Abstract
With the assistance of a rosin-based surfactant, dehydroabietyltrimethyl ammonium bromine (DTAB), well-dispersed hollow cube-like zirconia particles were firstly synthesized by the hydrothermal treatment of ZrOCl2 aqueous solutions. The introduction of DTAB is crucial for improving the dispersion and regularity of the as-synthesized sample. After calcination, the crystal size of the calcined samples increased, and the edge angle of the cube-like particles became round accordingly. Finally, a hollow spherical morphology was formed for the sample calcined at 923 K. The as-synthesized sample showed big surface area of 146.78 m2/g and large pore volume of 0.23 cm3/g. With the increase of calcination temperature, the surface area and pore volume of the samples decreased significantly, and the pore size increased accordingly.
1. Introduction
Zirconia has attracted a great deal of attention due to its unique physical and chemical properties, such as a high melting point and strength, excellent chemical stability and corrosion resistance, the presence of weak acid and basic sites on its surface [1,2,3]. In order to improve the properties of such a material, a great deal of work has focused on the adjustment of its crystal phase, pore structure, and surface properties [4,5,6]. Morphology is also a key factor determining the properties of zirconia. Chen et al. [7] successfully prepared flower-like zirconia nanostructures, which exhibited much better photocatalytic activity for the photodegradation of Rhodamine B in contrast to ordinary zirconia nanoparticles. Unique zirconia nanoshells were synthesized by Gole and co-workers [8]. The nanoshells showed a value of photoluminescence properties 100-times higher than that of irregularly shaped zirconia nanoparticles. Morphology-oriented zirconia (mesoporous, sphere, star, rod) supported vanadium oxides were fabricated by Royer et al. [9] for the NH3-SCR process. The morphology of zirconia showed a great influence on the catalytic performance of the catalysts. Therefore, it is of great importance to tailor the morphology of zirconia.
Various strategies have been employed for controlling the morphology of zirconia, including hydrothermal treatment, sol-gel processes, spray pyrolysis, magnetron sputtering and electrospinning methods [10,11,12,13,14]. Among them, hydrothermal treatment provides a relatively simple process. Until now, hydrothermal synthesis approaches were developed to prepare zirconia with different morphologies (needle, sphere, rod, spindle, star, flower) [7,8,15,16,17,18]. As far as we know, the morphology-controllable synthesis of zirconia through hydrothermal treatment assisted by rosin-based surfactants has not been reported. Different from traditional surfactants derived from fossil resources, rosin-based surfactants can be synthesized with raw resin acid, which is a kind of renewable resource from pine trees. The unique tricyclic phenanthrene-like structure endows the rosin-based surfactant with excellent surface activity, making it a good candidate for the morphology control of inorganic materials. In this thesis, by using the hydrothermal treatment of ZrOCl2 aqueous solutions with the assistance of a rosin-based surfactant, dehydroabietyltrimethyl ammonium bromine (DTAB), hollow cube-like zirconia was firstly synthesized. In order to effectively control the microstructure of the material, the effects of surfactant addition and calcination process on the material were investigated. The introduction of DTAB could improve the dispersion of the sample. As the calcination temperature increased, the morphology of the material progressively transformed into a hollow sphere.
2. Experimental Section
2.1. Chemicals
ZrOCl2·8H2O (AR) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China DTAB was self-synthesized according to the methodology of our previous study [19].
2.2. Methods
ZrOCl2·8H2O (6.445 g) and DTAB (0.408 g) with molar ratio of 20:1 were dissolved in deionized water (50 mL) at 308 K under stirring. Then, the solution was transferred to a Teflon-lined stainless steel autoclave for hydrothermal treatment at 393 K for 24 h. After cooling to room temperature, the product was washed and extracted in ethanol to remove the residual DTAB. After drying at 373 K for 8 h, the solid was calcined at 723 K or 923 K for 2 h at a heating rate of 1 K/min, respectively.
2.3. Characterization
XRD patterns of the samples were recorded using a Bucker D8 Focus X-ray diffractometer (Bucker AXS Inc., Karlsruhe, Germany) with Cu Ka radiation (λ = 0.154 nm). The operating target voltage and the current were set to 40 kV and 40 mA, respectively. The sample was powdered and scanned at 2θ angles ranging from 10 to 80°. TEM images were made of samples supported on carbon-coated copper grids using a JEOL JEM-2100 microscope (JEOL Ltd., Tokyo, Japan) operating at 200 kV. Field emission scanning electron microscopy (FESEM) images were obtained with a Hitachi S-4800 microscope (Hitachi High-Technologies Corp., Tokyo, Japan) operated at 5 kV. The samples were coated with gold before the measurements. Nitrogen physisorption isotherms were measured using a Micromeritics ASAP2020 instrument (Micromeritics Instrument Corp., Norcross, GA, USA) to determine the surface areas and pore size distributions of the samples.
3. Results and Discussion
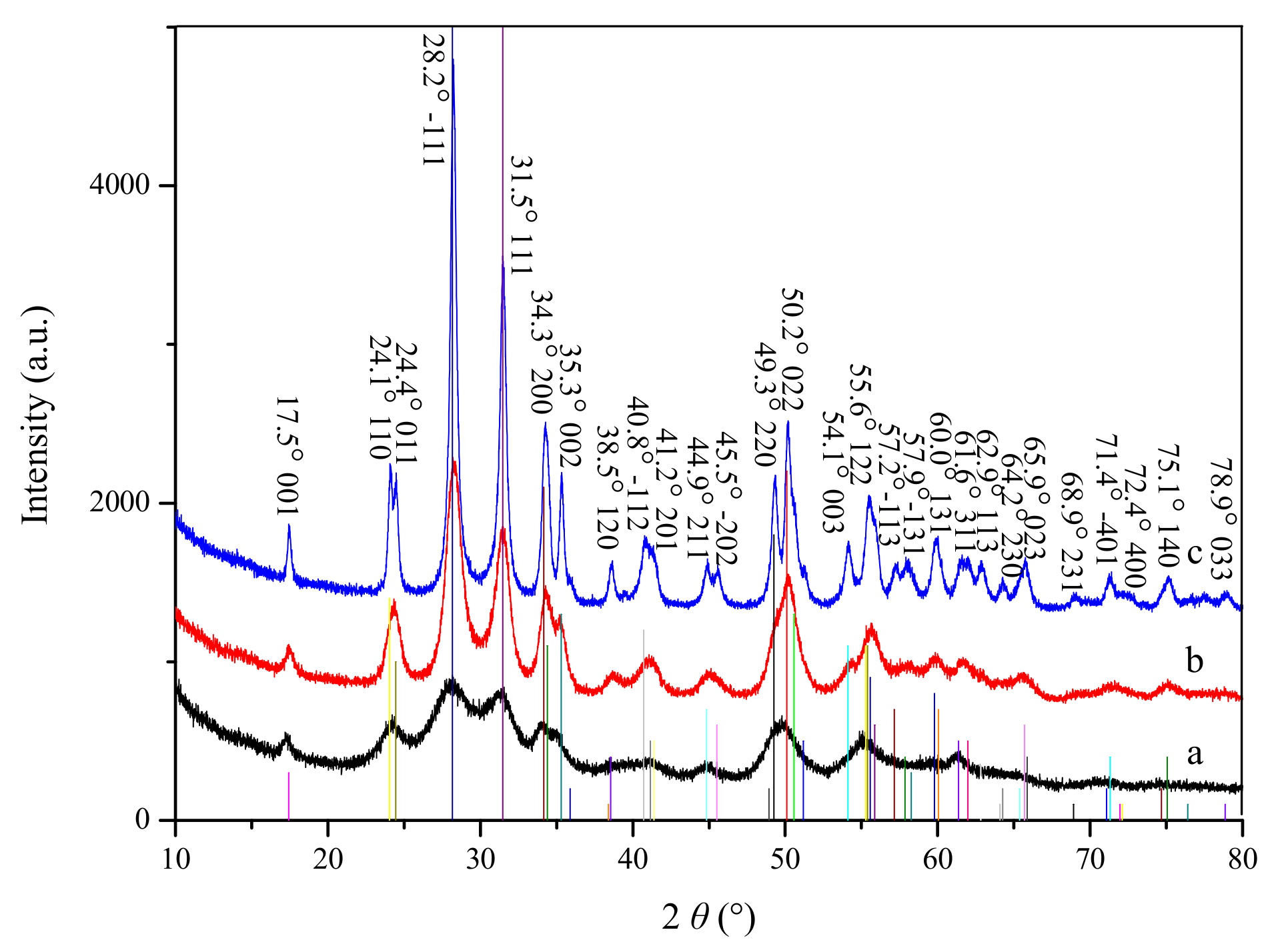
Figure 1 shows the XRD patterns of the as-synthesized and calcined samples. The vertical lines indicate the respective Joint Committee on Powder Diffraction Standards (JCPDS) card no. 37-1484, which can be related to monoclinic phase of zirconia. As can be seen in Figure 1, all the samples exhibited the characteristic diffraction peaks of monoclinic zirconia. No diffraction peaks of any other crystal phase can be observed in the samples, which indicates that all the samples are of pure monoclinic phase. With the increase of calcination temperature, the intensity of the diffraction peaks gradually increases, and the shape of the peaks becomes sharper. According to the Scherrer equation [20], the crystal size of the as-synthesized and calcined samples are 5.1 nm, 7.5 nm (calcined at 723 K) and 17.5 nm (calcined at 923 K), which indicates that the increase of calcination temperature can result in the increase the crystallinity and the crystal size of the materials.

Figure 1.
XRD patterns of as-synthesized (a) and calcined zirconia (b): calcined at 723 K; (c): calcined at 923 K, vertical line: PDF#37-1484.
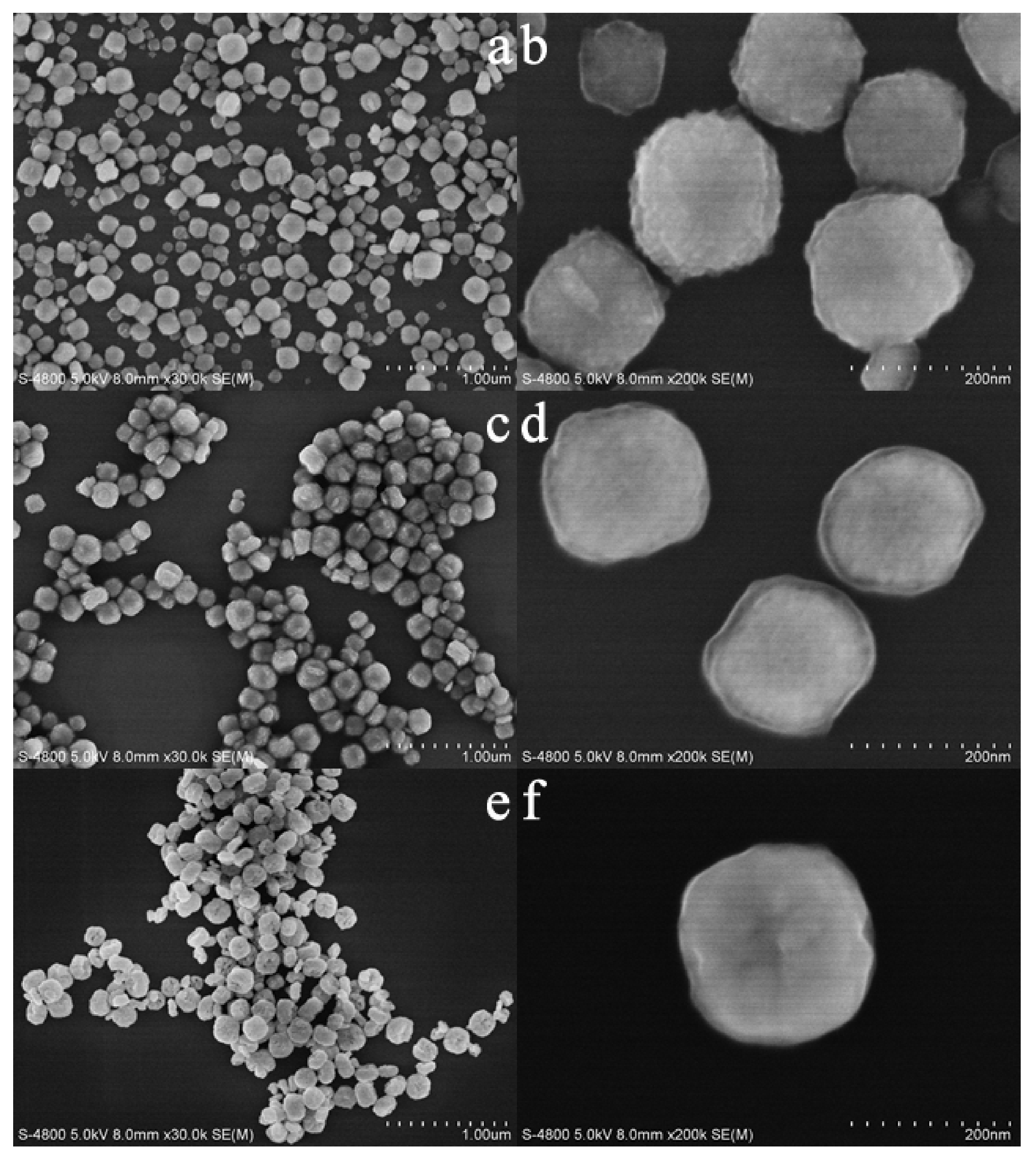
Figure 2 shows FESEM images of as-synthesized and calcined samples. The as-synthesized sample is composed of highly dispersed cube-like particles of 50–150 nm in size (Figure 2a,b). On the contrary, the sample synthesized without DTAB (Figure S1) shows obvious agglomeration, which indicates that the introduction of DTAB could improve the dispersion of the sample. After the calcination at 723 K, the sample shows a slight agglomeration, and the edge angle of the sample begins to become round (Figure 2c,d). When the calcination temperature increases to 923 K, the edge angle of the sample is further rounded, showing a spherical morphology. Meanwhile, the surface of the sample collapses to different degrees and becomes hollow (Figure 2e,f). It can be seen that the calcination has a great influence on the structure of the materials.
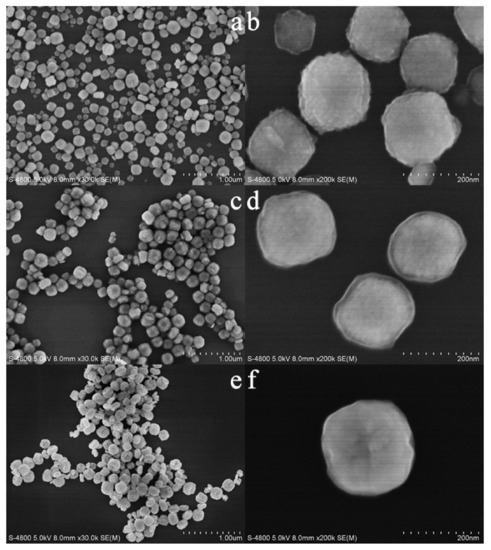
Figure 2.
FESEM images of as-synthesized (a,b) and calcined zirconias (c,d) calcined at 723 K; (e,f): calcined at 923 K.
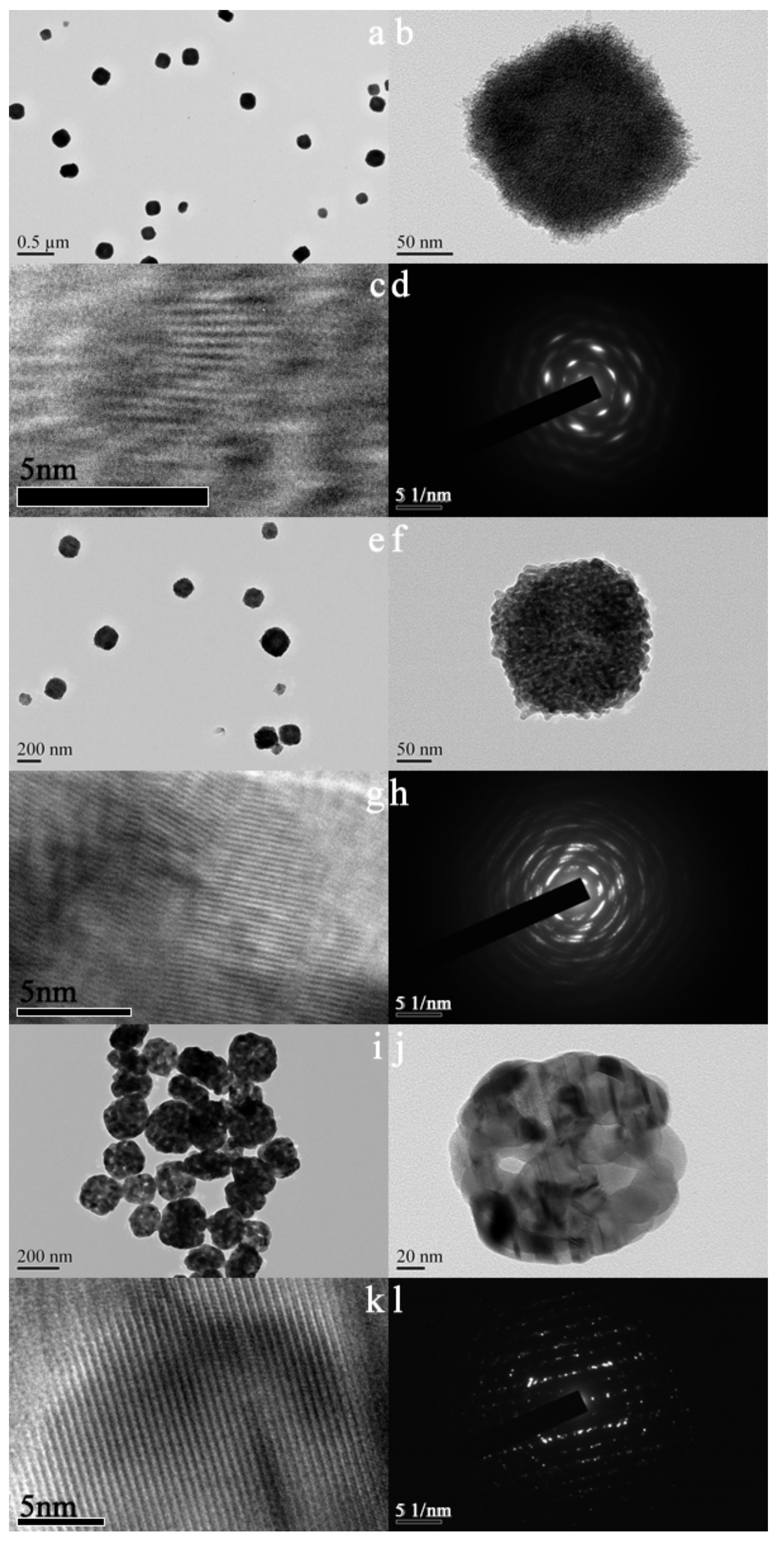
Figure 3 shows TEM images and electron diffraction patterns of as-synthesized and calcined samples. As can be seen in Figure 3a,b, the as-synthesized sample possesses cube-like morphology and each cube-like particle is made up of numerous crystals of about 5 nm in size. Under the compact accumulation of these nanocrystals, wormhole-like channels with a pore size of about 2 nm were formed for the as-synthesized sample. The color of the center of the particle (Figure 3b) is slightly lighter than that around it, which indicates that the sample may have a hollow structure. An array of blurred lattice fringes can be observed in Figure 3c, which indicates that the sample is in semi-crystallized state. Consistently with the SEM results, the sample synthesized without DTAB (Figure S2a) also shows obvious agglomeration. In addition, the shape of the particles is also not regular (Figure S2b). It can be seen that the introduction of DTAB can improve the dispersion and regularity of the as-synthesized sample. After calcination at 723 K, the morphology of the sample does not show obvious changes, but the edges of the corners become rounded. The size of the crystals increased to about 12 nm, which is a little bigger than the calculated value according to the Scherrer equation [20]. The pore size of the sample also accordingly increases to about 4 nm. The color difference between the center and the surroundings of the particle becomes more obvious, revealing that the sample has a hollow structure. When the calcination temperature increases to 923 K, the sample exhibits a hollow spherical structure and the crystal size increases to about 25 nm. Both the samples after calcination (Figure 3j,k) show clear lattice fringes, which indicates that the samples are in a highly crystallized state. As can be seen in Figure 3d,h,l, all the samples show characteristic diffraction pattern of monoclinic zirconia. The patterns undergo from blur to clarity, which suggests again that calcination can improve crystallinity.
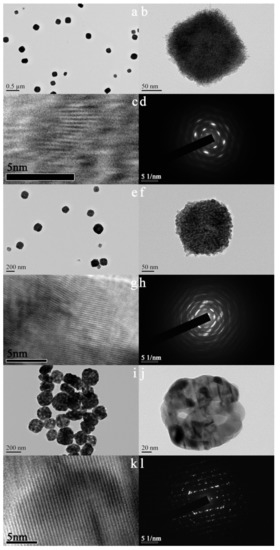
Figure 3.
TEM images (a–c,e–g,i–k) and electron diffraction patterns (d,h,l) of as-synthesized (a–d) and calcined zirconia (e–h) calcined at 723 K; (i–l): calcined at 923 K.
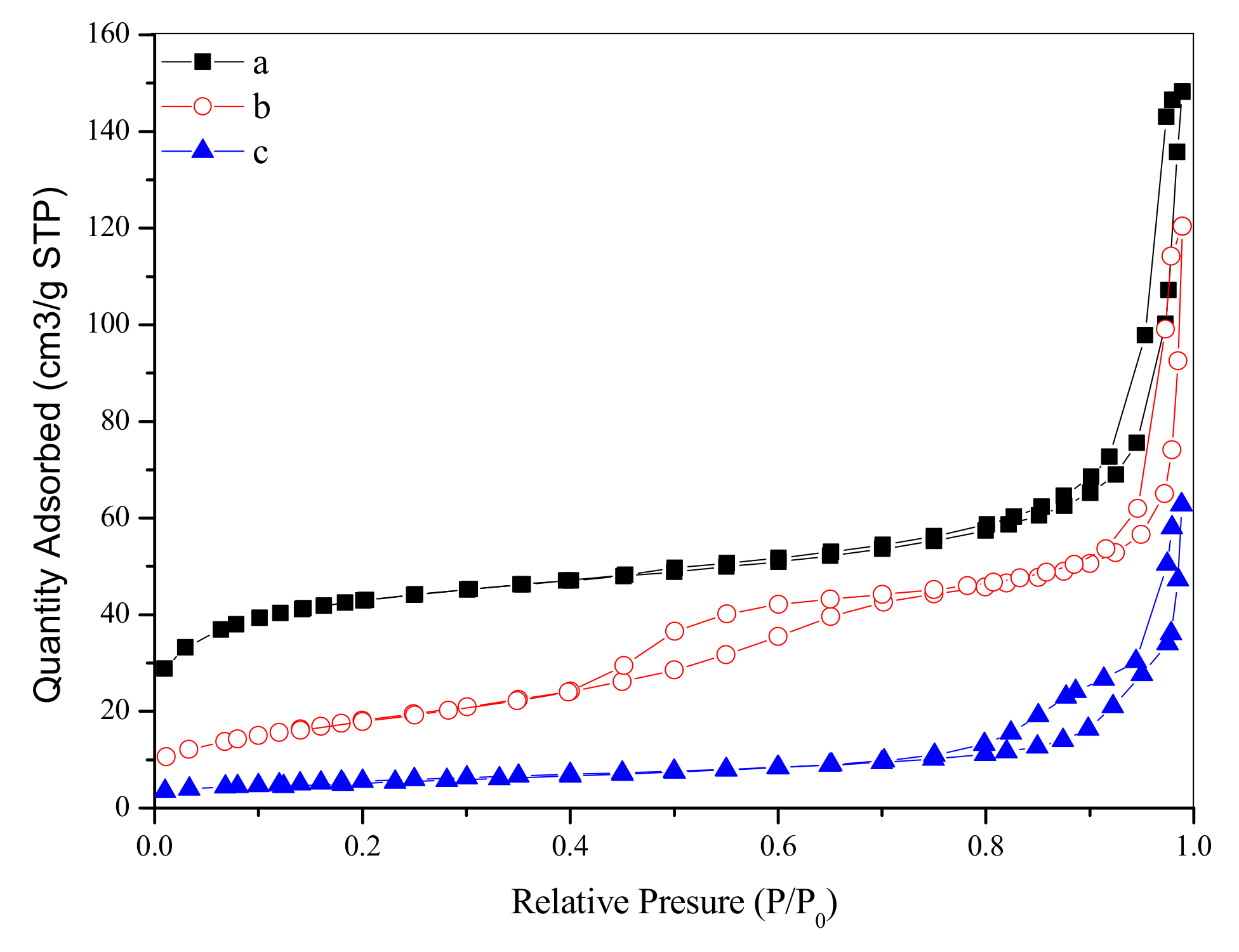
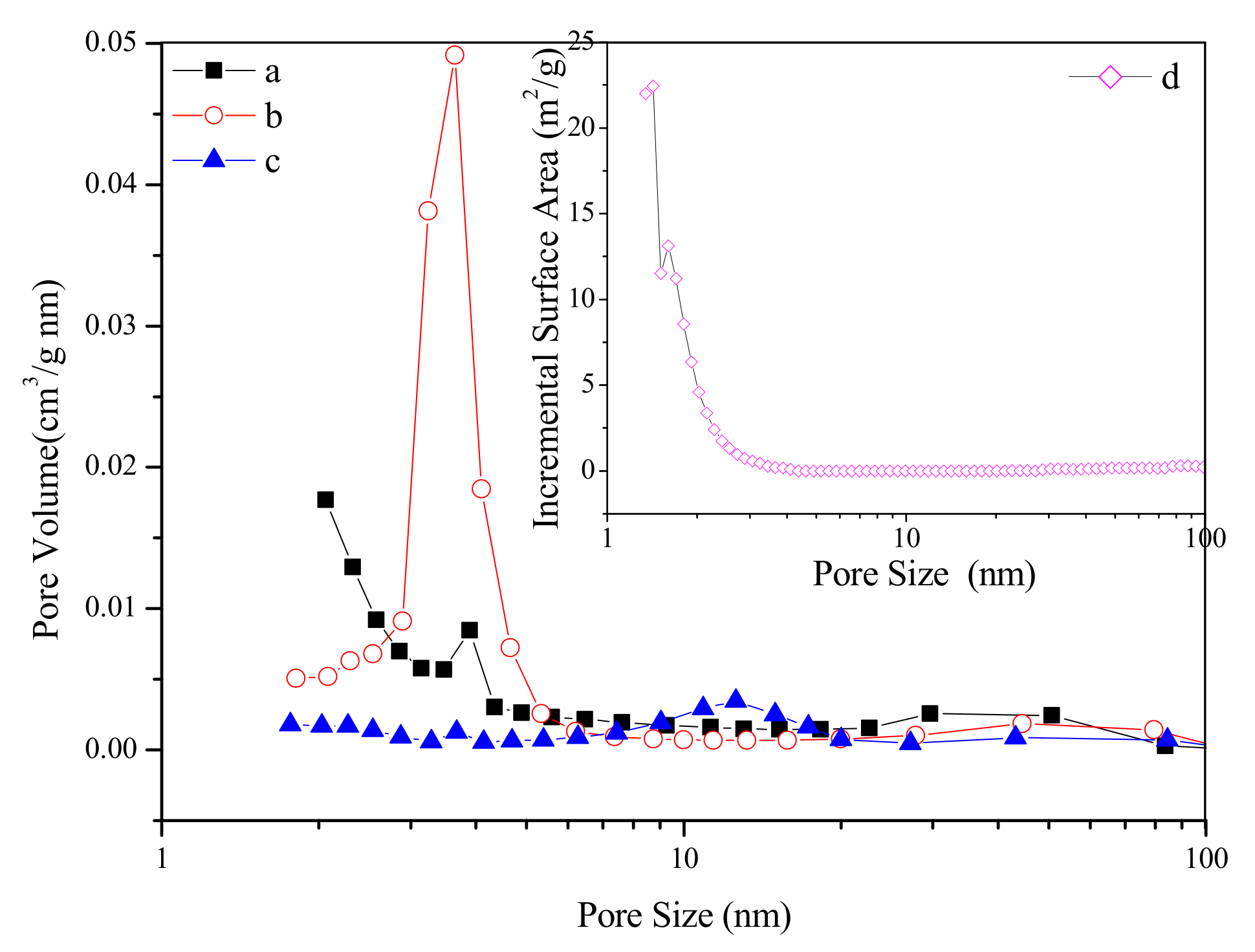
N2 adsorption–desorption isotherms and the pore size distributions of as-synthesized and calcined samples are plotted in Figure 4 and Figure 5. The as-synthesized sample shows a transitional type isotherm between the Type I (b) and Type II [21]. The hump in the range of P/P0 = 0–0.2 can be related to the micropore filling, and the steep uptake between P/P0 = 0.9–1.0 is due to the existence of the macropores in the sample, which indicates that the sample contains both micropores and macropores. The pore size distributions (Figure 5a,d, due to the limitation of BJH model in micropore calculation, we have added the DFT pore size distribution of the as-synthesized sample) of the as-synthesized sample proved the above conclusions. It can be seen that the pores of the sample are mainly micropores, with a certain amount of macropores. However, we cannot observe macropores in the TEM image (Figure 3b) of the outer surface of the as-synthesized sample. Therefore, such macropores are located inside the cube-like particles, suggesting that the as-synthesized sample is of hollow structure. After the calcination at 723 K, the sample shows a transitional type isotherm between the Type IV (a) and Type II. A distinct hysteresis loop can be observed between P/P0= 0.4–0.75, suggesting the existence of the mesopores. Like the as-synthesized sample, the sample calcined at 723 K also shows steep uptake between P/P0 = 0.9–1.0, revealing that the sample also has macropores. As shown in Figure 5b, due to the increase of crystal size by calcination, the pore size of the calcined sample increases from microporous to mesoporous range, mainly centering at 3.6 nm. In addition, there are a few macropores distributions in the range of 50–100 nm. When the calcination temperature increases to 923 K, the sample shows an isotherm of Type III. A hysteresis loop can be observed between P/P0 = 0.75–1.0, suggesting that the sample has a broad pore size distribution in the range of large-sized mesopores and macropores. As can be seen in Figure 5c, the pore channels of the sample are not well developed and the pore size is widely distributed in the range of 10–100 nm. At this time, the pore channels of the sample are only a simple accumulation of enlarged crystals by calcination at 923 K. The textural properties of as-synthesized and calcined zirconia are shown in Table 1. The as-synthesized sample shows big surface area of 146.78 m2/g and large pore volume of 0.23 cm3/g. With the increase of calcination temperature, the surface area and pore volume of the samples decrease significantly, and the pore size increases accordingly.
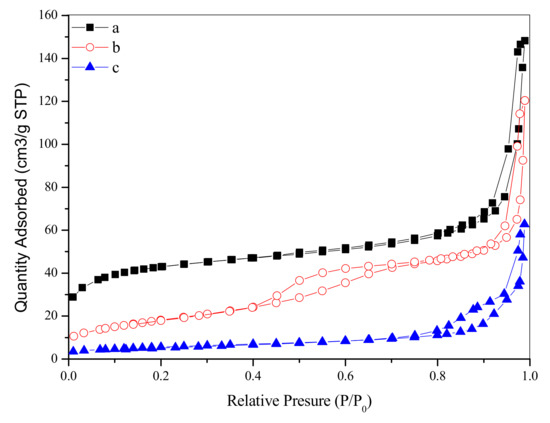
Figure 4.
N2 adsorption–desorption isotherms of as-synthesized (a,b) and calcined zirconia (b) calcined at 723 K; (c): calcined at 923 K.

Figure 5.
Pore size distributions of as-synthesized (a,b) and calcined zirconia (b) calcined at 723 K; (c): calcined at 923 K.

Table 1.
The textural properties of as-synthesized (a,b) and calcined zirconia (b) calcined at 723 K; (c): calcined at 923 K.
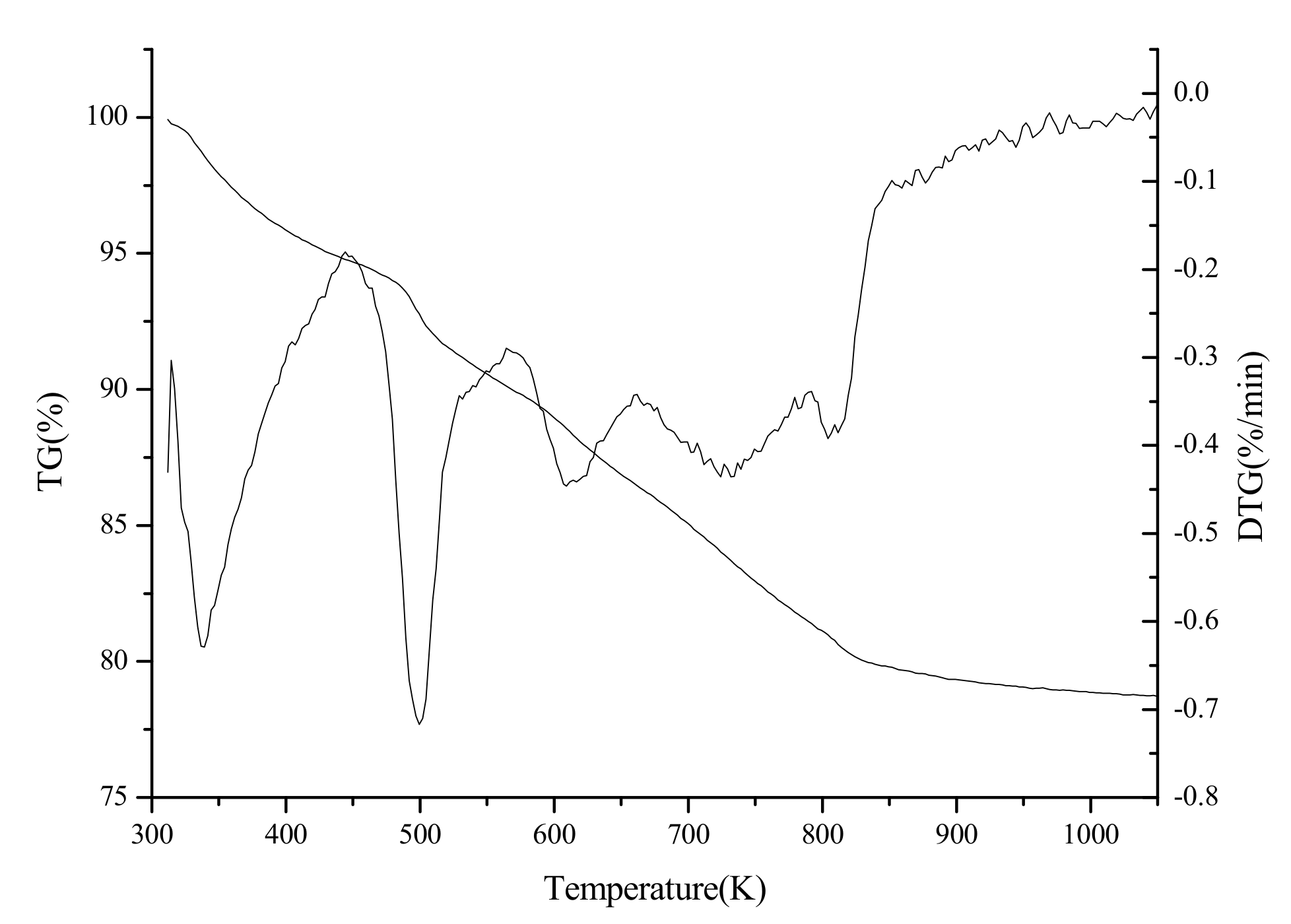
The TG-DTG curve of the as-synthesized sample is shown in Figure 6. The first weight loss of 5.2% below 444 K can be related to the removal of the physisorbed water. The second weight loss of 4.03% between the 444 K and 545 K corresponds to the decomposition of DTAB. The third weight loss of 10.8% in the range of 545 K to 840 K is due to the further decomposition of DTAB and the elimination of absorbed hydroxyl group, as well as non-structural carbonate group.
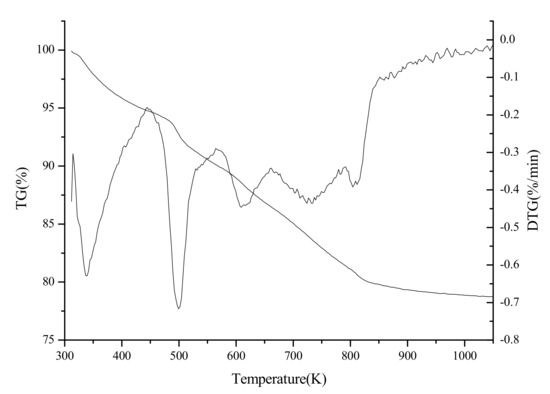
Figure 6.
TG-DTG curve of as-synthesized zirconia.
4. Conclusions
By the hydrothermal treatment of ZrOCl2 aqueous solutions with the assistance of a rosin-based surfactant, hollow cube-like zirconia with big surface area of 146.78 m2/g and large pore volume of 0.23 cm3/g was firstly synthesized. The introduction of the surfactant can improve the dispersion and regularity of the as-synthesized sample. Due to the increase of the crystal size by calcination, the edge angle of the cube-like particles became round gradually and finally a hollow spherical morphology was formed for the sample calcined at 923 K.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/9/19/4145/s1, Figure S1: FESEM image of as-synthesized zirconias without the DTAB assistance, Figure S2: TEM images of as-synthesized zirconias without the DTAB assistance.
Author Contributions
Conceptualization, P.W.; methodology, P.W. and S.L.; validation, S.L.; formal analysis, P.W.; investigation, C.G., H.S. and S.C.; resources, G.F.; writing—original draft preparation, C.G.; writing—review and editing, P.W.; supervision, Z.W.; project administration, P.W.; funding acquisition, P.W. and Z.W.
Funding
This work was funded by the [National Natural Science Foundation of China] grant number [31860191 and 31500484] and the [National Key Research and Development Program of China during the 13th Five-Year Plan Period] grant number [2017YFD0600704].
Acknowledgments
The authors would like to thank supports from Collaborative Innovation Center of Jiangxi Typical Trees Cultivation and Utilization.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hirvonen, A.; Nowak, R.; Yamamoto, Y.; Sekino, T.; Niihara, K. Fabrication, structure, mechanical and thermal properties of zirconia-based ceramic nanocomposites. J. Eur. Ceram. Soc. 2006, 26, 1497–1505. [Google Scholar] [CrossRef]
- Qin, W.; Nam, C.; Li, H.; Szpunar, J. Tetragonal phase stability in ZrO2 film formed on zirconium alloys and its effects on corrosion resistance. Acta Mater. 2007, 55, 1695–1701. [Google Scholar] [CrossRef]
- Yamaguchi, T. Application of ZrO2 as a catalyst and a catalyst support. Catal. Today 1994, 20, 199–217. [Google Scholar] [CrossRef]
- Morterra, C.; Cerrato, G.; Pinna, F.; Signoretto, M. Crystal phase, spectral features, and catalytic activity of sulfate-doped zirconia systems. J. Catal. 1995, 157, 109–123. [Google Scholar] [CrossRef]
- Blin, J.-L.; Flamant, R.; Su, B.-L. Synthesis of nanostructured mesoporous zirconia using CTMABr–ZrOCl2· 8H2O systems: A kinetic study of synthesis mechanism. Int. J. Inorg. Mater. 2001, 3, 959–972. [Google Scholar] [CrossRef]
- Jung, K.T.; Bell, A.T. The effects of synthesis and pretreatment conditions on the bulk structure and surface properties of zirconia. J. Mol. Catal. A Chem. 2000, 163, 27–42. [Google Scholar] [CrossRef]
- Shu, Z.; Jiao, X.; Chen, D. Synthesis and photocatalytic properties of flower-like zirconia nanostructures. CrystEngComm 2012, 14, 1122–1127. [Google Scholar] [CrossRef]
- Gole, J.L.; Prokes, S.M.; Stout, J.D.; Glembocki, O.J.; Yang, R. Unique properties of selectively formed zirconia nanostructures. Adv. Mater. 2006, 18, 664–667. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Wei, Y.; Zhang, R.; Royer, S. Morphology oriented ZrO2 supported vanadium oxide for NH3-SCR process: Importance of structural and textural properties. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Liang, J.; Deng, Z.; Jiang, X.; Li, F.; Li, Y. Photoluminescence of tetragonal ZrO2 nanoparticles synthesized by microwave irradiation. Inorg. Chem. 2002, 41, 3602–3604. [Google Scholar] [CrossRef]
- Kim, J.; Lin, Y. Sol-gel synthesis and characterization of yttria stabilized zirconia membranes. J. Membr. Sci. 1998, 139, 75–83. [Google Scholar] [CrossRef]
- Zhang, S.C.; Messing, G.L.; Borden, M. Synthesis of solid, spherical zirconia particles by spray pyrolysis. J. Am. Ceram. Soc. 1990, 73, 61–67. [Google Scholar] [CrossRef]
- Azad, A.-M. Fabrication of yttria-stabilized zirconia nanofibers by electrospinning. Mater. Lett. 2006, 60, 67–72. [Google Scholar] [CrossRef]
- Venkataraj, S.; Kappertz, O.; Weis, H.; Drese, R.; Jayavel, R.; Wuttig, M. Structural and optical properties of thin zirconium oxide films prepared by reactive direct current magnetron sputtering. J. Appl. Phys. 2002, 92, 3599–3607. [Google Scholar] [CrossRef]
- Nishizawa, H.; Yamasaki, N.; Matsuoka, K.; Mitsushio, H. Crystallization and transformation of zirconia under hydrothermal conditions. J. Am. Ceram. Soc. 1982, 65, 343–346. [Google Scholar] [CrossRef]
- Kumari, L.; Li, W.; Xu, J.; Leblanc, R.; Wang, D.; Li, Y.; Guo, H.; Zhang, J. Controlled hydrothermal synthesis of zirconium oxide nanostructures and their optical properties. Cryst. Growth Des. 2009, 9, 3874–3880. [Google Scholar] [CrossRef]
- Noh, H.-J.; Seo, D.-S.; Kim, H.; Lee, J.-K. Synthesis and crystallization of anisotropic shaped ZrO2 nanocrystalline powders by hydrothermal process. Mater. Lett. 2003, 57, 2425–2431. [Google Scholar] [CrossRef]
- Shu, Z.; Jiao, X.; Chen, D. Hydrothermal synthesis and selective photocatalytic properties of tetragonal star-like ZrO2 nanostructures. CrystEngComm 2013, 15, 4288–4294. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.-X.; Zhao, Z.-D.; Wang, Z.; Fan, G. Synthesis of ordered porous SiO2 with pores on the border between the micropore and mesopore regions using rosin-based quaternary ammonium salt. RSC Adv. 2015, 5, 11223–11228. [Google Scholar] [CrossRef]
- Patterson, A. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).