TiO2 Nanostructured Films for Electrochromic Paper Based-Devices
Abstract
1. Introduction
2. Experimental Details
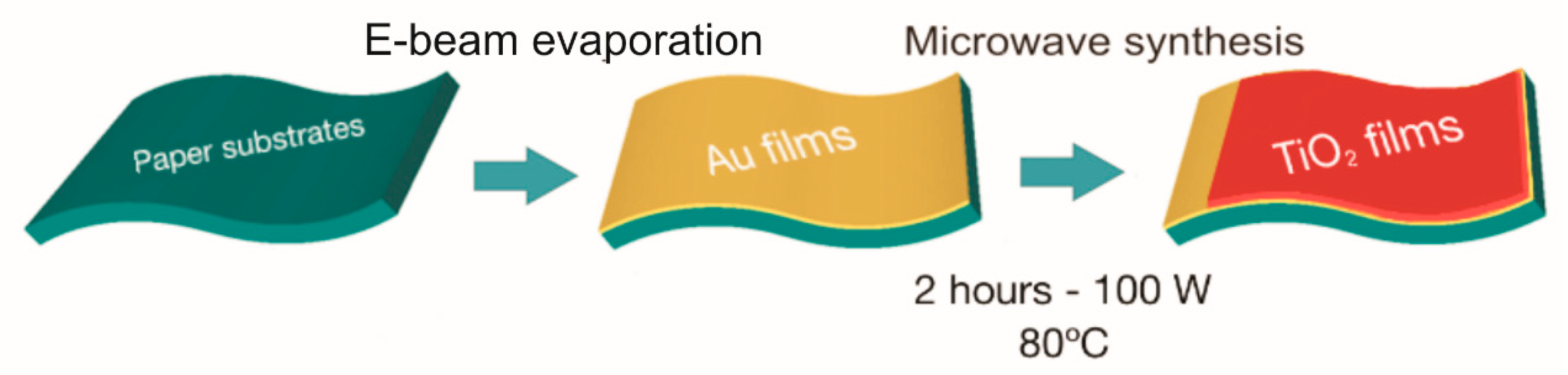
2.1. Synthesis of TiO2 Films and Gold Deposition
2.2. Characterization Techniques
2.3. Electrochromic Measurements
3. Results and Discussion
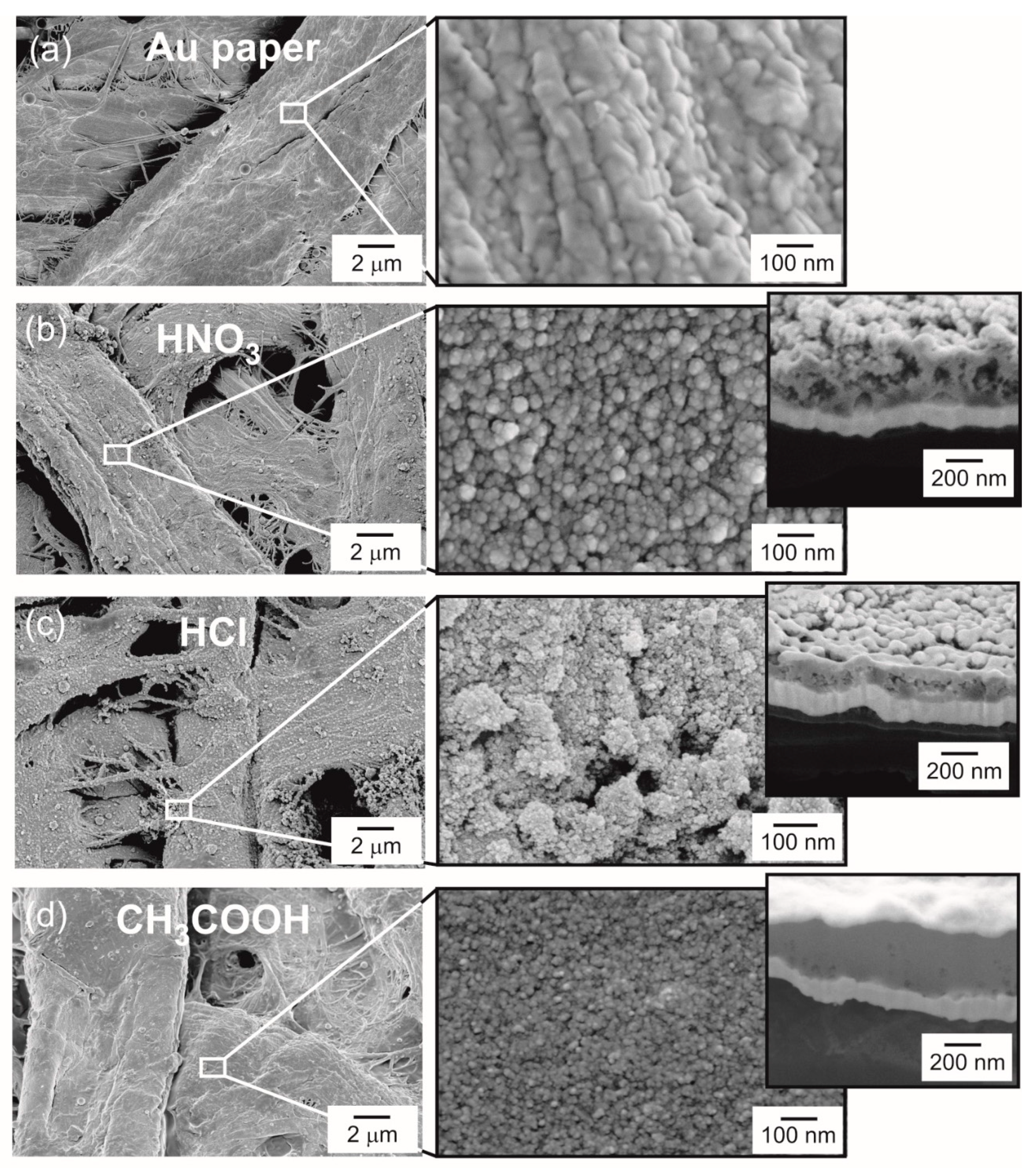
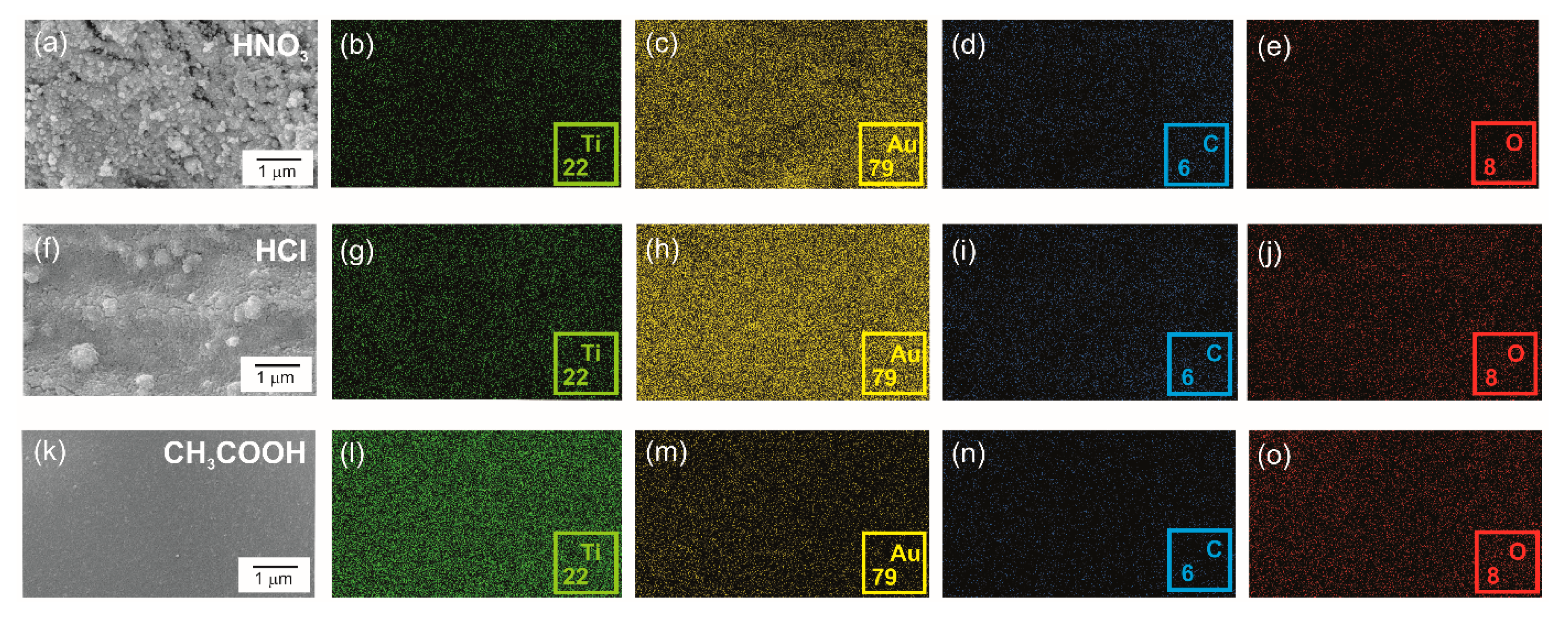
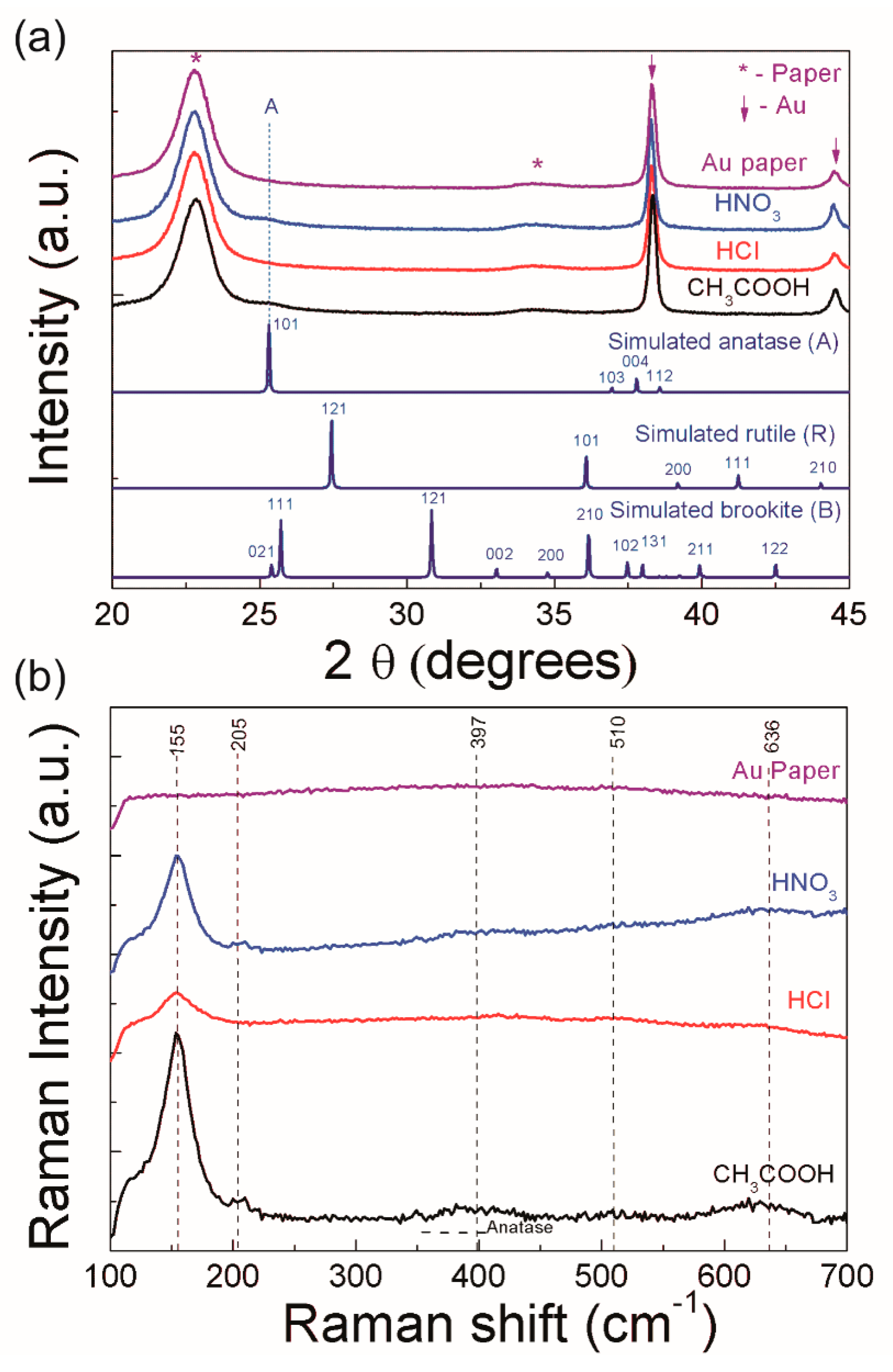
3.1. Structural Characterization
3.2. Electrochromic and Electrochemical Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Pinto, J.V.; Calmeiro, T.R.; Nandy, S.; Barquinha, P.; Pereira, L.; Carvalho, P.A.; Fortunato, E.; Martins, R. Photocatalytic behavior of TiO2 films synthesized by microwave irradiation. Catal. Today 2016, 278, 262–270. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Fortunato, E.; Martins, R. Photocatalytic TiO2 nanorod spheres and arrays compatible with flexible applications. Catalysts 2017, 7, 60. [Google Scholar] [CrossRef]
- Nunes, D.; Santos, L.; Pimentel, A.; Barquinha, P.; Pereira, L.; Fortunato, E.; Martins, R. Metal Oxide Nanostructures: Synthesis, Properties and Applications; Elsevier Science: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Heo, Y.-U.; Nattestad, A.; Sun, Z.; Wang, L.; Kim, J.H.; Dou, S.X. 3D hierarchical rutile TiO2 and metal-free organic sensitizer producing dye-sensitized solar cells 8.6% conversion efficiency. Sci. Rep. 2014, 4, 5769. [Google Scholar] [CrossRef] [PubMed]
- Park, N.-G.; van de Lagemaat, J.; Frank, A.J. Comparison of dye-sensitized rutile-and anatase-based TiO2 solar cells. J. Phys. Chem. B 2000, 104, 8989–8994. [Google Scholar] [CrossRef]
- Bernacka-Wojcik, I.; Senadeera, R.; Wojcik, P.J.; Silva, L.B.; Doria, G.; Baptista, P.; Aguas, H.; Fortunato, E.; Martins, R. Inkjet printed and “doctor blade” TiO2 photodetectors for DNA biosensors. Biosens. Bioelectron. 2010, 25, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhou, B. Titanium dioxide nanomaterials for sensor applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Pimentel, A.; Araujo, A.; Calmeiro, T.; Panigrahi, S.; Pinto, J.; Barquinha, P.; Gama, M.; Fortunato, E.; Martins, R. Enhanced UV flexible photodetectors and photocatalysts based on TiO2 nanoplatforms. Topics Catal. 2018, 61, 1591–1606. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Gonçalves, A.; Pereira, S.; Branquinho, R.; Barquinha, P.; Fortunato, E.; Martins, R. Metal oxide nanostructures for sensor applications. Semicond. Sci. Technol. 2019, 34, 043001. [Google Scholar] [CrossRef]
- Diasanayake, M.A.K.L.; Senadeera, G.K.R.; Sarangika, H.N.M.; Ekanayake, P.M.P.C.; Thotawattage, C.A.; Divarathne, H.K.D.W.M.N.R.; Kumari, J.M.K.W. TiO2 as a low cost, multi functional material. Mater. Today Proc. 2016, 3, S40–S47. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Gao, Z.-D.; Wang, J.-H.; Xia, X.-H.; Lynch, R. Multistage coloring electrochromic device based on TiO2 nanotube arrays modified with WO3 nanoparticles. Adv. Funct. Mater. 2011, 21, 1941–1946. [Google Scholar] [CrossRef]
- Chen, J.-Z.; Ko, W.-Y.; Yen, Y.-C.; Chen, P.-H.; Lin, K.-J. Hydrothermally processed TiO2 nanowire electrodes with antireflective and electrochromic properties. ACS Nano 2012, 6, 6633–6639. [Google Scholar] [CrossRef] [PubMed]
- Ghicov, A.; Albu, S.P.; Macak, J.M.; Schmuki, P. High-contrast electrochromic switching using transparent lift–off layers of self–organized TiO2 nanotubes. Small 2008, 4, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bi, Z.; Li, X.; Xu, X.; Zhang, S.; Hu, X. High-coloration efficiency electrochromic device based on novel porous TiO2@prussian blue core-shell nanostructures. Electrochim. Acta 2017, 224, 534–540. [Google Scholar] [CrossRef]
- Patil, R.A.; Devan, R.S.; Liou, Y.; Ma, Y.-R. Efficient electrochromic smart windows of one-dimensional pure brookite TiO2 nanoneedles. Sol. Energy Mater. Sol. Cells 2016, 147, 240–245. [Google Scholar] [CrossRef]
- Berger, S.; Ghicov, A.; Nah, Y.C.; Schmuki, P. Transparent TiO2 nanotube electrodes via thin layer anodization: Fabrication and use in electrochromic devices. Langmuir 2009, 25, 4841–4844. [Google Scholar] [CrossRef]
- Cai, G.; Zhou, D.; Xiong, Q.; Zhang, J.; Wang, X.; Gu, C.; Tu, J. Efficient electrochromic materials based on TiO2@ WO3 core/shell nanorod arrays. Sol. Energy Mater. Sol. Cells 2013, 117, 231–238. [Google Scholar] [CrossRef]
- Nah, Y.-C.; Ghicov, A.; Kim, D.; Berger, S.; Schmuki, P. TiO2-WO3 composite nanotubes by alloy anodization: Growth and enhanced electrochromic properties. J. Am. Chem. Soc. 2008, 130, 16154–16155. [Google Scholar] [CrossRef]
- Hashimoto, S.; Matsuoka, H. Prolonged lifetime of electrochromism of amorphous WO3–TiO2thin films. Surf. Interface Anal. 1992, 19, 464–468. [Google Scholar] [CrossRef]
- Reyes-Gil, K.R.; Stephens, Z.D.; Stavila, V.; Robinson, D.B. Composite WO3/TiO2 nanostructures for high electrochromic activity. ACS Appl. Mater. Interfaces 2015, 7, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Santos, L.; Pereira, S.; Emanuele, U.; Sinopoli, S.; Igreja, R.; Sales, G.; Martins, R.; Fortunato, E. A planar electrochromic device using WO3 nanoparticles and a modified paper-based electrolyte. Proceedings 2018, 2, 1065. [Google Scholar] [CrossRef]
- Wojcik, P.J.; Cruz, A.S.; Santos, L.; Pereira, L.; Martins, R.; Fortunato, E. Microstructure control of dual-phase inkjet-printed a-WO3/TiO2/WOX films for high-performance electrochromic applications. J. Mater. Chem. 2012, 22, 13268–13278. [Google Scholar] [CrossRef]
- Wang, J.M.; Sun, X.W.; Jiao, Z. Application of nanostructures in electrochromic materials and devices: Recent progress. Materials 2010, 3, 5029–5053. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, X.; Sun, P.; Wang, C.; Wei, Y.; Liu, Y. Enhanced electrochromic properties of a TiO2 nanowire array via decoration with anatase nanoparticles. J. Mater. Chem. C 2014, 2, 7891–7896. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.; Cab, C.; De Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Alesanco, Y.; Palenzuela, J.; Tena-Zaera, R.; Cabañero, G.; Grande, H.; Herbig, B.; Schmitt, A.; Schott, M.; Posset, U.; Guerfi, A.; et al. Plastic electrochromic devices based on viologen-modified TiO2 films prepared at low temperature. Sol. Energy Mater. Sol. Cells 2016, 157, 624–635. [Google Scholar] [CrossRef]
- Lang, A.W.; Österholm, A.M.; Reynolds, J.R. Paper-based electrochromic devices enabled by nanocellulose-coated substrates. Adv. Funct. Mater. 2019, 29, 1903487. [Google Scholar] [CrossRef]
- Vicente, A.; Araujo, A.; Mendes, M.J.; Nunes, D.; Oliveira, M.J.; Sanchez-Sobrado, O.; Ferreira, M.P.; Aguas, H.; Fortunato, E.; Martins, R. Multifunctional cellulose-paper for light harvesting and smart sensing applications. J. Mater. Chem. C 2018, 6, 3143–3181. [Google Scholar] [CrossRef]
- Tehrani, P.; Hennerdal, L.-O.; Dyer, A.L.; Reynolds, J.R.; Berggren, M. Improving the contrast of all-printed electrochromic polymer on paper displays. J. Mater. Chem. 2009, 19, 1799–1802. [Google Scholar] [CrossRef]
- Monk, P.M.; Delage, F.; Vieira, S.M.C. Electrochromic paper: Utility of electrochromes incorporated in paper. Electrochim. Acta 2001, 46, 2195–2202. [Google Scholar] [CrossRef]
- Brooke, R.; Edberg, J.; Crispin, X.; Berggren, M.; Engquist, I.; Jonsson, M.P. Greyscale and paper electrochromic polymer displays by UV patterning. Polymers 2019, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Miki, T.; Tanemura, S. TiO2 electrochromic thin films by reactive direct current magnetron sputtering. J. Vac. Sci. Technol. A Vac. Surf. Films 1997, 15, 2673–2676. [Google Scholar] [CrossRef]
- Weng, W.; Higuchi, T.; Suzuki, M.; Fukuoka, T.; Shimomura, T.; Ono, M.; Radhakrishnan, L.; Wang, H.; Suzuki, N.; Oveisi, H. A high-speed passive-matrix electrochromic display using a mesoporous TiO2 electrode with vertical porosity. Angew. Chem. Int. Edit. 2010, 49, 3956–3959. [Google Scholar] [CrossRef]
- Dinh, N.N.; Oanh, N.T.T.; Long, P.D.; Bernard, M.C.; Hugot-Le Goff, A. Electrochromic properties of TiO2 anatase thin films prepared by a dipping sol-gel method. Thin Solid Films 2003, 423, 70–76. [Google Scholar] [CrossRef]
- Pillai, S.; McCormack, D.; Periyat, P.; Leyland, N.; Corr, D.; Colreavy, J. Rapid microwave synthesis of mesoporous TiO2 for electrochromic displays. J. Mater. Chem. 2010, 20, 3650–3655. [Google Scholar]
- Pimentel, A.; Nunes, D.; Pereira, S.; Martins, R.; Fortunato, E. Photocatalytic activity of TiO2 nanostructured arrays prepared by microwave-assisted solvothermal method. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Araujo, A.; Pimentel, A.; Oliveira, M.J.; Mendes, M.J.; Franco, R.; Fortunato, E.; Águas, H.; Martins, R. Direct growth of plasmonic nanorod forests on paper substrates for low-cost flexible 3D SERS platforms. Flex. Print. Electron. 2017, 2, 014001. [Google Scholar] [CrossRef]
- Tsuji, M.; Hashimoto, M.; Nishizawa, Y.; Kubokawa, M.; Tsuji, T. Microwave-assisted synthesis of metallic nanostructures in solution. Chem. A Eur. J. 2005, 11, 440–452. [Google Scholar] [CrossRef]
- Joubert, T.; Bezuidenhout, P.; Chen, H.; Smith, S.; Land, K. Inkjet-printed silver tracks on different paper substrates. Mater. Today Proc. 2015, 2, 3891–3900. [Google Scholar] [CrossRef]
- Matias, M.L.; Nunes, D.; Pimentel, A.; Ferreira, S.H.; Agua, R.; Duarte, M.P.; Fortunato, E.; Martins, R. Paper-based nanoplatforms for multifunctional applications. J. Nanomater. 2019, 16. [Google Scholar] [CrossRef]
- Minges, M.L.; Committee, A.S.M.I.H. Electronic Materials Handbook: Packaging; Taylor & Francis: Oxfordshire, UK, 1989. [Google Scholar]
- Kim, K.-H.; Koo, B.-R.; Ahn, H.-J. Sheet resistance dependence of fluorine-doped tin oxide films for high-performance electrochromic devices. Ceram. Int. 2018, 44, 9408–9413. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Pearson, W.B.; Villars, P.; Calvert, L.D. Pearson’s Handbook of Crystallographic Data for Intermetallic Phases; American Society for Metals: Cleveland, OH, USA, 1985. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Li, L.; Qin, X.; Wang, G.; Qi, L.; Du, G.; Hu, Z. Synthesis of anatase TiO2 nanowires by modifying TiO2 nanoparticles using the microwave heating method. Appl. Surf. Sci. 2011, 257, 8006–8012. [Google Scholar] [CrossRef]
- Pimentel, A.; Rodrigues, J.; Duarte, P.; Nunes, D.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Effect of solvents on ZnO nanostructures synthesized by solvothermal method assisted by microwave radiation: A photocatalytic study. J. Mater. Sci. 2015, 50, 5777–5787. [Google Scholar] [CrossRef]
- Bregadiolli, B.A.; Fernandes, S.L.; Graeff, C.F.O. Easy and fast preparation of TiO2-based nanostructures using microwave assisted hydrothermal synthesis. Mater. Res. 2017, 20, 912–919. [Google Scholar] [CrossRef]
- Pimentel, A.; Samouco, A.; Nunes, D.; Araújo, A.; Martins, R.; Fortunato, E. Ultra-fast microwave synthesis of ZnO nanorods on cellulose substrates for UV sensor applications. Materials 2017, 10, 1308. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Huang, X.; Li, Q.; Li, G. New insights into fluorinated TiO2 (brookite, anatase and rutile) nanoparticles as efficient photocatalytic redox catalysts. RSC Adv. 2015, 5, 34302–34313. [Google Scholar] [CrossRef]
- Stagi, L.; Carbonaro, C.M.; Corpino, R.; Chiriu, D.; Ricci, P.C. Light induced TiO2 phase transformation: Correlation with luminescent surface defects. Phys. Status Solidi (b) 2015, 252, 124–129. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, L.; Shao, M.; Huang, J.; Ding, M.; Deng, X.; Wei, X.; Xu, X. Anodic oxidation synthesis of one-dimensional TiO2 nanostructures for photocatalytic and field emission properties. J. Nanomater. 2014, 2014, 14. [Google Scholar] [CrossRef]
- Vásquez, G.C.; Peche-Herrero, M.A.; Maestre, D.; Alemán, B.; Ramírez-Castellanos, J.; Cremades, A.; González-Calbet, J.M.; Piqueras, J. Influence of Fe and Al doping on the stabilization of the anatase phase in TiO2 nanoparticles. J. Mater. Chem. C 2014, 2, 10377–10385. [Google Scholar] [CrossRef]
- Castellanos-Leal, E.L.; Acevedo-Peña, P.; Güiza-Argüello, V.R.; Córdoba-Tuta, E.M. N and F codoped TiO2 thin films on stainless steel for photoelectrocatalytic removal of cyanide ions in aqueous solutions. J. Mater. Res. 2017, 20, 487–495. [Google Scholar] [CrossRef]
- Nezar, S.; Saoula, N.; Sali, S.; Faiz, M.; Mekki, M.; Laoufi, N.; Tabet, N. Properties of TiO2 thin films deposited by rf reactive magnetron sputtering on biased substrates. Appl. Surf. Sci. 2017, 395, 172–179. [Google Scholar] [CrossRef]
- Bhandari, S.; Deepa, M.; Srivastava, A.K.; Lakshmikumar, S.T.; RamaKant. Electrochromic response, structure optimization and ion transfer behavior in viologen adsorbed titanium oxide films. Solid State Ion. 2009, 180, 41–49. [Google Scholar] [CrossRef]
- Nah, Y.-C.; Ghicov, A.; Kim, D.; Schmuki, P. Enhanced electrochromic properties of self-organized nanoporous WO3. Electrochem. Commun. 2008, 10, 1777–1780. [Google Scholar] [CrossRef]
- Mjejri, I.; Doherty, C.M.; Rubio-Martinez, M.; Drisko, G.L.; Rougier, A. Double-sided electrochromic device based on metal-organic frameworks. ACS Appl. Mater. Interfaces 2017, 9, 39930–39934. [Google Scholar] [CrossRef]
- Pereira, S.; Gonçalves, A.; Correia, N.; Pinto, J.; Pereira, L.; Martins, R.; Fortunato, E. Electrochromic behavior of NiO thin films deposited by e-beam evaporation at room temperature. Sol. Energy Mater. Sol. Cells 2014, 120, 109–115. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Schanze, K.S. Semiconductor Photochemistry and Photophysics/Volume Ten; Taylor & Francis: Marcel Dekker, NY, USA, 2003. [Google Scholar]
- Barawi, M.; De Trizio, L.; Giannuzzi, R.; Veramonti, G.; Manna, L.; Manca, M. Dual band electrochromic devices based on Nb-doped TiO2 nanocrystalline electrodes. ACS Nano 2017, 11, 3576–3584. [Google Scholar] [CrossRef]
- Wen, R.-T.; Niklasson, G.A.; Granqvist, C.G. Eliminating electrochromic degradation in amorphous TiO2 through Li-Ion detrapping. ACS Appl. Mater. Interfaces 2016, 8, 5777–5782. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, D.; Freire, T.; Barranger, A.; Vieira, J.; Matias, M.; Pereira, S.; Pimentel, A.; Cordeiro, N.J.A.; Fortunato, E.; Martins, R. TiO2 Nanostructured Films for Electrochromic Paper Based-Devices. Appl. Sci. 2020, 10, 1200. https://doi.org/10.3390/app10041200
Nunes D, Freire T, Barranger A, Vieira J, Matias M, Pereira S, Pimentel A, Cordeiro NJA, Fortunato E, Martins R. TiO2 Nanostructured Films for Electrochromic Paper Based-Devices. Applied Sciences. 2020; 10(4):1200. https://doi.org/10.3390/app10041200
Chicago/Turabian StyleNunes, Daniela, Tomas Freire, Andrea Barranger, João Vieira, Mariana Matias, Sonia Pereira, Ana Pimentel, Neusmar J. A. Cordeiro, Elvira Fortunato, and Rodrigo Martins. 2020. "TiO2 Nanostructured Films for Electrochromic Paper Based-Devices" Applied Sciences 10, no. 4: 1200. https://doi.org/10.3390/app10041200
APA StyleNunes, D., Freire, T., Barranger, A., Vieira, J., Matias, M., Pereira, S., Pimentel, A., Cordeiro, N. J. A., Fortunato, E., & Martins, R. (2020). TiO2 Nanostructured Films for Electrochromic Paper Based-Devices. Applied Sciences, 10(4), 1200. https://doi.org/10.3390/app10041200









