Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion
Abstract
:1. Introduction
2. The Role of Phytochemicals in Oxidative Stress
2.1. Phytochemicals as Antioxidant Agents
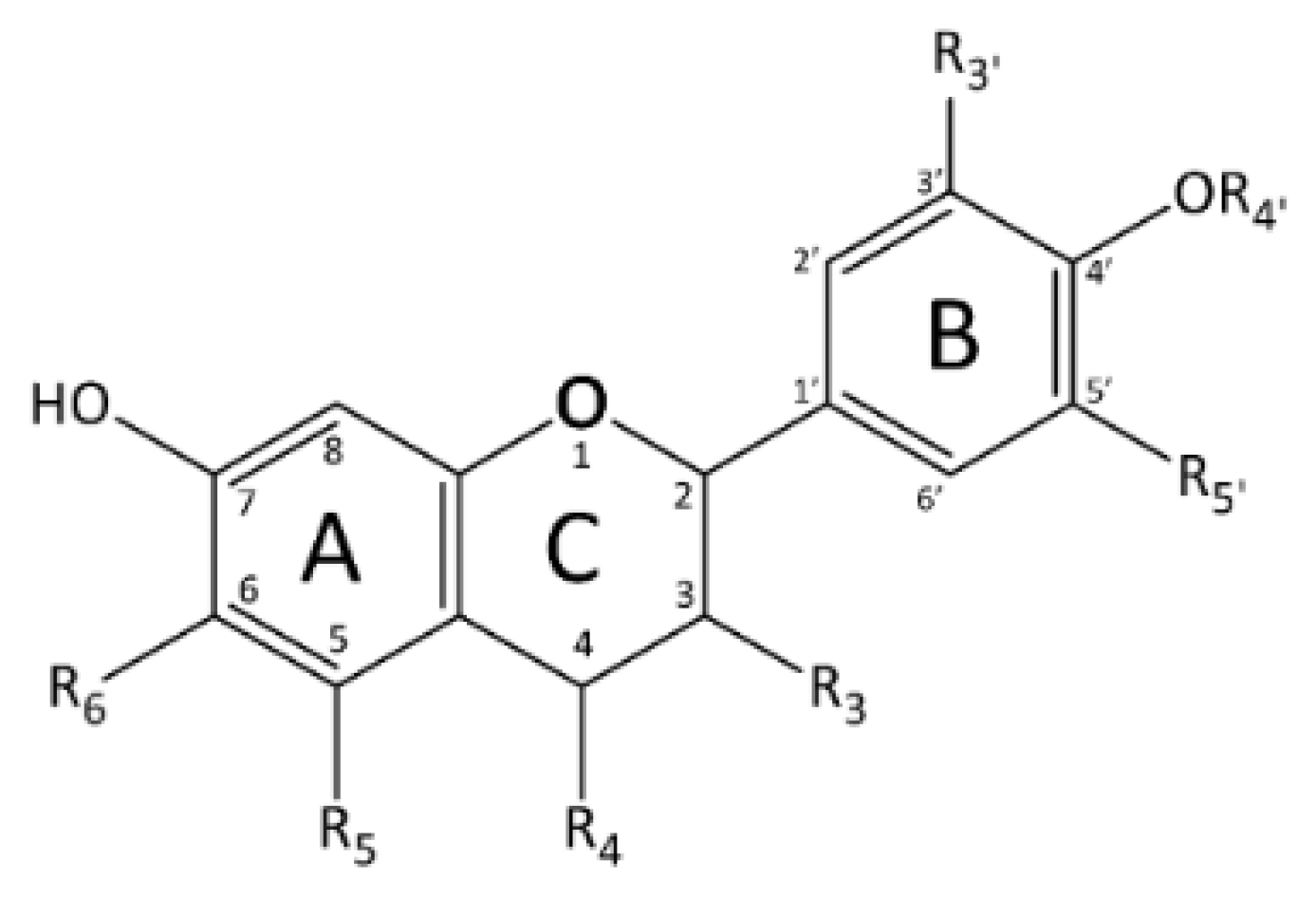
2.2. Case Study: Antioxidant Activity of Flavonoids
2.2.1. Free-Radical Scavenging
2.2.2. Metal-Ion Chelating
2.2.3. Inhibition of Pro-Oxidant Enzymes
2.2.4. Activation of Antioxidant Enzymes
2.3. Phytochemicals as Pro-Oxidant Agents: Friend or Foe?
3. Therapeutic Relevance of Plant-Derived Antioxidants in Aging and Aging-Related Diseases
3.1. Anti-Aging
3.2. Aging-Related Diseases
3.2.1. Cardiovascular Diseases
3.2.2. Cancer
3.2.3. Diabetes
3.2.4. Neurodegenerative Disorders
4. The Evidence in Humans
4.1. Cardiovascular Risk
4.2. Diabetes Mellitus
5. Plant Compounds and Longevity
6. Concluding Remarks and Upcoming Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sharifi-Rad, M.; Salehi, B.; Iriti, M.; Roointan, A.; Mnayer, D.; Soltani-Nejad, A.; Afshari, A. In vitro and in vivo assessment of free radical scavenging and antioxidant activities of Veronica persica Poir. Cell. Mol. Biol. 2018, 64, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Noctor, G. Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface between Stress Perception and Physiological Responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Martorell, M.; Arbiser, J.L.; Sureda, A.; Martins, N.; Maurya, P.K.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or Negative Actors? Biomolecules 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghi Aminjan, H.; Abtahi, S.R.; Hazrati, E.; Chamanara, M.; Jalili, M.; Paknejad, B. Targeting of oxidative stress and inflammation through ROS/NF-kappaB pathway in phosphine-induced hepatotoxicity mitigation. Life Sci. 2019, 232, 116607. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A.M. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79. [Google Scholar] [CrossRef] [Green Version]
- Myburgh, K.H. Polyphenol supplementation: Benefits for exercise performance or oxidative stress? Sport Med. 2014, 44, 57–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V.; Dumas, F.; Alscher, R.G.; Erturk, N.; Heath, L.S.; Couée, I.; Sulmon, C.; Gouesbet, G.; et al. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2002, 91, 174–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Flavonoids: A Re-Run of the carotenoids story? Novartis Found Symp. 2007, 282, 93–104. [Google Scholar] [PubMed]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.C.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, 310–329. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Jajic, I.; Sarna, T.; Strzalka, K. Senescence, Stress, and Reactive Oxygen Species. Plants 2015, 4, 393–411. [Google Scholar] [CrossRef] [Green Version]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Kjølsrud-Bøhn, S.; Sandvik, L.; Blomhoff, R. Anthocyanins Inhibit Nuclear Factor-κB Activation in Monocytes and Reduce Plasma Concentrations of Pro-Inflammatory Mediators in Healthy Adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jiao, H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radical’s, and singlet oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef]
- Leoncini, E.; Nedovic, D.; Panic, N.; Pastorino, R.; Edefonti, V.; Boccia, S. Carotenoid intake from natural sources and head and neck cancer: A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Takeda, A.; Nyssen, O.P.; Syed, A.; Jansen, E.; Bueno-De-Mesquita, B.; Gallo, V. Vitamin A and carotenoids and the risk of parkinson’s disease: A systematic review and meta-analysis. Neuroepidemiology 2014, 42, 25–38. [Google Scholar] [CrossRef]
- Soares, N. da C.P.; Teodoro, A.J.; Lotsch, P.F.; Granjeiro, J.M.; Borojevic, R. Anticancer properties of carotenoids in prostate cancer. A review. Histol. Histopathol. 2015, 30, 1143–1154. [Google Scholar]
- Chajès, V.; Romieu, I. Nutrition and breast cancer. Maturitas 2014, 77, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase-derived reactive species: Physiological and pathological effects. Oxid. Med. Cell. Longev. 2016, 3527579. [Google Scholar] [CrossRef] [Green Version]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase in cancer: More than a differentiation marker. Cancer Med. 2016, 5, 546–557. [Google Scholar] [CrossRef]
- Battelli, M.G.; Bolognesi, A.; Polito, L. Pathophysiology of circulating xanthine oxidoreductase: New emerging roles for a multi-tasking enzyme. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1502–1517. [Google Scholar] [CrossRef] [Green Version]
- Gutteridge, J.C.; Halliwell, B. Free Radicals and Antioxidants in the Year 2000: A Historical Look to the Future. Ann. N. Y. Acad. Sci. 2006, 899, 136–147. [Google Scholar] [CrossRef]
- McGregor, G.P.; Biesalski, H.K. Rationale and impact of vitamin C in clinical nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 697–703. [Google Scholar] [CrossRef]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Radical chemistry of flavonoid antioxidants. In Antioxidants in Therapy and Preventative Medicine; Springer: Berlin/Heidelberg, Germany, 1990; pp. 165–170. ISBN 978-1-4684-5732-2. [Google Scholar]
- Bors, W.; Michel, C. Antioxidant capacity of flavanols and gallate esters: Pulse radiolysis studies. Free Radic. Biol. Med. 1999, 27, 1413–1426. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H. Bin Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Londhe, J.S.; Devasagayam, T.P.A.; Foo, L.Y.; Ghaskadbi, S.S. Radioprotective Properties of Polyphenols from Phyllanthus amarus Linn. J. Radiat. Res. 2009, 50, 303–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moharram, F.A.; Marzouk, M.S.A.; Ibrahim, M.T.; Mabry, T.J. Antioxidant galloylated flavonol glycosides from Calliandra haematocephala. Nat. Prod. Res. 2006, 20, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Trouillas, P.; Marsal, P.; Siri, D.; Lazzaroni, R.; Duroux, J.L. A DFT study of the reactivity of OH groups in quercetin and taxifolin antioxidants: The specificity of the 3-OH site. Food Chem. 2006, 97, 679–688. [Google Scholar] [CrossRef]
- Bubols, G.B.; Vianna, D. da R.; Medina-Remon, A.; von Poser, G.; Lamuela-Raventos, R.M.; Eifler-Lima, V.L.; Garcia, S.C. The Antioxidant Activity of Coumarins and Flavonoids. Mini-Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of the Antioxidant Activity of Flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef]
- Moalin, M.; Van Strijdonck, G.P.F.; Beckers, M.; Hagemen, G.J.; Borm, P.J.; Bast, A.; Haenen, G.R.M.M. A planar conformation and the hydroxyl groups in the B and C rings play a pivotal role in the antioxidant capacity of quercetin and quercetin derivatives. Molecules 2011, 16, 9636–9650. [Google Scholar] [CrossRef] [Green Version]
- Stepanić, V.; Trošelj, K.G.; Lučić, B.; Marković, Z.; Amić, D. Bond dissociation free energy as a general parameter for flavonoid radical scavenging activity. Food Chem. 2013, 141, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.L.; Li, X.; Gu, Y.; Wright, P.M.; Chang, D.Y. Repair of oxidative DNA damage: Mechanisms and functions. Cell Biochem. Biophys. 2001, 35, 141–170. [Google Scholar] [CrossRef]
- Rai, P.; Wemmer, D.E.; Linn, S. Preferential binding and structural distortion by Fe2+at RGGG-containing DNA sequences correlates with enhanced oxidative cleavage at such sequences. Nucleic Acids Res. 2005, 33, 497–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Lin, C.M.; Chen, C.S.; Chen, C.T.; Liang, Y.C.; Lin, J.K. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem. Biophys. Res. Commun. 2002, 294, 167–172. [Google Scholar] [CrossRef]
- Van Hoorn, D.E.C.; Nijveldt, R.J.; Van Leeuwen, P.A.M.; Hofman, Z.; M’Rabet, L.; De Bont, D.B.A.; Van Norren, K. Accurate prediction of xanthine oxidase inhibition based on the structure of flavonoids. Eur. J. Pharmacol. 2002, 451, 111–118. [Google Scholar] [CrossRef]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- O’Leary, K.A.; De Pascual-Tereasa, S.; Needs, P.W.; Bao, Y.P.; O’Brien, N.M.; Williamson, G. Effect of flavonoids and Vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 551, 245–254. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.L.; Tsushida, T.; Terao, J. Inhibition of mammalian 15-lipoxygenase-dependent lipid peroxidation in low-density lipoprotein by quercetin and quercetin monoglucosides. Arch. Biochem. Biophys. 1998, 349, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-lipoxygenases by flavonoids: Structure-activity relations and mode of action. Biochem. Pharmacol. 2003, 65, 773–781. [Google Scholar] [CrossRef]
- Batteli, M.G. Xanthine Oxidoreductase in Drug Metabolism: Beyond a Role as a Detoxifying Enzyme. Curr. Med. Chem. 2016, 23, 4027–4036. [Google Scholar] [CrossRef] [Green Version]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Döring, F.; Wolffram, S.; Rimbach, G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar] [CrossRef]
- Miyamoto, N.; Izumi, H.; Miyamoto, R.; Bin, H.; Kondo, H.; Tawara, A.; Sasaguri, Y.; Kohno, K. Transcriptional regulation of activating transcription factor 4 under oxidative stress in retinal pigment epithelial ARPE-19/HPV-16 cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1226–1234. [Google Scholar] [CrossRef] [Green Version]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.N.T. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef]
- Lee-Hilz, Y.Y.; Boerboom, A.M.J.F.; Westphal, A.H.; Van Berkel, W.J.H.; Aarts, J.M.M.J.G.; Rietjens, I.M.C.M. Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem. Res. Toxicol. 2006, 19, 1499–1505. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Fazal, F.; Rahman, A.; Hadi, S.M.; Parish, J.H. Activities of flavonoids for the cleavage of DNA in the presence of cu(II): Correlation with generation of active oxygen species. Carcinogenesis 1992, 13, 605–608. [Google Scholar] [CrossRef]
- Cherubini, A.; Ruggiero, C.; Polidori, M.C.; Mecocci, P. Potential markers of oxidative stress in stroke. Free Radic. Biol. Med. 2005, 39, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Flora, S.J.S. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid. Med. Cell. Longev. 2009, 2, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, N.; Arakawa, T.; Ohnishi, M.; Furuno, K. Anti- and pro-oxidative effects of flavonoids on metal-induced lipid hydroperoxide-dependent lipid peroxidation in cultured hepatocytes loaded with α-linolenic acid. Free Radic. Biol. Med. 1999, 27, 1313–1323. [Google Scholar] [CrossRef]
- Yordi, E.G.; Pérez, E.M.; Matos, M.J.; Villares, E.U. Antioxidant and Pro-Oxidant Effects of Polyphenolic Compounds and Structure-Activity Relationship Evidence. In Nutrition, Well-Being and Health; IntechOpen Limited: London, UK, 2012; ISBN 978-953-51-0125-3. [Google Scholar]
- Azmi, A.S.; Sarkar, F.H.; Hadi, S. Pro-oxidant activity of dietary chemopreventive agents: An under-appreciated anti-cancer property. F1000Research 2013, 2, 135. [Google Scholar] [CrossRef] [Green Version]
- Arif, H.; Sohail, A.; Farhan, M.; Rehman, A.A.; Ahmad, A.; Hadi, S.M. Flavonoids-induced redox cycling of copper ions leads to generation of reactive oxygen species: A potential role in cancer chemoprevention. Int. J. Biol. Macromol. 2018, 106, 569–578. [Google Scholar] [CrossRef]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [Green Version]
- Akagawa, M.; Shigemitsu, T.; Suyama, K. Production of Hydrogen Peroxide by Polyphenols and Polyphenol-rich Beverages under Quasi -physiological Conditions. Biosci. Biotechnol. Biochem. 2003, 67, 2632–2640. [Google Scholar] [CrossRef]
- Chai, P.C.; Long, L.H.; Halliwell, B. Contribution of hydrogen peroxide to the cytotoxicity of green tea and red wines. Biochem. Biophys. Res. Commun. 2003, 304, 650–654. [Google Scholar] [CrossRef]
- Kachadourian, R.; Day, B.J. Flavonoid-induced glutathione depletion: Potential implications for cancer treatment. Free Radic. Biol. Med. 2006, 41, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.N.T. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: Antioxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharmacol. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Palliyaguru, D.L.; Kensler, T.W. Frugal chemoprevention: Targeting Nrf2 with foods rich in sulforaphane. Semin. Oncol. 2016, 43, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, T.; Ishikawa, M.; Miyoshi, N.; Yasunaga, M.; Akagawa, M.; Uchida, K.; Nakamura, Y. Catechol type polyphenol is a potential modifier of protein sulfhydryls: Development and application of a new probe for understanding the dietary polyphenol actions. Chem. Res. Toxicol. 2009, 22, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Na, H.K.; Surh, Y.J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef]
- Copple, I.M.; Shelton, L.M.; Walsh, J.; Kratschmar, D.V.; Lister, A.; Odermatt, A.; Goldring, C.E.; Dinkova-Kostova, A.T.; Honda, T.; Park, B.K. Chemical tuning enhances both potency toward Nrf2 and in vitro therapeutic index of triterpenoids. Toxicol. Sci. 2014, 140, 462–469. [Google Scholar] [CrossRef] [Green Version]
- Sirota, R.; Gibson, D.; Kohen, R. The role of the catecholic and the electrophilic moieties of caffeic acid in Nrf2/Keap1 pathway activation in ovarian carcinoma cell lines. Redox Biol. 2015, 4, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.P.; Hayashi, T.; Cottam, H.B.; Jin, G.; Yao, S.; Wu, C.C.N.; Rosenbach, M.D.; Corr, M.; Schwab, R.B.; Carson, D.A. Nrf2 responses and the therapeutic selectivity of electrophilic compounds in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2010, 107, 7479–7484. [Google Scholar] [CrossRef] [Green Version]
- Reynaert, N.L.; van der Vliet, A.; Guala, A.S.; McGovern, T.; Hristova, M.; Pantano, C.; Heintz, N.H.; Heim, J.; Ho, Y.-S.; Matthews, D.E.; et al. Dynamic redox control of NF- B through glutaredoxin-regulated S-glutathionylation of inhibitory B kinase beta. Proc. Natl. Acad. Sci. USA 2006, 103, 13086–13091. [Google Scholar] [CrossRef] [Green Version]
- Heyninck, K.; Lahtela-Kakkonen, M.; Van Der Veken, P.; Haegeman, G.; Berghe, W. Vanden Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKβ. Biochem. Pharmacol. 2014, 91, 501–509. [Google Scholar] [CrossRef]
- Son, P.S.; Park, S.A.; Na, H.K.; Jue, D.M.; Kim, S.; Surh, Y.J. Piceatannol, a catechol-type polyphenol, inhibits phorbol ester-induced NF-κB activation and cyclooxygenase-2 expression in human breast epithelial cells: Cysteine 179 of IKKβ as a potential target. Carcinogenesis 2010, 31, 1442–1449. [Google Scholar] [CrossRef] [Green Version]
- Weseler, A.R.; Bast, A. Oxidative stress and vascular function: Implications for pharmacologic treatments. Curr. Hypertens. Rep. 2010, 12, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Gastell, P.L.P.; De Alejo, J.L.P. Métodos para medir el daño oxidativo. Rev. Cuba. Med. Mil. 2000, 29, 192–198. [Google Scholar]
- Harman, D. Aging: A Theory based on Free Radical and Radiation Chemestry. J Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Aitbaev, K.; Murkamilov, I.; Fomin, V. Molecular mechanisms of aging: The role of oxidative stress and epigenetic modifications. Adv. Gerontol. 2019, 32, 20–28. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.-Z.; No, M.-H.; Heo, J.-W.; Park, D.-H.; Kang, J.-H.; Kim, S.H.; Kwak, H.-B. Role of exercise in age-related sarcopenia. J Exerc. Rehabil. 2018, 14, 551–558. [Google Scholar] [CrossRef]
- Gilca, M.; Stoian, I.; Atanasiu, V.; Virgolici, B. The oxidative hypothesis of senescence. J. Postgrad. Med. 2007, 53, 207–213. [Google Scholar] [CrossRef]
- Hutcheson, R.; Rocic, P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: The great exploration. Exp. Diabetes Res. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Milisav, I.; Ribari, S.; Poljsak, B. Antioxidant Vitamins and Ageing; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9789811328350. [Google Scholar]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Booth, S.L.; Sadowski, J.A.; Prior, R.L. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am. J. Clin. Nutr. 1998, 68, 1081–1087. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Lau, F.C.; Josep, J.A. Berry fruit supplementation and the aging brain. J. Agric. Food Chem. 2008, 56, 636–641. [Google Scholar] [CrossRef]
- Seeram, N.P.; Cichewicz, R.H.; Chandra, A.; Nair, M.G. Cyclooxygenase inhibitory and antioxidant compounds from crabapple fruits. J. Agric. Food Chem. 2003, 51, 1948–1951. [Google Scholar] [CrossRef]
- Maurya, P.K.; Rizvi, S.I. Protective role of tea catechins on erythrocytes subjected to oxidative stress during human aging. Nat. Prod. Res. 2009, 23, 1072–1079. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle 2008, 7, 1020–1037. [Google Scholar] [CrossRef] [Green Version]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites—Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Xu, J.; Rottinghaus, G.E.; Simonyi, A.; Lubahn, D.; Sun, G.Y.; Sun, A.Y. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002, 958, 439–447. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Del Mar Bibiloni, M.; Tur, J.; Pons, A.; Sureda, A. Cardioprotective Effects of the Polyphenol Hydroxytyrosol from Olive Oil. Curr Drug Targets 2017, 18, 1477–1486. [Google Scholar] [CrossRef]
- Fabiani, R.; Rosignoli, P.; De Bartolomeo, A.; Fuccelli, R.; Servili, M.; Montedoro, G.F.; Morozzi, G. Oxidative DNA Damage Is Prevented by Extracts of Olive Oil, Hydroxytyrosol, and Other Olive Phenolic Compounds in Human Blood Mononuclear Cells and HL60 Cells. J. Nutr. 2008, 138, 1411–1416. [Google Scholar] [CrossRef] [Green Version]
- Bolli, R. Oxygen-derived free radicals and myocardial reperfusion injury: An overview. Cardiovasc. Drugs Ther. 1991, 5, 249–268. [Google Scholar] [CrossRef]
- Dubick, M.A.; Omaye, S.T. Evidence for grape, wine and tea polyphenols as modulators of atherosclerosis and ischemic heart disease in humans. J. Nutraceuticals Funct. Med. Foods 2001, 3, 67–93. [Google Scholar] [CrossRef]
- Zhao, Y.; Shu, P.; Zhang, Y.; Lin, L.; Zhou, H.; Xu, Z.; Suo, D.; Xie, A.; Jin, X. Effect of centella asiatica on oxidative stress and lipid metabolism in hyperlipidemic animal models. Oxid. Med. Cell. Longev. 2014, 2014, 154295. [Google Scholar] [CrossRef] [Green Version]
- Higano, T.; Kuroda, M.; Sakagami, H.; Mimaki, Y. Convallasaponin A, a New 5β-Spirostanol Triglycoside from the Rhizomes of Convallaria majalis. Chem. Pharm. Bull. (Tokyo) 2007, 55, 337–339. [Google Scholar] [CrossRef] [Green Version]
- Pirola, L.; Fröjdö, S. Resveratrol: One molecule, many targets. IUBMB Life 2008, 60, 323–332. [Google Scholar] [CrossRef]
- Montó, F.; Arce, C.; Noguera, M.A.; Ivorra, M.D.; Flanagan, J.; Roller, M.; Issaly, N.; D’Ocon, P. Action of an extract from the seeds of Fraxinus excelsior L. on metabolic disorders in hypertensive and obese animal models. Food Funct. 2014, 5, 786–796. [Google Scholar] [CrossRef]
- da Chunga, A.P. Digitalis purpurea, Digitalis lanata. In Plantas e Produtos Vegetais em Fitoterapia; Fundação Calouste Gulbenkian: Lisboa, Portugal, 2006; pp. 274–275. [Google Scholar]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of Carcinogenesis by Dietary Polyphenolic Compounds. Ann. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [Green Version]
- Johnson, I.T.; Williamson, G.; Musk, S.R.R. Anticarcinogenic Factors in Plant Foods: A New Class of Nutrients? Nutr. Res. Rev. 1994, 7, 175. [Google Scholar] [CrossRef] [Green Version]
- Ezzat, S.; Hegazy, A.; Amer, A.M.; Kamel, G.; El-Alfy, T. Isolation of biologically active constituents from Moringa peregrina (Forssk.) Fiori. (family: Moringaceae) growing in Egypt. Pharmacogn. Mag. 2011, 7, 109. [Google Scholar] [CrossRef] [Green Version]
- Bachmeier, B.E.; Mirisola, V.; Romeo, F.; Generoso, L.; Esposito, A.; Dell’Eva, R.; Blengio, F.; Killian, P.H.; Albini, A.; Pfeffer, U. Reference profile correlation reveals estrogen-like trancriptional activity of curcumin. Cell. Physiol. Biochem. 2010, 26, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Senft, C.; Polacin, M.; Priester, M.; Seifert, V.; Kögel, D.; Weissenberger, J. The nontoxic natural compound Curcumin exerts anti-proliferative, anti-migratory, and anti-invasive properties against malignant gliomas. BMC Cancer 2010, 10, 491. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Bower, K.A.; Wang, S.; Frank, J.A.; Chen, G.; Ding, M.; Wang, S.; Shi, X.; Ke, Z.; Luo, J. Cyanidin-3-Glucoside inhibits ethanol-induced invasion of breast cancer cells overexpressing ErbB2. Mol. Cancer 2010, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Acharya, A.; Das, I.; Singh, S.; Saha, T. Chemopreventive properties of indole-3-carbinol, diindolylmethane and other constituents of cardamom against carcinogenesis. Recent Patents Food Nutr. Agric. 2010, 2, 166–177. [Google Scholar]
- Das, A.; Banik, N.L.; Ray, S.K. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer 2010, 116, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, T.C.; Wu, J.M. Targeting CWR22Rv1 prostate cancer cell proliferation and gene expression by combinations of the phytochemicals EGCG, genistein and quercetin. Anticancer Res. 2009, 29, 4025–4032. [Google Scholar]
- Qiao, Y.; Cao, J.; Xie, L.; Shi, X. Cell growth inhibition and gene expression regulation by (-)-epigallocatechin-3-gallate in human cervical cancer cells. Arch. Pharm. Res. 2009, 32, 1309–1315. [Google Scholar] [CrossRef]
- Imran, A.; Butt, M.S.; Xiao, H.; Imran, M.; Rauf, A.; Mubarak, M.S.; Ramadan, M.F. Inhibitory effect of black tea (Camellia sinensis) theaflavins and thearubigins against HCT 116 colon cancer cells and HT 460 lung cancer cells. J. Food Biochem. 2019, 43, e12822. [Google Scholar] [CrossRef]
- Sánchez, Y.; Amrán, D.; de Blas, E.; Aller, P. Regulation of genistein-induced differentiation in human acute myeloid leukaemia cells (HL60, NB4). Protein kinase modulation and reactive oxygen species generation. Biochem. Pharmacol. 2009, 77, 384–396. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Oo Khor, T.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Lee, J.-H.; Tony Kong, A.-N. Plants vs. Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anticancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Ahmed, M.; Khan, M.I.; Muhammad, M.R.; Khan, A.U.; Khan, R.A. Role of medicinal plants in oxidative stress and cancer. Sci. Rep. 2013, 2, 641. [Google Scholar]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta 2014, 1840, 2709–2729. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Kumar, N.V.A.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Das Gupta, P.; Amartya, D. Diabetes Mellitus and its herbal treatment. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 706–721. [Google Scholar]
- Hegazy, G.A.; Alnoury, A.M.; Gad, H.G. The role of Acacia Arabica extract as an antidiabetic, antihyperlipidemic, and antioxidant in streptozotocin-induced diabetic rats. Saudi Med. J. 2013, 34, 727–733. [Google Scholar]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef]
- Benard, O.; Chi, Y. Medicinal Properties of Mangiferin, Structural Features, Derivative Synthesis, Pharmacokinetics and Biological Activities. Mini-Rev. Med. Chem. 2015, 15, 582–594. [Google Scholar] [CrossRef]
- Lampariello, L.; Cortelazzo, A.; Guerranti, R.; Sticozzi, C.; Valacchi, G. The magic velvet bean of mucuna pruriens. J. Tradit. Complement. Med. 2012, 2, 331–339. [Google Scholar] [CrossRef]
- Iriti, M.; Vitalini, S.; Fico, G.; Faoro, F. Neuroprotective herbs and foods from different traditional medicines and diets. Molecules 2010, 15, 3517–3555. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.C.; Zhou, Y.C.; Chen, Y.; Zhu, Y.G.; Fang, F.; Chen, L.M. Ginsenoside Rg1 reduces MPTP-induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol. Sin. 2005, 26, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudakewich, M.; Ba, F.; Benishin, C.G. Neurotrophic and neuroprotective actions of ginsenosides Rb1and Rg1. Planta Med. 2001, 67, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Teter, B.; Frautschy, S.A. Neuroprotective effects of curcumin. Adv. Exp. Med. Biol. 2007, 595, 197–212. [Google Scholar] [PubMed] [Green Version]
- Wang, M.Y.; Peng, L.; Weidenbacher-Hoper, V.; Deng, S.; Anderson, G.; West, B.J. Noni juice improves serum lipid profiles and other risk markers in cigarette smokers. Sci. World J. 2012, 2012, 594657. [Google Scholar] [CrossRef]
- Babish, J.G.; Dahlberg, C.J.; Ou, J.J.; Keller, W.J.; Gao, W.; Kaadige, M.R.; Brabazon, H.; Lamb, J.; Soudah, H.C.; Kou, X.; et al. Synergistic in vitro antioxidant activity and observational clinical trial of F105, a phytochemical formulation including Citrus bergamia, in subjects with moderate cardiometabolic risk factors. Can. J. Physiol. Pharmacol. 2016, 94, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Silveira, J.Q.; Dourado, G.K.Z.S.; Cesar, T.B. Red-fleshed sweet orange juice improves the risk factors for metabolic syndrome. Int. J. Food Sci. Nutr. 2015, 66, 830–836. [Google Scholar] [CrossRef]
- Kardum, N.; Milovanović, B.; Šavikin, K.; Zdunić, G.; Mutavdžin, S.; Gligorijević, T.; Spasić, S. Beneficial Effects of Polyphenol-Rich Chokeberry Juice Consumption on Blood Pressure Level and Lipid Status in Hypertensive Subjects. J. Med. Food 2015, 18, 1231–1238. [Google Scholar] [CrossRef]
- Han, H.J.; Jung, U.J.; Kim, H.-J.; Cho, S.-J.; Kim, A.H.; Han, Y.; Choi, M.-S. Combined Supplementation with Grape Pomace and Omija Fruit Ethanol Extracts Dose-Dependently Improves Body Composition, Plasma Lipid Profiles, Inflammatory Status, and Antioxidant Capacity in Overweight and Obese Subjects. J. Med. Food 2016, 19, 170–180. [Google Scholar] [CrossRef]
- Ivanova, D.; Tasinov, O.; Kiselova-Kaneva, Y. Improved lipid profile and increased serum antioxidant capacity in healthy volunteers after Sambucus ebulus L. fruit infusion consumption. Int. J. Food Sci. Nutr. 2014, 65, 740–744. [Google Scholar] [CrossRef]
- Dostal, A.M.; Samavat, H.; Espejo, L.; Arikawa, A.Y.; Stendell-Hollis, N.R.; Kurzer, M.S. Green Tea Extract and Catechol-O-Methyltransferase Genotype Modify Fasting Serum Insulin and Plasma Adiponectin Concentrations in a Randomized Controlled Trial of Overweight and Obese Postmenopausal Women. J. Nutr. 2016, 146, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.K.; Choi, S.W.; Siu, P.M.; Benzie, I.F.F. Effects of single dose and regular intake of green tea (Camellia sinensis) on DNA damage, DNA repair, and heme oxygenase-1 expression in a randomized controlled human supplementation study. Mol. Nutr. Food Res. 2014, 58, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Argani, H.; Ghorbanihaghjo, A.; Vatankhahan, H.; Rashtchizadeh, N.; Raeisi, S.; Ilghami, H. The effect of red grape seed extract on serum paraoxonase activity in patients with mild to moderate hyperlipidemia. Sao Paulo Med. J. 2016, 134, 234–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janiques, A.G.; Leal Vde, O.; Stockler-Pinto, M.B.; Moreira, N.X.; Mafra, D. Effects of grape powder supplementation on inflammatory and antioxidant markers in hemodialysis patients: A randomized double-blind study. J. Bras. Nefrol. 2014, 36, 461–501. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Shen, Y.C.; Huang, T.Y.; Venkatakrishnan, K.; Wang, C.K. Cardioprotective Efficacy of Red Wine Extract of Onion in Healthy Hypercholesterolemic Subjects. Phyther. Res. 2016, 30, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Ahn, Y.; Kwon, O.; Lee, M.Y.; Lee, C.H.; Lee, S.; Park, T.; Kwon, S.W.; Kim, J.Y. Dietary wolfberry extract modifies oxidative stress by controlling the expression of inflammatory mrnas in overweight and hypercholesterolemic subjects: A randomized, double-blind, placebo-controlled trial. J. Agric. Food Chem. 2017, 65, 309–316. [Google Scholar] [CrossRef]
- Verma, S.K.; Jain, V.; Singh, D.P. Effect of Pueraria tuberosa DC. (Indian Kudzu) on blood pressure, fibrinolysis and oxidative stress in patients with stage 1 hypertension. Pakistan J. Biol. Sci. 2012, 15, 742–747. [Google Scholar] [CrossRef]
- Panahi, Y.; Dadjou, Y.; Pishgoo, B.; Akbari, A.; Sahebkar, A. Antioxidant Activity of Heracleum persicum Fruit Extract: Evidence from a Randomized Controlled Trial. J. Diet. Suppl. 2016, 13, 530–537. [Google Scholar] [CrossRef]
- Urquiaga, I.; Ávila, F.; Echeverria, G.; Perez, D.; Trejo, S.; Leighton, F. A Chilean Berry Concentrate Protects against Postprandial Oxidative Stress and Increases Plasma Antioxidant Activity in Healthy Humans. Oxid. Med. Cell. Longev. 2017, 2017, 8361493. [Google Scholar] [CrossRef] [Green Version]
- McAnulty, L.S.; Collier, S.R.; Landram, M.J.; Whittaker, D.S.; Isaacs, S.E.; Klemka, J.M.; Cheek, S.L.; Arms, J.C.; McAnulty, S.R. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014, 34, 577–584. [Google Scholar] [CrossRef]
- Wu, P.-T.; Fitschen, P.J.; Kistler, B.M.; Jeong, J.H.; Chung, H.R.; Aviram, M.; Phillips, S.A.; Fernhall, B.; Wilund, K.R. Effects of Pomegranate Extract Supplementation on Cardiovascular Risk Factors and Physical Function in Hemodialysis Patients. J. Med. Food 2015, 18, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, P.; Crowe, W.; Bahar, B.; Harnedy, P.A.; Brown, E.S.; Taylor, S.S.; Smyth, T.J.; Soler-Vila, A.; Magee, P.J.; Gill, C.I.R.; et al. The effect of consuming Palmaria palmata-enriched bread on inflammatory markers, antioxidant status, lipid profile and thyroid function in a randomised placebo-controlled intervention trial in healthy adults. Eur. J. Nutr. 2016, 55, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Yeung, V.T.F.; Collins, A.R.; Benzie, I.F.F. Redox-linked effects of green tea on DNA damage and repair, and influence of microsatellite polymorphism in HMOX-1: Results of a human intervention trial. Mutagenesis 2015, 30, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shidfar, F.; Rajab, A.; Rahideh, T.; Khandouzi, N.; Hosseini, S.; Shidfar, S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J. Complement. Integr. Med. 2015, 12, 165–170. [Google Scholar] [CrossRef]
- Kaatabi, H.; Bamosa, A.O.; Badar, A.; Al-Elq, A.; Abou-Hozaifa, B.; Lebda, F.; Al-Khadra, A.; Al-Almaie, S. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: Placebo controlled participant blinded clinical trial. PLoS ONE 2015, 10, e0113486. [Google Scholar] [CrossRef]
- Mesaik, M.A.; Ahmed, A.; Khalid, A.S.; Jan, S.; Siddiqui, A.A.; Perveen, S.; Azim, M.K. Effect of Grewia asiatica fruit on glycemic index and phagocytosis tested in healthy human subjects. Pak. J. Pharm. Sci. 2013, 26, 85–89. [Google Scholar]
- Seo, S.K.; Hong, Y.; Yun, B.H.; Chon, S.J.; Jung, Y.S.; Park, J.H.; Cho, S.; Choi, Y.S.; Lee, B.S. Antioxidative effects of Korean red ginseng in postmenopausal women: A double-blind randomized controlled trial. J. Ethnopharmacol. 2014, 154, 753–757. [Google Scholar] [CrossRef]
- Mercken, E.M.; Carboneau, B.A.; Krzysik-Walker, S.M.; De Cabo, R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev. 2012, 11, 390–398. [Google Scholar] [CrossRef] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194. [Google Scholar] [CrossRef] [Green Version]
- Niso-Santano, M.; González-Polo, R.A.; Paredes-Barquero, M.; Fuentes, J.M.; Aschner, M. Natural Products in the Promotion of Healthspan and Longevity. Clin. Pharmacol. Transl. Med. 2019, 3, 149–151. [Google Scholar]
- Esposito, K.; Di Palo, C.; Maiorino, M.I.; Petrizzo, M.; Bellastella, G.; Siniscalchi, I.; Giugliano, D. Long-term effect of mediterranean-style diet and calorie restriction on biomarkers of longevity and oxidative stress in overweight men. Cardiol. Res. Pract. 2011, 1, 293916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckland, G.; Agudo, A.; Travier, N.; María Huerta, J.; Cirera, L.; Tormo, M.J.; Navarro, C.; Dolores Chirlaque, M.; Moreno-Iribas, C.; Ardanaz, E.; et al. Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Br. J. Nutr. 2011, 106, 1581–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Dietary patterns, Mediterranean diet, and cardiovascular disease. Curr. Opin. Lipidol. 2014, 25, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N.D.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A.D. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [Green Version]
- Pes, G.M.; Tolu, F.; Dore, M.P.; Sechi, G.P.; Errigo, A.; Canelada, A.; Poulain, M. Male longevity in Sardinia, a review of historical sources supporting a causal link with dietary factors. Eur. J. Clin. Nutr. 2015, 69, 411–418. [Google Scholar] [CrossRef]
- Eleftheriou, D.; Benetou, V.; Trichopoulou, A.; La Vecchia, C.; Bamia, C. Mediterranean diet and its components in relation to all-cause mortality: Meta-analysis. Br. J. Nutr. 2018, 120, 1081–1097. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, T.; Byles, J.; Martin, S.; Avery, J.C.; Taylor, A.W. Food habits, lifestyle factors and mortality among oldest old Chinese: The Chinese longitudinal healthy longevity survey (CLHLS). Nutrients 2015, 7, 7562–7579. [Google Scholar] [CrossRef] [Green Version]
- Vlahchev, T.; Zhivkov, Z.T. Hunza—A Healthy and a Long Living People. Asklepii 2002, 15, 96–97. [Google Scholar]
- Schmid, A. The Dom of Hunza (Northern Areas of Pakistan). In Disappearing Peoples? Routledge: London, UK, 2016; pp. 107–128. ISBN 9781315430416. [Google Scholar]
- Zarse, K.; Bossecker, A.; Müller-Kuhrt, L.; Siems, K.; Hernandez, M.A.; Berendsohn, W.G.; Birringer, M.; Ristow, M. The phytochemical glaucarubinone promotes mitochondrial metabolism, reduces body fat, and extends lifespan of Caenorhabditis elegans. Horm. Metab. Res. 2011, 43, 241–243. [Google Scholar] [CrossRef]
- Shukla, V.; Yadav, D.; Phulara, S.C.; Gupta, M.M.; Saikia, S.K.; Pandey, R. Longevity-promoting effects of 4-hydroxy-E-globularinin in Caenorhabditis elegans. Free Radic. Biol. Med. 2012, 53, 1848–1856. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, K.; Saharia, K.; Pradhan, D.; Subramaniam, J.R. Withania somnifera root extract extends lifespan of Caenorhabditis elegans. Ann. Neurosci. 2013, 20, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; An, H.S.; Jung, Y.W.; Lee, E.J.; Lee, H.Y.; Choi, E.S.; An, S.W.; Son, H.; Lee, S.J.; Kim, J.B.; et al. Korean mistletoe (Viscum album coloratum) extract extends the lifespan of nematodes and fruit flies. Biogerontology 2014, 15, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Zamberlan, D.C.; Amaral, G.P.; Arantes, L.P.; Machado, M.L.; Mizdal, C.R.; Campos, M.M.A.; Soares, F.A.A. Rosmarinus officinalis L. increases caenorhabditis elegans stress resistance and longevity in a DAF-16, HSF-1 and SKN-1-dependent manner. Braz. J. Med. Biol. Res. 2016, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liu, J.; Li, T.; Liu, R.H. Blueberry extract promotes longevity and stress tolerance via DAF-16 in Caenorhabditis elegans. Food Funct. 2018, 9, 5273–5282. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Han, Y.T.; Jeon, H.; Cha, D.S. Antiageing properties of Damaurone D in Caenorhabditis elegans. J. Pharm. Pharmacol. 2018, 70, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Phulara, S.C.; Mishra, S.K.; Bajpai, R.; Kumar, A.; Niranjan, A.; Lehri, A.; Upreti, D.K.; Chauhan, P.S. Betula utilis extract prolongs life expectancy, protects against amyloid-β toxicity and reduces Alpha Synuclien in Caenorhabditis elegans via DAF-16 and SKN-1. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 228, 108647. [Google Scholar] [CrossRef]
- Piegholdt, S.; Rimbach, G.; Wagner, A.E. The phytoestrogen prunetin affects body composition and improves fitness and lifespan in male Drosophila melanogaster. FASEB J. 2016, 30, 948–958. [Google Scholar] [CrossRef] [Green Version]
- Chatzigeorgiou, S.; Thai, Q.D.; Tchoumtchoua, J.; Tallas, K.; Tsakiri, E.N.; Papassideri, I.; Halabalaki, M.; Skaltsounis, A.L.; Trougakos, I.P. Isolation of natural products with anti-ageing activity from the fruits of Platanus orientalis. Phytomedicine 2017, 33, 53–61. [Google Scholar] [CrossRef]
- Niraula, P.; Ghimire, S.; Lee, H.; Kim, M.S. Ilex paraguariensis extends lifespan and increases an ability to resist environmental stresses in drosophila. Rejuvenation Res. 2018, 21, 497–505. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, A.; Dong, J.; Sigears, A.; Lu, B. Skin extract improves muscle function and extends lifespan of a Drosophila model of Parkinson’s disease through activation of mitophagy. Exp. Gerontol. 2018, 113, 10–17. [Google Scholar] [CrossRef]
- Arabit, J.G.J.; Elhaj, R.; Schriner, S.E.; Sevrioukov, E.A.; Jafari, M. Rhodiola rosea Improves Lifespan, Locomotion, and Neurodegeneration in a Drosophila melanogaster Model of Huntington’s Disease. Biomed Res. Int. 2018, 2018, 6726874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona-Gutierrez, D.; Zimmermann, A.; Kainz, K.; Pietrocola, F.; Chen, G.; Maglioni, S.; Schiavi, A.; Nah, J.; Mertel, S.; Beuschel, C.B.; et al. The flavonoid 4,4′-dimethoxychalcone promotes autophagy-dependent longevity across species. Nat. Commun. 2019, 10, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Bedmar, Z.; Demyda-Peyrás, S.; Merinas-Amo, T.; Del Río-Celestino, M. Nutraceutic potential of two allium species and their distinctive organosulfur compounds: A multi-assay evaluation. Foods 2019, 8, 222. [Google Scholar] [CrossRef] [Green Version]
- Tomobe, K.; Fujii, H.; Sun, B.; Nishioka, H.; Aruoma, O.I. Modulation of infection-induced inflammation and locomotive deficit and longevity in senescence-accelerated mice-prone (SAMP8) model by the oligomerized polyphenol Oligonol. Biomed. Pharmacother. 2007, 61, 427–434. [Google Scholar] [CrossRef]
- Porquet, D.; Casadesús, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegrí, C.; Sanfeliu, C.; Camins, A.; Pallàs, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age (Omaha) 2013, 35, 1851–1865. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Sun, H.; Liu, D.; Jiao, Z.; Yue, S.; He, X.; Xia, W.; Ji, J.; Xiang, L. Protective effect of a phenolic extract containing indoline amides from Portulaca oleracea against cognitive impairment in senescent mice induced by large dose of D-galactose /NaNO2. J. Ethnopharmacol. 2017, 203, 252–259. [Google Scholar] [CrossRef]
- Dutta, K.; Patel, P.; Julien, J.P. Protective effects of Withania somnifera extract in SOD1 G93A mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2018, 309, 193–204. [Google Scholar] [CrossRef]
- Aires, V.; Labbé, J.; Deckert, V.; Pais de Barros, J.P.; Boidot, R.; Haumont, M.; Maquart, G.; Le Guern, N.; Masson, D.; Prost-Camus, E.; et al. Healthy adiposity and extended lifespan in obese mice fed a diet supplemented with a polyphenol-rich plant extract. Sci. Rep. 2019, 9, 9134. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.H.; Wu, J.C.; Ho, C.T.; Badmaev, V. Effects of water extract of Curcuma longa (L.) roots on immunity and telomerase function. J. Complement. Integr. Med. 2017, 14. [Google Scholar] [CrossRef]
- Souza-Monteiro, J.R.; Arrifano, G.P.F.; Queiroz, A.I.D.G.; Mello, B.S.F.; Custódio, C.S.; Macêdo, D.S.; Hamoy, M.; Paraense, R.S.O.; Bittencourt, L.O.; Lima, R.R.; et al. Antidepressant and Antiaging Effects of Açaí (Euterpe oleracea Mart.) in Mice. Oxid. Med. Cell. Longev. 2019, 2019, 3614960. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.; V. Anil Kumar, N.; Dini, L.; Panzarini, E.; Rajkovic, J.; Valere Tsouh Fokou, P.; Peluso, I.; et al. Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Appl. Sci. 2020, 10, 947. https://doi.org/10.3390/app10030947
Salehi B, Azzini E, Zucca P, Maria Varoni E, V. Anil Kumar N, Dini L, Panzarini E, Rajkovic J, Valere Tsouh Fokou P, Peluso I, et al. Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Applied Sciences. 2020; 10(3):947. https://doi.org/10.3390/app10030947
Chicago/Turabian StyleSalehi, Bahare, Elena Azzini, Paolo Zucca, Elena Maria Varoni, Nanjangud V. Anil Kumar, Luciana Dini, Elisa Panzarini, Jovana Rajkovic, Patrick Valere Tsouh Fokou, Ilaria Peluso, and et al. 2020. "Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion" Applied Sciences 10, no. 3: 947. https://doi.org/10.3390/app10030947
APA StyleSalehi, B., Azzini, E., Zucca, P., Maria Varoni, E., V. Anil Kumar, N., Dini, L., Panzarini, E., Rajkovic, J., Valere Tsouh Fokou, P., Peluso, I., Prakash Mishra, A., Nigam, M., El Rayess, Y., El Beyrouthy, M., N. Setzer, W., Polito, L., Iriti, M., Sureda, A., Magdalena Quetglas-Llabrés, M., ... Sharifi-Rad, J. (2020). Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Applied Sciences, 10(3), 947. https://doi.org/10.3390/app10030947


















