Removal of Pb(II) Ions from Wastewater by Using Polyethyleneimine-Functionalized Fe3O4 Magnetic Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation s of Fe3O4 MNPs
2.3. Characterization
2.4. Batch Adsorption Experiments
3. Results and Discussion
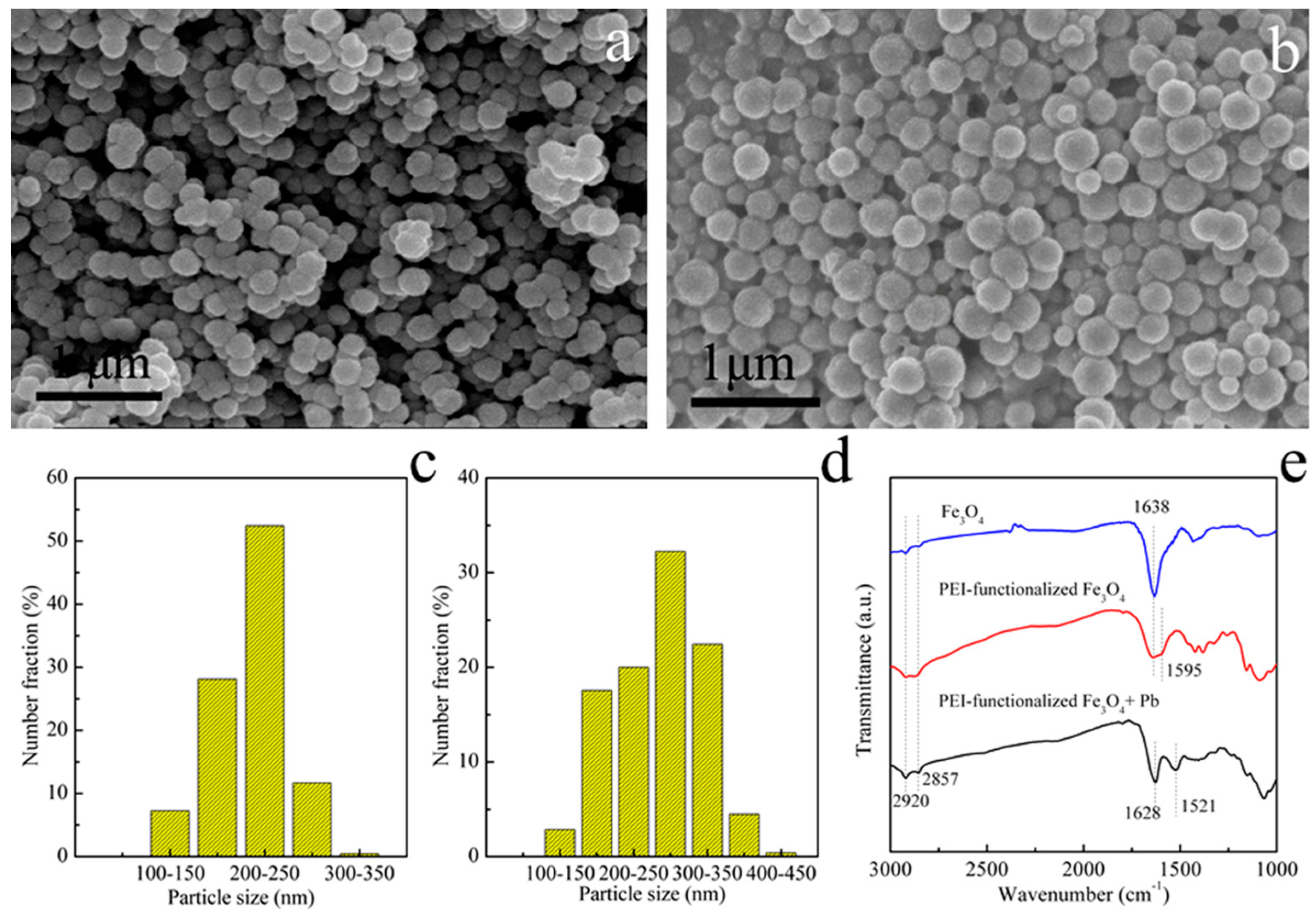
3.1. Characterizations
3.2. Adsorption Kinetics
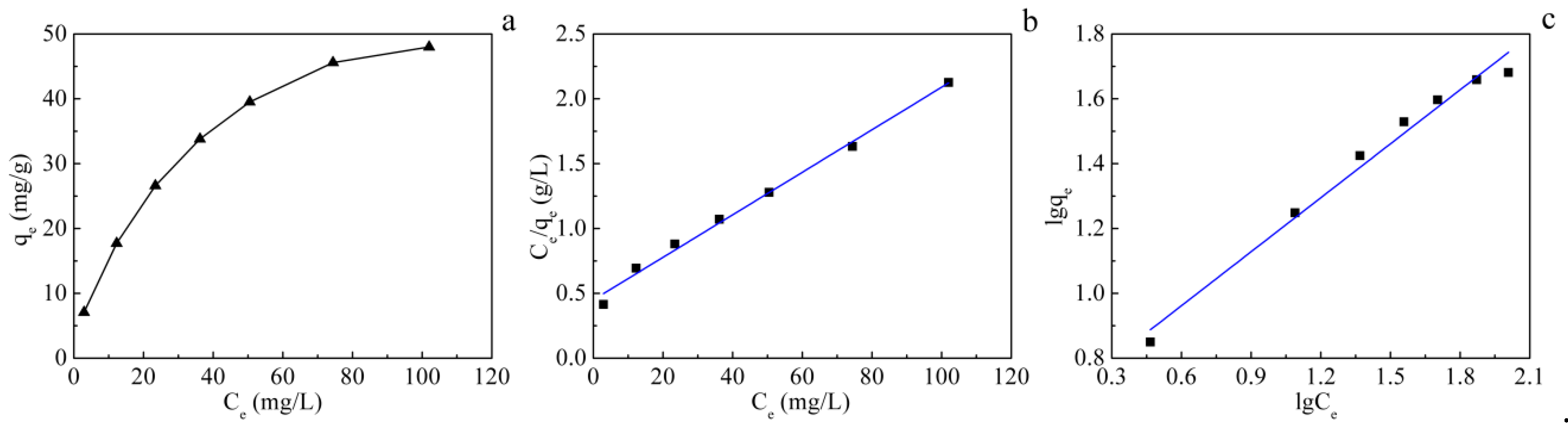
3.3. Adsorption Isotherm
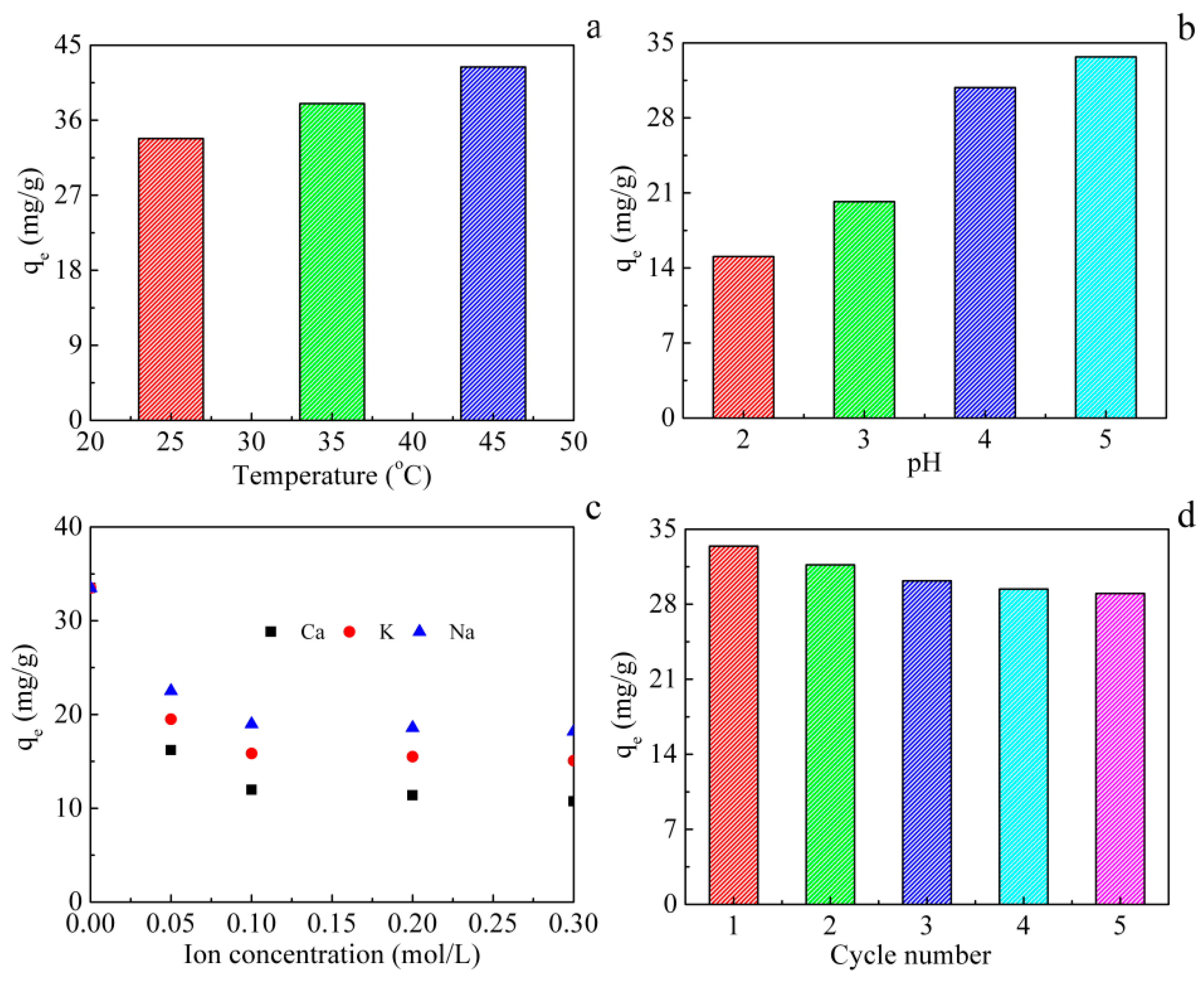
3.4. Factors Affecting Adsorption
3.5. Reusability of MNPs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liang, X.; Quyang, X.; Wang, S.; Yang, L.; Huang, F.; Ji, C.; Chen, X. Efficient adsorption of Pb(II) from aqueous solutions using aminopropyltriethoxysilane-modified magnetic attapulgite@chitosan (APTS-Fe3O4/APT@CS) composite hydrogel beads. Int. J. Biol. Macromol. 2019, 137, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, R.; Liu, H.; Li, M.; Chen, T.; Chen, D.; Zou, X.; Frost, R.L. Green preparation of magnetic biochar for the effective accumulation of Pb(II): Performance and mechanism. Chem. Eng. J. 2019, 375, 122011. [Google Scholar] [CrossRef]
- Huang, X.; Wei, D.; Zhang, X.; Fan, D.; Sun, X.; Du, B.; Wei, Q. Synthesis of amino-functionalized magnetic aerobic granular sludge-biochar for Pb(II) removal: Adsorption performance and mechanism studies. Sci. Total Environ. 2019, 685, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Le, V.T.; Pham, T.M.; Doan, V.D.; Lebedeva, O.E.; Nguyen, H.T. Removal of Pb(II) ions from aqueous solution using a novel composite adsorbent of Fe3O4/PVA/spent coffee grounds. Sep. Sci. Technol. 2019, 54, 3070–3081. [Google Scholar] [CrossRef]
- Jiang, R.; Tian, J.; Zheng, H.; Qi, J.; Sun, S.; Li, X. A novel magnetic adsorbent based on waste litchi peels for removing Pb(II) from aqueous solution. J. Environ. Manag. 2015, 155, 24–30. [Google Scholar] [CrossRef]
- Ge, H.; Hua, T.; Chen, X. Selective adsorption of lead on grafted and crosslinked chitosan nanoparticles prepared by using Pb2+ as template. J. Hazard. Mater. 2016, 308, 225–232. [Google Scholar] [CrossRef]
- Niu, Y.; Qu, R.; Sun, C.; Wang, C.; Chen, H.; Ji, C.; Zhang, Y.; Shao, X.; Bu, F. Adsorption of Pb(II) from aqueous solution by Silica-gel supported hyperbranched polyamidoamine dendrimers. J. Hazard. Mater. 2013, 244, 276–286. [Google Scholar] [CrossRef]
- Wang, P.; Du, M.; Zhu, H.; Bao, S.; Yan, T.; Zou, M. Structure regulation of silica nanotubes and their adsorption behaviors for heavy metal ions: pH effect, kinetics, isotherms and mechanism. J. Hazard. Mater. 2015, 286, 533–544. [Google Scholar] [CrossRef]
- Ahrouch, M.; Gatica, J.M.; Draoui, K.; Bellido, D.; Vidal, H. Lead removal from aqueous solution by means of integral natural clays honeycomb monoliths. J. Hazard. Mater. 2019, 365, 519–530. [Google Scholar] [CrossRef]
- Yadav, V.B.; Gadi, R.; Kalra, S. Clay based nanocomposites for removal of heavy metals from water: A review. J. Environ. Manag. 2019, 232, 803–817. [Google Scholar] [CrossRef]
- Pandey, P.K.; Sharma, S.K.; Sambi, S.S. Removal of lead (II) from waste water on zeolite-NaX. J. Environ. Chem. Eng. 2015, 3, 2604–2610. [Google Scholar] [CrossRef]
- Acharya, J.; Sahu, J.N.; Mohanty, C.R.; Meikap, B.C. Removal of lead(II) from wastewater by activated carbon developed from tamarind wood by zinc chloride activation. Chem. Eng. J. 2009, 149, 249–262. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K.; Al-Saadi, A.A. Adsorption of lead ions from aqueous solution using porous carbon derived from rubber tires: Experimental and computational study. J. Colloid Interface Sci. 2013, 396, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, G.; Zheng, H.; Li, F.; Ngo, H.H.; Guo, W.; Liu, C.; Chen, L.; Xing, B. Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour. Technol. 2015, 177, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Amritphale, S.S.; Chandra, N. Removal of lead from aqueous solution by hybrid precursor prepared by rice hull. J. Hazard. Mater. 2009, 163, 1194–1198. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Le, D.H.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef]
- Gatabi, J.; Sarrafi, Y.; Lakouraj, M.M.; Taghavi, M. Facile and efficient removal of Pb(II) from aqueous solution by chitosan-lead ion imprinted polymer network. Chemosphere 2020, 240, 124772. [Google Scholar] [CrossRef]
- Deng, S.B.; Bai, R.B.; Chen, J.P. Aminated polyacrylonitrile fibers for lead and copper removal. Langmuir 2003, 19, 5058–5064. [Google Scholar] [CrossRef]
- Liu, C.K.; Bai, R.B.; Ly, Q.S. Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: Behaviors and mechanisms. Water Res. 2008, 42, 1511–1522. [Google Scholar] [CrossRef]
- Xin, X.; Wei, Q.; Yang, J.; Yan, L.; Feng, R.; Chen, G.; Du, B.; Li, H. Highly efficient removal of heavy metal ions by amine-functionalized mesoporous Fe3O4 nanoparticles. Chem. Eng. J. 2012, 184, 132–140. [Google Scholar] [CrossRef]
- Zou, X.; Yin, Y.; Zhao, Y.; Chen, D.; Dong, S. Synthesis of ferriferrous oxide/l-cysteine magnetic microspheres and their adsorption capacity for Pb(II) ions. Mater. Lett. 2015, 150, 59–61. [Google Scholar] [CrossRef]
- Fu, Q.; Hu, B.; Zhou, X.; Hu, Q.; Sheng, J. Impact of key geochemical parameters on the attenuation of Pb(II) from water using a novel magnetic nanocomposite: Fulvic acid-coated magnetite nanoparticles. Desalin. Water Treat. 2016, 57, 26063–26072. [Google Scholar] [CrossRef]
- Yang, B.; Wei, Y.; Liu, Q.; Luo, Y.; Qiu, S.; Shi, Z. Polyvinylpyrrolidone functionalized magnetic graphene-based composites for highly efficient removal of lead from wastewater. Colloid Surf. A 2019, 582, 123927. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Chen, R.; Sun, M.; Tong, H.; Xu, J. Preparation of ion imprinted magnetic Fe3O4 nanoparticles for selective remediation of Pb(II). J. Taiwan Inst. Chem. Eng. 2017, 80, 184–191. [Google Scholar] [CrossRef]
- Zendehdel, M.; Ramezani, M.; Shoshtari-Yeganeh, B.; Cruciani, G.; Salmani, A. Simultaneous removal of Pb(II), Cd (II) and bacteria from aqueous solution using amino functionalized Fe3O4/NaP zeolite nanocomposites. Environ. Technol. 2019, 40, 3689–3704. [Google Scholar] [CrossRef] [PubMed]
- Bee, A.; Talbot, D.; Abramson, S.; Dupuis, V. Magnetic alginate beads for Pb(II) ions removal from wastewater. J. Colloid Interface Sci. 2011, 362, 486–492. [Google Scholar] [CrossRef]
- Lü, T.; Qi, D.; Zhang, D.; Zhang, C.; Zhao, H. One-step synthesis of versatile magnetic nanoparticles for efficiently removing emulsified oil droplets and cationic and anionic heavy metal ions from the aqueous environment. Environ. Sci. Pollut. Res. 2019, 26, 6153–6166. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, F.; Zhang, Y.; Wei, M.; Zhang, X.; Ling, C.; Li, A. Nitrogen-doped chitosan-Fe(III) composite as a dual-functional material for synergistically enhanced co-removal of Cu(II) and Cr(VI) based on adsorption and redox. Chem. Eng. J. 2016, 306, 579–587. [Google Scholar] [CrossRef]
- Rehman1, M.; Rehman1, W.; Waseem, M.; Hussain, S.; Haq, S.; Anees-ur Rehman, M. Adsorption mechanism of Pb2+ ions by Fe3O4, SnO2, and TiO2 nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 19968–19981. [Google Scholar] [CrossRef]
- Ji, J.; Chen, G.; Zhao, J. Preparation and characterization of amino/thiol bifunctionalized magnetic nanoadsorbent and its application in rapid removal of Pb(II) from aqueous system. J. Hazard. Mater. 2019, 368, 255–263. [Google Scholar] [CrossRef]

| qe,exp (mg/g) | Pseudo First-Order Equation | Pseudo Second-Order Equation | ||||
|---|---|---|---|---|---|---|
| k1 (1/min) | qe (mg/g) | R2 | k2 (g/(mg∙min)) | qe (mg/g) | R2 | |
| 33.651 | 0.033 | 10.199 | 0.702 | 0.023 | 34.014 | 0.999 |
| Langmuir Parameters | Freundlich Parameters | ||||
|---|---|---|---|---|---|
| qm (mg/g) | b(L/mg) | R2 | n | k (L/mg) | R2 |
| 60.976 | 0.036 | 0.992 | 1.802 | 4.254 | 0.980 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Zhang, C.; Lü, T.; Zhao, H. Removal of Pb(II) Ions from Wastewater by Using Polyethyleneimine-Functionalized Fe3O4 Magnetic Nanoparticles. Appl. Sci. 2020, 10, 948. https://doi.org/10.3390/app10030948
Tao Y, Zhang C, Lü T, Zhao H. Removal of Pb(II) Ions from Wastewater by Using Polyethyleneimine-Functionalized Fe3O4 Magnetic Nanoparticles. Applied Sciences. 2020; 10(3):948. https://doi.org/10.3390/app10030948
Chicago/Turabian StyleTao, Yu, Chuan Zhang, Ting Lü, and Hongting Zhao. 2020. "Removal of Pb(II) Ions from Wastewater by Using Polyethyleneimine-Functionalized Fe3O4 Magnetic Nanoparticles" Applied Sciences 10, no. 3: 948. https://doi.org/10.3390/app10030948
APA StyleTao, Y., Zhang, C., Lü, T., & Zhao, H. (2020). Removal of Pb(II) Ions from Wastewater by Using Polyethyleneimine-Functionalized Fe3O4 Magnetic Nanoparticles. Applied Sciences, 10(3), 948. https://doi.org/10.3390/app10030948





