Effect of Short Fermentation Times with Lactobacillus paracasei in Rheological, Physical and Chemical Composition Parameters in Cassava Dough and Biscuits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flour Production
2.2. Dough Fermentation
2.3. Dough and Biscuit Preparation
2.4. Rheological Assays
2.5. Physical Assays
2.6. Chemical Composition Evaluation
2.7. Experimental Design and Statistical Analyzes
3. Results and Discussion
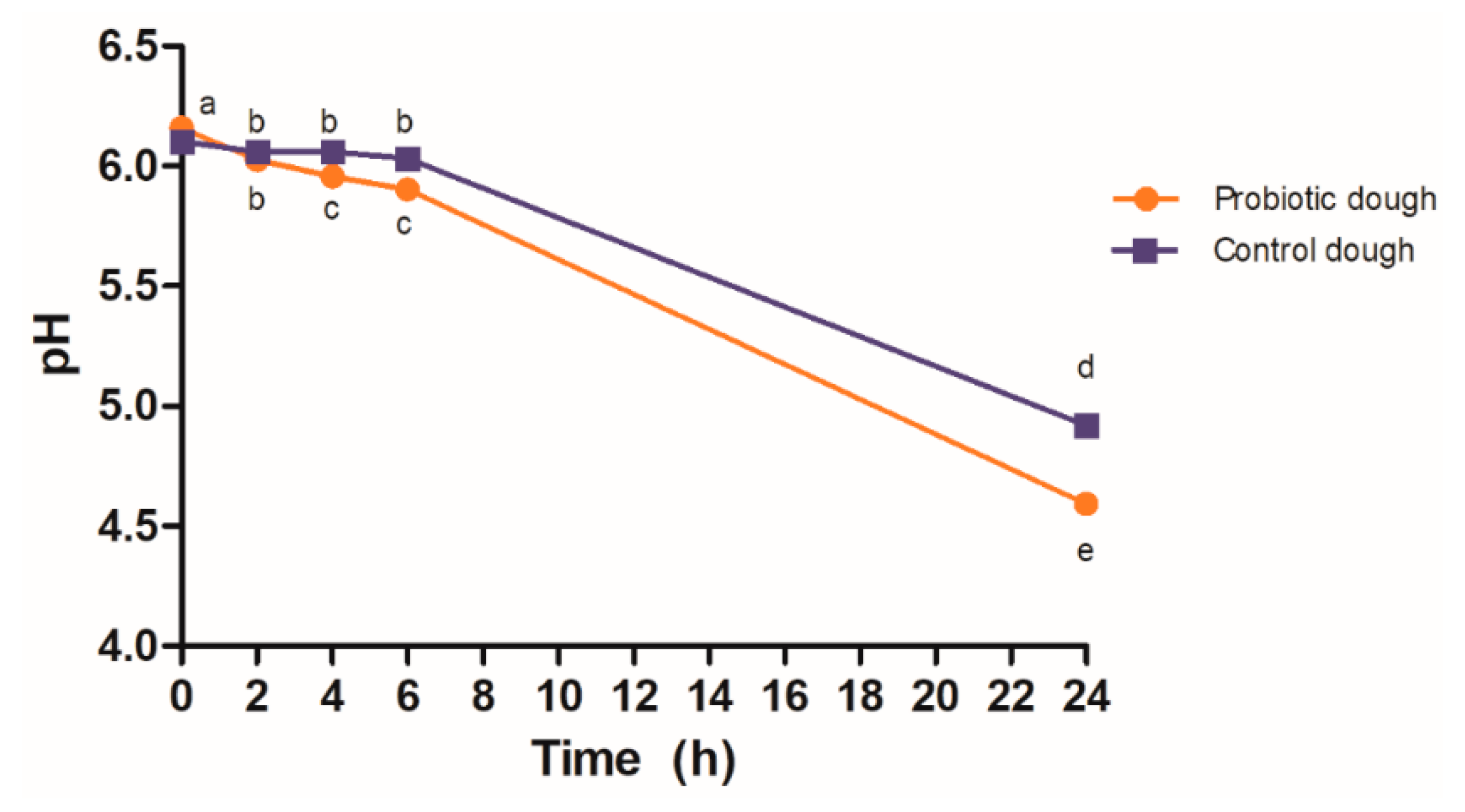
3.1. Dough Fermentation
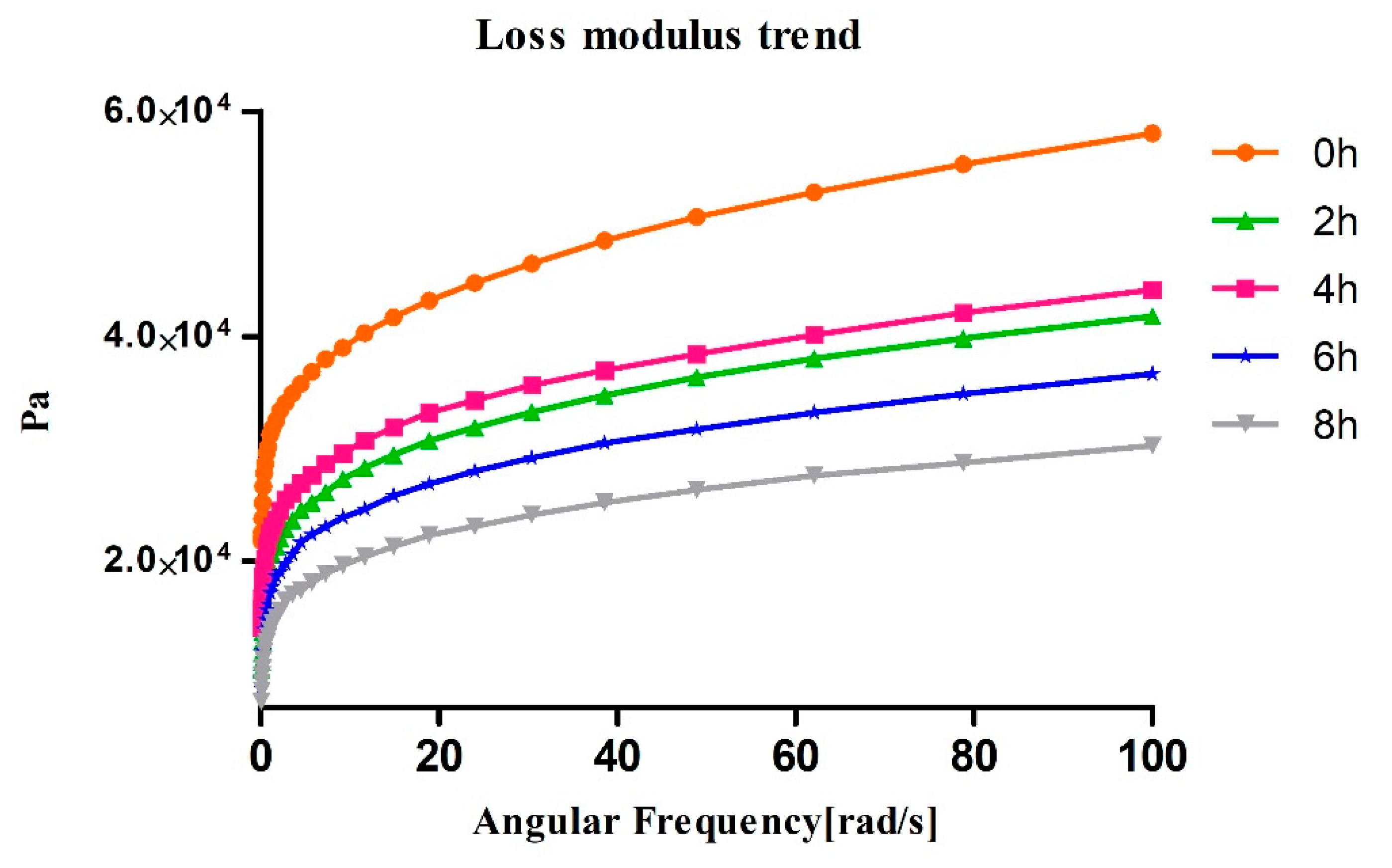
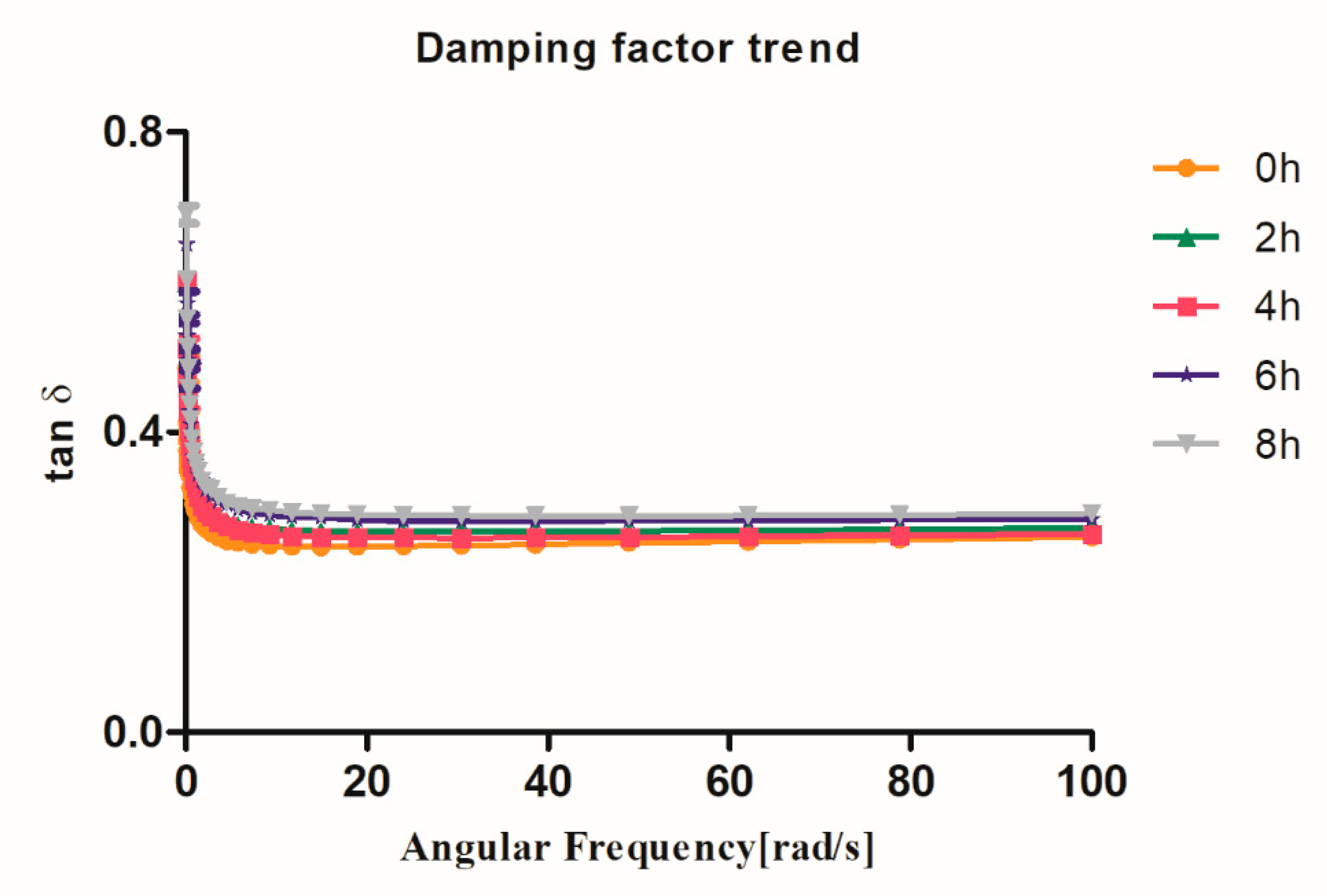
3.2. Rheology Assays
3.3. Physical Assay
3.4. Chemical Composition Assays
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagi, H.P.S.; Kuar, J.; Dar, B.N.; Sharma, S. Effect of Storage Period and Packaging on the Shelf Life of Cereal Bran Incorporated Biscuits. Am. J. Food Technol. 2012, 7, 301–310. [Google Scholar]
- Cauvain, S. Technology of Breadmaking, 3rd ed.; Springer International Publishing: Witney, UK, 2015; pp. 306–309. [Google Scholar]
- Morais, E.C.; Cruz, A.G.; Faria, J.A.F.; Bolini, H.M.A. Prebiotic gluten-free bread: Sensory profiling and drivers of liking. LWT Food Sci. Technol. 2014, 55, 248–254. [Google Scholar] [CrossRef]
- Scherf, K.A.; Wieser, H.; Koehler, P. Novel approaches for enzymatic gluten degradation to create high-quality gluten-free products. Food Res. Int. 2016, 110, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.E.; Rosell, C.M. Quality Indicators of Rice-Based Gluten-Free Bread-Like Products: Relationships between Dough Rheology and Quality Characteristics. Food Bioprocess Technol. 2013, 6, 2331–2341. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, N.; Albanell, E.; Miñarro, B.; Capellas, M. Chestnut flour sourdough for gluten-free bread making. Eur. Food Res. Technol. 2016, 242, 1795–1802. [Google Scholar] [CrossRef]
- Pasqualone, A.; Caponio, F.; Summo, C.; Paradiso, V.M.; Bottega, G.; Pagani, M.A. Gluten-Free Bread Making Trials from Cassava (Manihot esculenta crantz) Flour and Sensory Evaluation of the Final Product. Int. J. Food Prop. 2010, 13, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, H.; NeBambi, L.; Graeme, T. Save and Grow Cassava a Guide to Sustainable Production Intensification; FAO: Rome, Italy, 2013; pp. 30–31. [Google Scholar]
- Peñas, E.; Diana, M.; Frias, J.; Quílez, J.; Martínez-Villaluenga, C. A Multistrategic Approach in the Development of Sourdough Bread Targeted Towards Blood Pressure Reduction. Plant Foods Hum. Nutr. 2015, 70, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Czerny, M.; Arendt, E.K. Impact of sourdough fermented with Lactobacillus plantarum FST 1.7 on baking and sensory properties of gluten-free breads. Eur. Food Res. Technol. 2014, 239, 1–12. [Google Scholar] [CrossRef]
- Huang, L.; Shan, Y.-J.; He, C.-X.; Ren, M.-H.; Tian, P.-J.; Song, W. Effects of L. paracasei subp. paracasei X12 on cell cycle of colon cancer HT-29 cells and regulation of mTOR signalling pathway. J. Funct. Foods 2016, 21, 431–439. [Google Scholar] [CrossRef]
- Mantzourani, I.; Plessas, S.; Odatzidou, M.; Alexopoulos, A.; Galanis, A.; Bezirtzoglou, E.; Bekatorou, A. Effect of a novel Lactobacillus paracasei starter on sourdough bread quality. Food Chem. 2019, 271, 259–265. [Google Scholar] [CrossRef]
- Dirección General de Normas. Determinacion de pH en Alimentos; Gobierno Federal Mexicano: Mexico City, Mexico, 1978; pp. 3–6. [Google Scholar]
- Sulieman, A.A.; Zhu, K.X.; Peng, W.; Hassan, H.A.; Obadi, M.; Siddeeg, A.; Zhou, H.M. Rheological and quality characteristics of composite gluten-free dough and biscuits supplemented with fermented and unfermented Agaricus bisporus polysaccharide flour. Food Chem. 2019, 271, 193–203. [Google Scholar] [CrossRef] [PubMed]
- WHO/FAO. Food Energy Methods of Analysis and Conversion Factors; WHO: Geneva, Switzerland; FAO: Rome Italy, 2002; p. 12. [Google Scholar]
- Dastmalchi, F.; Razavi, S.H.; Faraji, M.; Labbafi, M. Effect of Lactobacillus casei- casei and Lactobacillus reuteri on acrylamide formation in flat bread and Bread roll. J. Food Sci. Technol. 2016, 53, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Paucean, A.; Vodnar, D.C.; Socaci, S.A.; Socaciu, C. Carbohydrate metabolic conversions to lactic acid and volatile derivatives, as influenced by Lactobacillus plantarum ATCC 8014 and Lactobacillus casei ATCC 393 efficiency during in vitro and sourdough fermentation. Eur. Food Res. Technol. 2013, 237, 679–689. [Google Scholar] [CrossRef]
- Guyot, J.P.; Morlon-Guyot, J. Effect of different cultivation conditions on Lactobacillus manihotivorans OND32T, an amylolytic lactobacillus isolated from sour starch cassava fermentation. Int. J. Food Microbiol. 2001, 67, 217–225. [Google Scholar] [CrossRef]
- Torrieri, E.; Pepe, O.; Ventorino, V.; Masi, P.; Cavella, S. Effect of sourdough at different concentrations on quality and shelf life of bread. LWT Food Sci. Technol. 2014, 56, 508–516. [Google Scholar] [CrossRef]
- Ramos, C.L.; Sousa, E.S.O.D.; Ribeiro, J.; Almeida, T.M.M.; Santos, C.C.A.D.A.; Abegg, M.A.; Schwan, R.F. Microbiological and chemical characteristics of tarubá, an indigenous beverage produced from solid cassava fermentation. Food Microbiol. 2015, 49, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Xie, Y.; Peng, C.; Liu, Y.; Wang, H. A Novel Antidiabetic Food Produced via Solid-State Fermentation of Tartary Buckwheat using L. plantarum TK9 and L. paracasei TK1501. Food Technol. Biotechnol. 2018, 56, 3. [Google Scholar] [CrossRef] [PubMed]
- Vogelmann, S.A.; Seitter, M.; Singer, U.; Brandt, M.J.; Hertel, C. Adaptability of lactic acid bacteria and yeasts to sourdoughs prepared from cereals, pseudocereals and cassava and use of competitive strains as starters. Int. J. Food Microbiol. 2009, 130, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Ashraf Khan, A.; Gani, A.; Masoodi, F.A.; Mushtaq, U.; Silotry Naik, A. Structural, rheological, antioxidant, and functional properties of β-glucan extracted from edible mushrooms Agaricus bisporus, Pleurotus ostreatus and Coprinus attrimentarius. Bioact. Carbohydr. Diet. Fibre 2017, 11, 67–74. [Google Scholar] [CrossRef]
- Ronda, F.; Pérez-Quirce, S.; Villanueva, M. Rheological Properties of Gluten-Free Bread Doughs: Relationship with Bread Quality. In Advances in Food Rheology and Its Applications; Ahmed, J., Pstazek, P., Banu, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 297–334. [Google Scholar]
- Sarabhai, S.; Sudha, M.L.; Prabhasankar, P. Rheological characterization and biscuit making potential of gluten free flours. J. Food Meas. Charact. 2017, 11, 1449–1461. [Google Scholar] [CrossRef]
- Rinaldi, M.; Paciulli, M.; Caligiani, A.; Sgarbi, E.; Cirlini, M.; Dall’Asta, C.; Chiavaro, E. Durum and soft wheat flours in sourdough and straight-dough bread-making. J. Food Sci. Technol. 2015, 52, 6254–6265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhassan, M.S.M.; Emmambux, M.N.; Taylor, J.R.N. Transgenic sorghum with suppressed synthesis of kafirin subclasses: Effects on flour and dough rheological characteristics. J. Cereal Sci. 2017, 75, 69–76. [Google Scholar] [CrossRef]
- Olojede, A.O.; Banwo, K.; Sanni, A.I. Rheological, textural and nutritional properties of gluten-free sourdough made with functionally important lactic acid bacteria and yeast from Nigerian sorghum. LWT Food Sci. Technol. 2020, 120, 108875. [Google Scholar] [CrossRef]
- Pontonio, E.; Nionelli, L.; Curiel, J.A.; Sadeghi, A.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G. Iranian wheat flours from rural and industrial mills: Exploitation of the chemical and technology features, and selection of autochthonous sourdough starters for making breads. Food Microbiol. 2015, 47, 99–110. [Google Scholar] [CrossRef]
- Klupsaite, D.; Juodeikiene, G.; Bartkiene, E.; Maknickiene, Z.; Liutkute, G.; Zadeike, D. The influence of lactic acid fermentation on functional properties of narrow-leaved lupine protein as functional additive for higher value wheat bread. LWT Food Sci. Technol. 2017, 75, 180–186. [Google Scholar] [CrossRef]
- Monthe, O.C.; Grosmaire, L.; Nguimbou, R.M.; Dahdouh, L.; Riccie, J.; Tran, T.; Ndjouenkeu, R. Rheological and textural properties of gluten-free doughs and breads based on fermented cassava, sweet potato and sorghum mixed flours. LWT Food Sci. Technol. 2019, 101, 575–582. [Google Scholar] [CrossRef]
- Abedfar, A.; Sadeghi, A. Response surface methodology for investigating the effects of sourdough fermentation conditions on Iranian cup bread properties. Heliyon 2019, 5, e02608. [Google Scholar] [CrossRef] [Green Version]
- Shittu, T.; Raji, A.O.; Sanni, L.O. Bread from composite cassava-wheat flour: I. effect of baking time and temperature on some physical properties of bread loaf. Food Res. Int. 2007, 40, 280–290. [Google Scholar] [CrossRef]
- Scarnato, L.; Montanari, C.; Serrazanetti, D.I.; Aloisi, I.; Balestra, F.; Del Duca, S.; Lanciotti, R. New bread formulation with improved rheological properties and longer shelf-life by the combined use of transglutaminase and sourdough. LWT Food Sci. Technol. 2017, 81, 101–110. [Google Scholar] [CrossRef]
- Habibi Najafi, M.B.; Pourfarzad, A.; Zahedi, H.; Ahmadian-Kouchaksaraie, Z.; Haddad Khodaparast, M.H. Development of sourdough fermented date seed for improving the quality and shelf life of flat bread: Study with univariate and multivariate analyses. J. Food Sci. Technol. 2016, 53, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Rathnayake, H.A.; Navaratne, S.B.; Navaratne, C.M. Porous Crumb Structure of Leavened Baked Products. Int. J. Food Sci. 2018, 2018, 8187318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz-Penella, J.M.; Tamayo-Ramos, J.A.; Haros, M. Application of Bifidobacteria as Starter Culture in Whole Wheat Sourdough Breadmaking. Food Bioprocess Technol. 2012, 5, 2370–2380. [Google Scholar] [CrossRef] [Green Version]
- Cremon, C.; Guglielmetti, S.; Gargari, G.; Taverniti, V.; Castellazzi, A.M.; Valsecchi, C.; Tagliacarne, C.; Fiore, W.; Bellini, M.; Bertani, L.; et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United Eur. Gastroenterol. J. 2018, 6, 604–613. [Google Scholar] [CrossRef] [Green Version]
- Axel, C.; Brosnan, B.; Zannini, E.; Peyer, L.C.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal activities of three different Lactobacillus species and their production of antifungal carboxylic acids in wheat sourdough. Appl. Microbiol. Biotechnol. 2016, 100, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Pontonio, E.; Waters, D.M.; Arendt, E.K. Applications of microbial fermentations for production of gluten-free products and perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 473–485. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Obadina, A.O.; Adebo, O.A.; Kayitesi, E. Comparison of nutritional quality and sensory acceptability of biscuits obtained from native, fermented, and malted pearl millet (Pennisetum glaucum) flour. Food Chem. 2017, 232, 210–217. [Google Scholar] [CrossRef] [PubMed]



| Fermentation Time | Crude Fiber (%) | Fats (%) | Carbohydrates (%) | Ash (%) | Protein (%) | Moisture (%) |
|---|---|---|---|---|---|---|
| 0 h | 0.40 ± 0.05 a | 8.66 ± 1.66 b | 56.76 ± 3.16 a | 0.08 ± 0.03 a | 9.43 ± 0.64 a | 24.6 ± 2.31 a |
| 2 h | 0.68 ± 0.02 a | 14.3 ± 1.08 a | 32.9 ± 1.71 b | 4.63 ± 0.09 b | 12.2 ± 2.08 a | 35.1 ± 1.01 b |
| 4 h | 0.57 ± 0.05 a | 14.2 ± 0.45 a | 29.4 ± 7.27 b | 4.82 ± 0.14 b | 12.2 ± 1.13 a | 38.6 ± 6.43 b |
| 6 h | 0.40 ± 0.07 a | 13.2 ± 0.84 a | 35.4 ± 1.10 b | 4.79 ± 0.10 b | 10.8 ± 0.35 a | 35.3 ± 1.15 b |
| 8 h | 0.42 ± 0.02 a | 12.4 ± 0.16 a | 38.0 ± 1.52 b | 4.76 ± 0.13 b | 10.7 ± 2.16 a | 33.5 ± 0.40 b |
| Fermentation Time | Color | ||
|---|---|---|---|
| L* | a* | b* | |
| 0 h | 73.92 ±3.88 a | 13.78 ±1.41 a | 33.14 ±1.43 a |
| 2 h | 54.99 ±3.17 b | 18.39 ±1.71 a | 30.92 ±1.73 a |
| 4 h | 54.88 ±2.16 b | 20.12 ±1.51 a | 32.65 ±1.12 a |
| 6 h | 51.71 ±3.28 b | 19.20 ±2.65 a | 30.96 ±2.73 a |
| 8 h | 52.28 ±8.64 b | 19.69 ±4.63 a | 30.66 ±0.54 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longoria, S.; Contreras, J.; Belmares, R.; Cruz, M.; Flores, M. Effect of Short Fermentation Times with Lactobacillus paracasei in Rheological, Physical and Chemical Composition Parameters in Cassava Dough and Biscuits. Appl. Sci. 2020, 10, 1383. https://doi.org/10.3390/app10041383
Longoria S, Contreras J, Belmares R, Cruz M, Flores M. Effect of Short Fermentation Times with Lactobacillus paracasei in Rheological, Physical and Chemical Composition Parameters in Cassava Dough and Biscuits. Applied Sciences. 2020; 10(4):1383. https://doi.org/10.3390/app10041383
Chicago/Turabian StyleLongoria, Samuel, Juan Contreras, Ruth Belmares, Mario Cruz, and Mildred Flores. 2020. "Effect of Short Fermentation Times with Lactobacillus paracasei in Rheological, Physical and Chemical Composition Parameters in Cassava Dough and Biscuits" Applied Sciences 10, no. 4: 1383. https://doi.org/10.3390/app10041383







