice. Nat. Protoc. 2006, 1, 2900–2904. [Google Scholar] [CrossRef]
- Low, K.B.; Ittensohn, M.; Le, T.; Platt, J.; Sodi, S.; Amoss, M.; Ash, O.; Carmichael, E.; Chakraborty, A.; Fischer, J.; et al. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat. Biotechnol. 1999, 17, 37–41. [Google Scholar] [CrossRef]
- Mascolo, M.; Staibano, S.; Ilardi, G.; Siano, M.; Vecchione, M.L.; Esposito, D.; De Rosa, G.; De Palma, G.D. Probe-based confocal laser endomicroscopy evaluation of colon preneoplastic lesions, with particular attention to the aberrant crypt foci, and comparative assessment with histological features obtained by conventional endoscopy. Gastroenterol. Res. Pract. 2012, 2012, 645173. [Google Scholar] [CrossRef]
- Li, C.Q.; Yu, T.; Zuo, X.L.; Xie, X.J.; Li, W.B.; Chu, C.L.; Zuo, F.; Li, Y.Q. Effects on confocal laser endomicroscopy image quality by different acriflavine concentrations. J. Interv. Gastroenterol. 2011, 1, 59–63. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, S.W.; Nguyen, D.-h.; Park, S.; Lee, H.; Lee, C.-M.; Lee, C.; Min, J.-J. Development of Dual-Scale Fluorescence Endoscopy for In Vivo Bacteria Imaging in an Orthotopic Mouse Colon Tumor Model . Appl. Sci. 2020, 10, 844. https://doi.org/10.3390/app10030844
Yoo SW, Nguyen D-h, Park S, Lee H, Lee C-M, Lee C, Min J-J. Development of Dual-Scale Fluorescence Endoscopy for In Vivo Bacteria Imaging in an Orthotopic Mouse Colon Tumor Model . Applied Sciences. 2020; 10(3):844. https://doi.org/10.3390/app10030844
Chicago/Turabian StyleYoo, Su Woong, Dinh-huy Nguyen, Suhyeon Park, Hyeri Lee, Chang-Moon Lee, Changho Lee, and Jung-Joon Min. 2020. "Development of Dual-Scale Fluorescence Endoscopy for In Vivo Bacteria Imaging in an Orthotopic Mouse Colon Tumor Model " Applied Sciences 10, no. 3: 844. https://doi.org/10.3390/app10030844
APA StyleYoo, S. W., Nguyen, D.-h., Park, S., Lee, H., Lee, C.-M., Lee, C., & Min, J.-J. (2020). Development of Dual-Scale Fluorescence Endoscopy for In Vivo Bacteria Imaging in an Orthotopic Mouse Colon Tumor Model . Applied Sciences, 10(3), 844. https://doi.org/10.3390/app10030844






