Color Enhancement Strategies for 3D Printing of X-ray Computed Tomography Bone Data for Advanced Anatomy Teaching Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Processing
2.1.1. For Poly-Jet Split Processes
2.1.2. For Binder-Jet Painting Processes
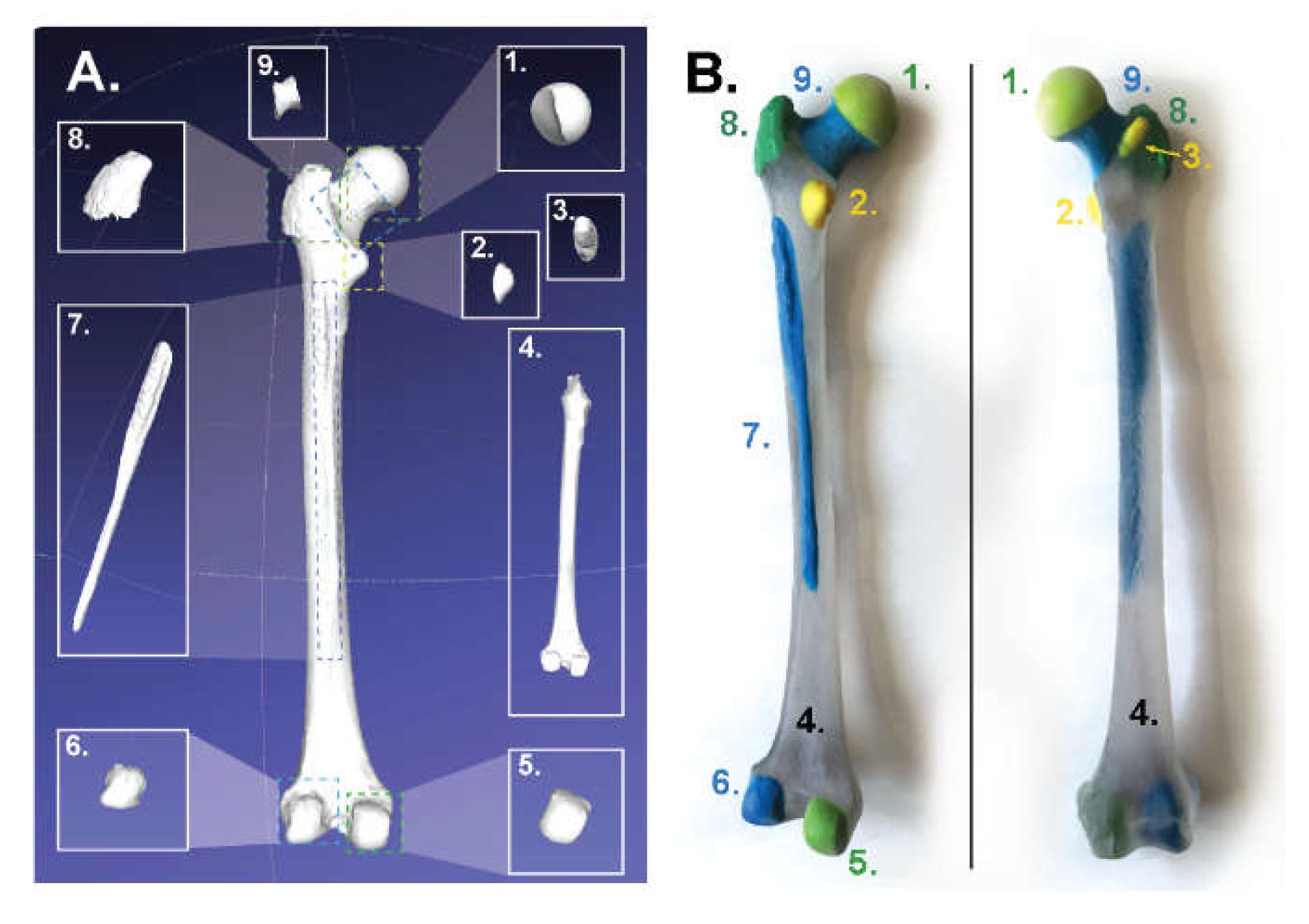
- Femur: https://skfb.ly/6Mt8w;
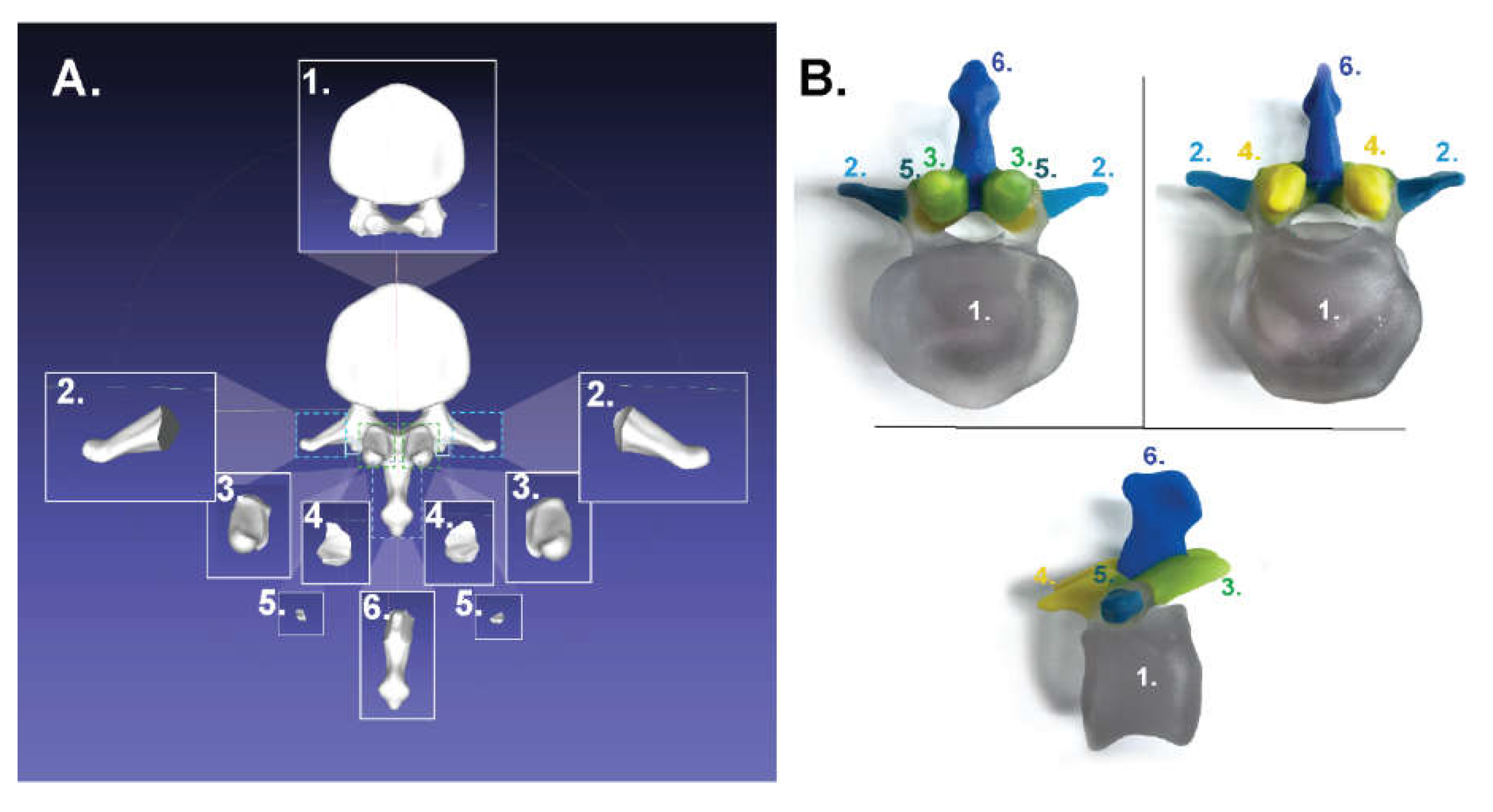
- Lumbar: https://skfb.ly/6MYTw;
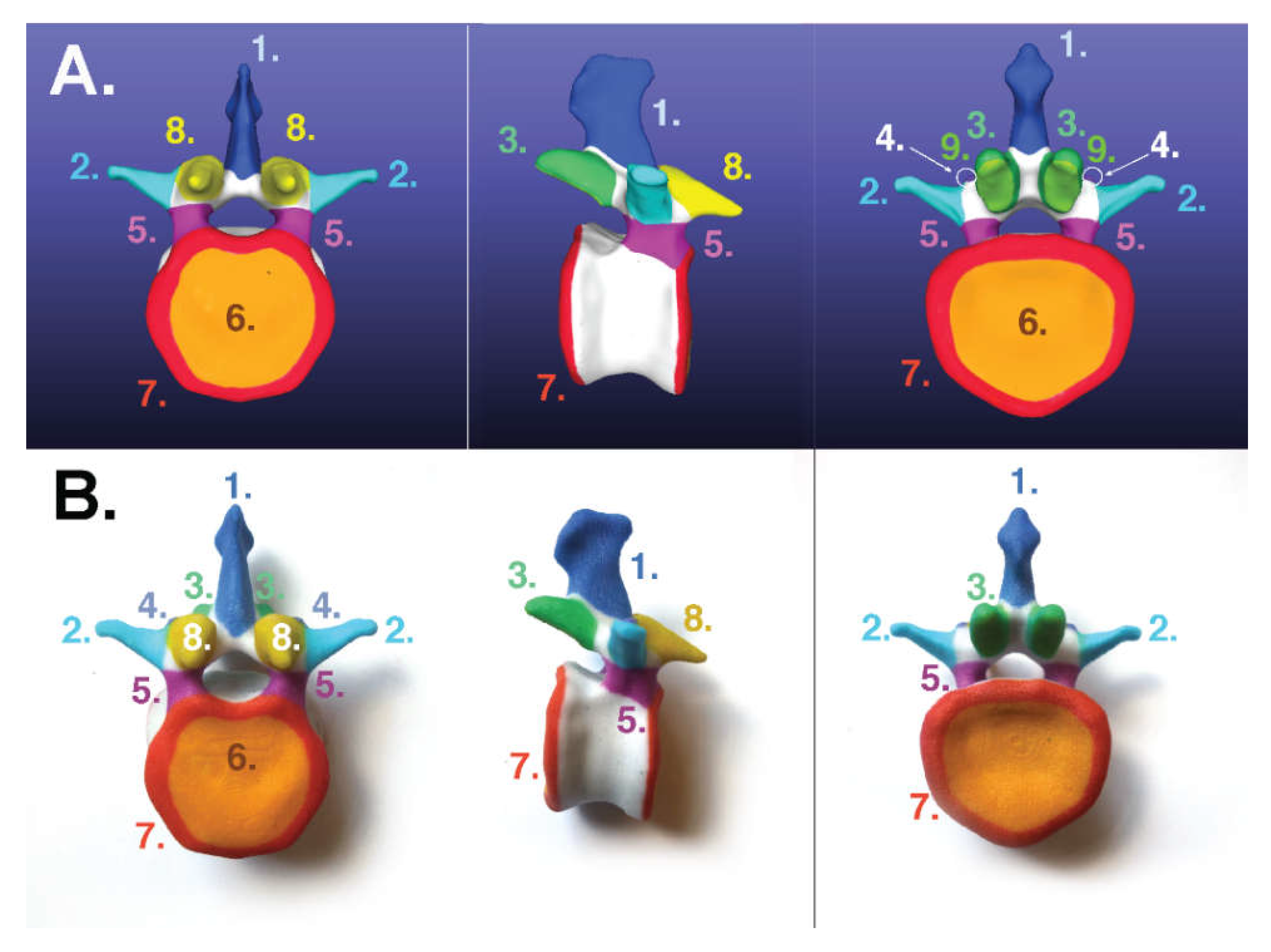
- Scapula: https://skfb.ly/6MYTL;
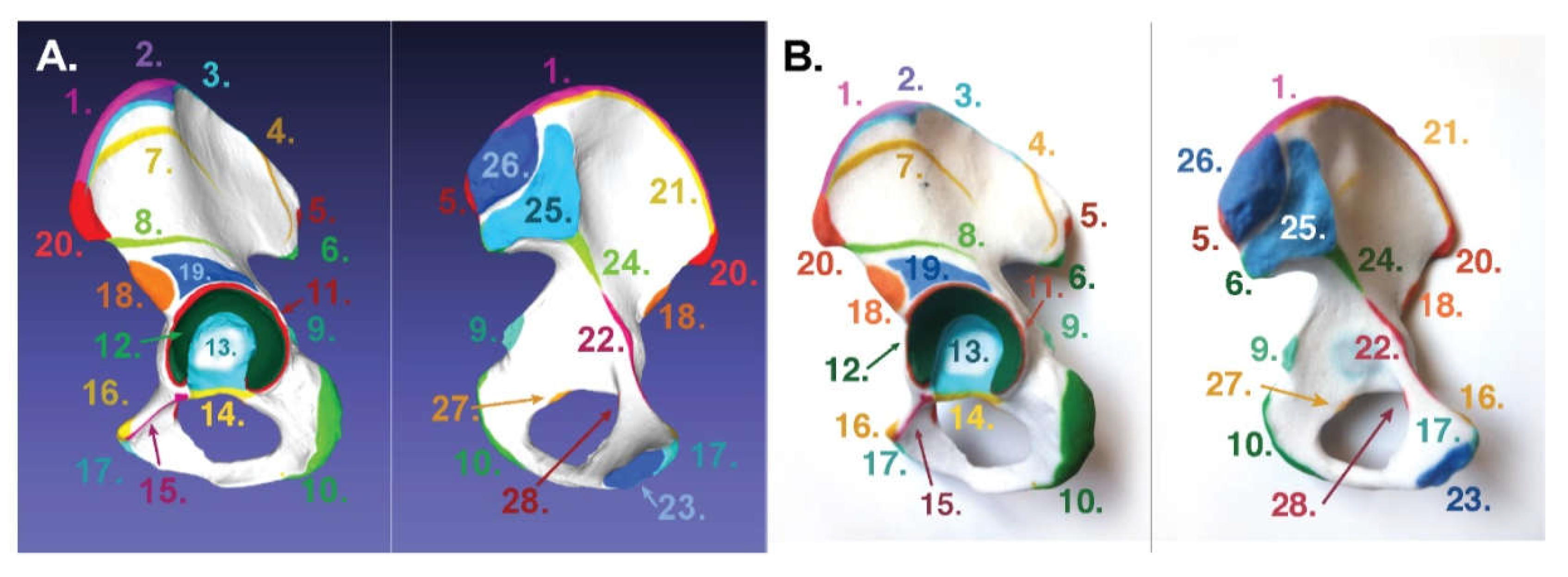
- Innominate bone: https://skfb.ly/6NotO.
2.2. 3D Printing
2.2.1. For Poly-Jet Processes
2.2.2. For Binder-Jet Processes
3. Results
3.1. For Poly-Jet Split Processes
3.2. For Binder-Jet Paint Processes
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Femur | Lumbar | Scapula | Innominate Bone | |
|---|---|---|---|---|
| Dimensions | X: 35.11 mm Y: 30.66 mm Z: 200.83 mm | X: 45.16 mm Y: 32.47 mm Z: 56.3 mm | X: 94.15 mm Y: 113.4 mm Z: 121.2 mm | X: 61.6 mm Y: 58.76 mm Z: 89.09 mm |
| Model volume: 34.77 cm3 | Model volume: 13.47 cm3 | Model Volume: 54.06 cm3 | Model volume: 20.88 cm3 | |
| Sandstone printing (full color) | $33.58 | $17.60 | $48.04 | $23.16 |
| Multi-color poly-jet printing | $382.92 | $191.19 | $556.5 | $257.92 |
References
- Smith, C.F.; Tollemache, N.; Covill, D.; Johnston, M. Take away body parts! An investigation into the use of 3D-printed anatomical models in undergraduate anatomy education. Anat. Sci. Educ. 2018, 11, 44–53. [Google Scholar] [CrossRef]
- AbouHashem, Y.; Dayal, M.; Savanah, S.; Strkalj, G. The application of 3D printing in anatomy education. Med. Educ. Online 2018, 20. [Google Scholar] [CrossRef]
- Sander, I.M.; Liepert, T.T.; Doney, E.L.; Leevy, W.M.; Liepert, D.R. Patient education for endoscopic sinus surgery: Preliminary experience using 3D-printed clinical imaging data. J. Funct. Biomater. 2017, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Mogali, S.R.; Yeong, W.Y.; Tan, H.K.J.; Tan, G.J.S.; Abrahams, P.H.; Zary, N.; Low-Beer, N.; Ferenczi, M.A. Evaluation by medical students of the educational value of multi-material and multi-colored three dimensional printed models of the upper limb for anatomical education. Anat. Sci. Educ. 2018, 11, 54–64. [Google Scholar] [CrossRef]
- Pawlina, W.; Drake, R.L. Anatomical models: Don’t banish them from the anatomy laboratory yet. Anat. Sci. Educ. 2013, 6, 209–210. [Google Scholar] [CrossRef]
- Lauridsen, H.; Hansen, K.; Ørum, N.M.; Wang, T.; Pedersen, M. From tissue to silicon to plastic: Three-dimensional printing in comparative anatomy and physiology. R. Soc. Open Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khot, Z.; Quinlan, K.; Norman, G.R.; Wainman, B. The relative effectiveness of computer-based and traditional resources for education in anatomy. Anat. Sci. Educ. 2013, 6, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Preece, D.; Williams, S.B.; Lam, R.; Weller, R. “Let’s get physical”: Advantages of a physical model over 3D computer models and textbooks in learning imaging anatomy. Anat. Sci. Educ. 2013, 6, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Garas, M.; Vaccarezza, M.; Newland, G.; McVay-Doornbusch, K.; Hasani, J. 3D-printed specimens as a valuable tool in anatomy education: A pilot study. Ann. Anat. 2018, 219, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Stratasys. Connex Objet500 and Objet350. Available online: https://www.stratasys.com/3d-printers/objet-350-500-connex3 (accessed on 7 January 2020).
- Ejaz, F.; Ryan, J.; Henriksen, M.; Osborn, M.; Frakes, D. Color-coded patient specific models of congenital heart disease. Rapid Prototyp. J. 2014, 20, 336–343. [Google Scholar] [CrossRef]
- Bernhard, J.C.; Isotani, S.; Matsugasumi, T.; Duddalwar, V.; Hung, A.J.; Suer, E.; Baco, E.; Satkunasivam, R.; Djaladat, H.; Metcalfe, C.; et al. Personalized 3D printed model of kidney and tumor anatomy: A useful tool for patient education. World J. Urol. 2016, 34, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Kang, J.W.; Kim, N.; Song, J.K.; Lee, J.W.; Lim, T.H. Myocardial 3-dimensional printing for septal myectomy guidance in a patient with obstructive hypertrophic cardiomyopathy. Circulation 2015, 132, 300–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Jabbari, O.; Abu Saleh, W.K.; Patel, A.P.; Igo, S.R.; Reardon, M.J. Use of three-dimensional models to assist in the resection of malignant tumors. J. Card. Surg. 2016, 31, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.P.; Olivieri, L.J.; Su, L.; Krieger, A.; Alfares, F.; Thabit, O.; Marshall, M.B.; Yoo, S.J.; Kim, P.C.; Jonas, R.A.; et al. Incorporating three-dimensional printing into a simulation-based congenital heart disease and critical care training curriculum for resident physicians. Congenit. Heart Dis. 2015, 10, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Loughborough University. About Additive Manufacturing—Binder Jetting. Available online: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing/binderjetting/ (accessed on 23 November 2019).
- 3D Hubs. Full Color Sandstone. Available online: https://www.3dhubs.com/material-group/full-color-sandstone (accessed on 23 November 2019).
- McMenamin, P.G.; Quayle, M.R.; McHenry, C.R.; Adams, J.W. The production of anatomical teaching resources using three-dimensional (3D) printing technology. Anat. Sci. Educ. 2014, 7, 479–486. [Google Scholar] [CrossRef]
- Adams, J.W.; Paxton, L.; Dawes, K.; Burlak, K.; Quayle, M.; McMenamin, P.G. 3D printed reproductions of orbital dissections: A novel mode of visualising anatomy for trainees in ophthalmology or optometry. Br. J. Ophthalmol. 2015, 99, 1162–1167. [Google Scholar] [CrossRef]
- Adams, F.; Qiu, T.; Mark, A.; Fritz, B.; Kramer, L.; Schlager, D.; Wetterauer, U.; Miernik, A.; Fischer, P. Soft 3D-printed phantom of the human kidney with collecting system. Ann. Biomed. Eng. 2017, 45, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Perica, E.; Sun, Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant. Imaging Med. Surg. 2017, 7, 668–677. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Li, C.; Chen, G.; Song, G.; Zhang, Y. Three-Dimensional Printed Skull Base Simulation for Transnasal Endoscopic Surgical Training. World Neurosurg. 2018, 111, 773–782. [Google Scholar] [CrossRef]
- Dauber, W.; Feneis, H. Pocket Atlas of Human Anatomy, 5th ed.; Thieme Publishing: Tuebingen, Germany, 2006. [Google Scholar]
- Shapeways. Material Information—Sandstone. Available online: https://www.shapeways.com/materials/sandstone (accessed on 23 November 2019).
- Blender. UV Editor—Introduction. Available online: https://docs.blender.org/manual/en/latest/editors/uv/introduction.html (accessed on 23 November 2019).
- Fang, B.; Wu, Y.; Chu, C.; Li, Y.; Luo, N.; Liu, K.; Tan, L.; Zhang, S. Creation of a Virtual Anatomy System based on Chinese Visible Human data sets. Surg Radiol Anat. 2017, 39, 441–449. [Google Scholar] [CrossRef]
- 3D4Medical. Complete Anatomy 2020. Available online: https://3d4medical.com (accessed on 23 November 2019).
- Education Mobile. Visual Anatomy. Available online: https://www.edumobapp.com/product.html (accessed on 23 November 2019).
- Erolin, C. Interactive 3D digital models for anatomy and medical education. Biomed. Vis. 2019, 2, 1–16. [Google Scholar]
- Estevez, M.E.; Lindgren, K.A.; Bergethon, P.R. A novel three- dimensional tool for teaching human neuroanatomy. Anat. Sci. Educ. 2010, 3, 309–317. [Google Scholar] [CrossRef] [PubMed]


| Durability | Software Processing | Pricing | Build Size | |
|---|---|---|---|---|
| Poly-jet | High | Relatively complex (time-consuming, requires high computational power, stepwise planning) | 10X | 255 × 252 × 200 mm for Objet 260 Connex3. 342 × 342 × 200 mm for Objet 350 Connex3 |
| Binder-jet | Relatively fragile | Relatively simple | X | 250 × 380 × 200 mm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, M.; Freel, T.; Van Avermaete, A.; Leevy, W.M. Color Enhancement Strategies for 3D Printing of X-ray Computed Tomography Bone Data for Advanced Anatomy Teaching Models. Appl. Sci. 2020, 10, 1571. https://doi.org/10.3390/app10051571
Inoue M, Freel T, Van Avermaete A, Leevy WM. Color Enhancement Strategies for 3D Printing of X-ray Computed Tomography Bone Data for Advanced Anatomy Teaching Models. Applied Sciences. 2020; 10(5):1571. https://doi.org/10.3390/app10051571
Chicago/Turabian StyleInoue, Megumi, Tristan Freel, Anthony Van Avermaete, and W. Matthew Leevy. 2020. "Color Enhancement Strategies for 3D Printing of X-ray Computed Tomography Bone Data for Advanced Anatomy Teaching Models" Applied Sciences 10, no. 5: 1571. https://doi.org/10.3390/app10051571
APA StyleInoue, M., Freel, T., Van Avermaete, A., & Leevy, W. M. (2020). Color Enhancement Strategies for 3D Printing of X-ray Computed Tomography Bone Data for Advanced Anatomy Teaching Models. Applied Sciences, 10(5), 1571. https://doi.org/10.3390/app10051571




