Expression of Insulin Receptor and c-MET Is Associated with Clinicopathologic Characteristics and Molecular Subtypes in Premenopausal Breast Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Tumor Samples
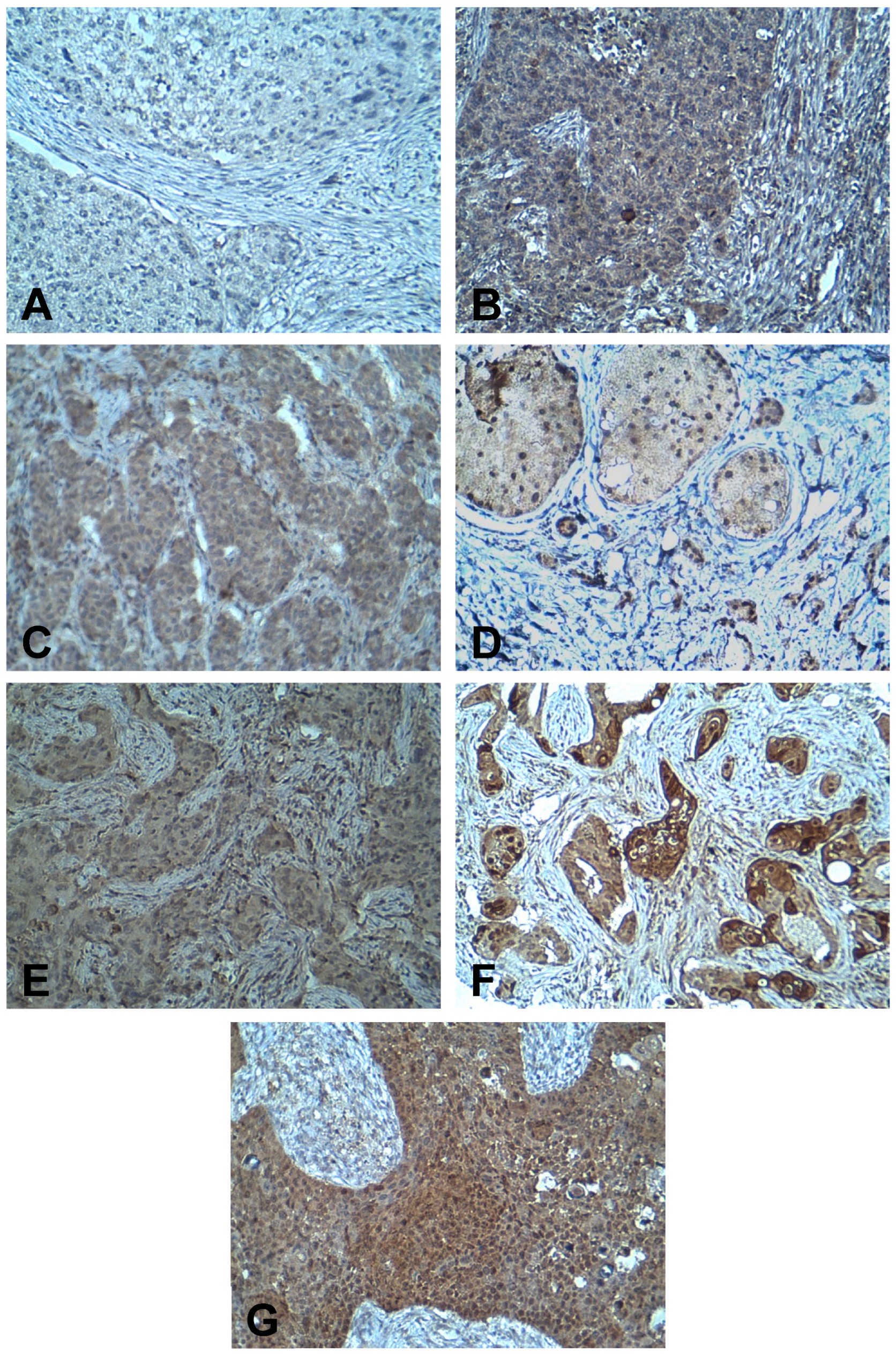
2.2. Immunohistochemistry
2.3. Evaluation of Immunostaining
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Expression of IR and c-MET in Breast Cancer Tissues and Association with Clinicopathologic Characteristics
3.3. Correlation between IR and c-MET Expression with Clinicopathologic Characteristics Based on Menopausal Status
3.4. Expression of IR and c-MET among Breast Cancer Molecular Subtypes
3.5. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hynes, N.E.; Watson, C.J. Mammary gland growth factors: Roles in normal development and in cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a003186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, D.R.; Wu, Y.M.; Lin, S.F. The protein tyrosine kinase family of the human genome. Oncogene 2000, 19, 5548–5557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangwan, V.; Park, M. Receptor tyrosine kinases: Role in cancer progression. Curr. Oncol. 2006, 13, 191–193. [Google Scholar] [PubMed]
- Favoni, R.E.; de Cupis, A. The role of polypeptide growth factors in human carcinomas: New targets for a novel pharmacological approach. Pharmacol. Rev. 2000, 52, 179–206. [Google Scholar]
- Malaguarnera, R.; Belfiore, A. The insulin receptor: A new target for cancer therapy. Front. Endocrinol. 2011, 2, 93. [Google Scholar] [CrossRef] [Green Version]
- Clayton, p.E.; Banerjee, I.; Murray, P.G.; Renehan, A.G. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat. Rev. Endocrinol. 2011, 7, 11–24. [Google Scholar] [CrossRef]
- Fox, E.M.; Miller, T.W.; Balko, J.M.; Kuba, M.G.; Sanchez, V.; Smith, R.A.; Liu, S.; Gonzalez-Angulo, A.M.; Mills, G.B.; Ye, F.; et al. A kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res. 2011, 71, 6773–6784. [Google Scholar] [CrossRef] [Green Version]
- Belfiore, A.; Frasca, F. IGF and insulin receptor signaling in breast cancer. J. Mammary Gland. Biol. Neoplasia. 2008, 13, 381–406. [Google Scholar] [CrossRef]
- Kalla Singh, S.; Brito, C.; Tan, Q.W.; De Leon, M.; De Leon, D. Differential expression and signaling activation of insulin receptor isoforms A and B: A link between breast cancer and diabetes. Growth Factors 2011, 29, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Papa, V.; Pezzino, V.; Costantino, A.; Belfiore, A.; Giuffrida, D.; Frittitta, L.; Vannelli, G.B.; Brand, R.; Goldfine, I.D.; Vigneri, R. Elevated insulin receptor content in human breast cancer. J. Clin. Investig. 1990, 86, 1503–1510. [Google Scholar] [CrossRef] [Green Version]
- Ligibel, J.A.; Strickler, H.D. Obesity and its impact on breast cancer: Tumor incidence, recurrence, survival, and possible interventions. Am. Soc. Clin. Oncol. Educ. Book. 2013, 33, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Novosyadlyy, R.; Lann, D.E.; Vijayakumar, A.; Rowzee, A.; Lazzarino, D.A.; Fierz, Y.; Carboni, J.M.; Gottardis, M.M.; Pennisi, P.A.; Molinolo, A.A.; et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010, 70, 741–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaaks, R.; Lukanova, A. Effects of weight control and physical activity in cancer prevention: Role of endogenous hormone metabolism. Ann. N. Y. Acad. Sci. 2002, 963, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Strickler, H.D.; Wylie-Rosett, J.; Rohan, T.; Hoover, D.R.; Smoller, S.; Burk, R.D.; Yu, H. The relation of type 2 diabetes and cancer. Diabetes Technol. Ther. 2001, 3, 263–274. [Google Scholar] [CrossRef]
- Vigneri, P.; Frasca, F.; Sciacca, L.; Pandini, G.; Vigneri, R. Diabetes and cancer. Endocr. Relat. Cancer 2009, 16, 1103–1123. [Google Scholar] [CrossRef] [Green Version]
- Vigneri, P.; Frasca, F.; Sciacca, L.; Frittitta, L.; Vigneri, R. Obesity and cancer. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 1–7. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S. S961, a biosynthetic insulin receptor antagonist, downregulates insulin receptor expression & suppresses the growth of breast cancer cells. Indian J. Med Res. 2018, 147, 545–551. [Google Scholar] [CrossRef]
- Dowling, R.J.; Niraula, S.; Chang, M.C.; Done, S.J.; Ennis, M.; McCready, D.R.; Leong, W.L.; Escallon, J.M.; Reedijk, M.; Goodwin, P.J.; et al. Changes in insulin receptor signaling underlie neoadjuvant metformin administration in breast cancer: A prospective window of opportunity neoadjuvant study. Breast Cancer Res. 2015, 17, 32. [Google Scholar] [CrossRef] [Green Version]
- Gastaldi, S.; Comoglio, P.M.; Trusolino, L. The Met oncogene and basal-like breast cancer: Another culprit to watch out for? Breast Cancer Res. 2010, 12, 208. [Google Scholar] [CrossRef] [Green Version]
- Sattler, M.; Salgia, R. c-Met and hepatocyte growth factor: Potential as novel targets in cancer therapy. Curr. Oncol. Rep. 2007, 9, 102–108. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Chen, H.; Karuturi, M.S.; Chavez-MacGregor, M.; Tsavachidis, S.; Meric-Bernstam, F.; Do, K.A.; Hortobagyi, G.N.; Thompson, P.A.; Mills, G.B.; et al. Frequency of mesenchymal-epithelial transition factor gene (MET) and the catalytic subunit of phosphoinositide-3-kinase (PIK3CA) copy number elevation and correlation with outcome in patients with early stage breast cancer. Cancer 2013, 119, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponzo, M.G.; Lesurf, R.; Petkiewicz, S.; O’Malley, F.P.; Pinnaduwage, D.; Andrulis, I.L.; Bull, S.B.; Chughtai, N.; Zuo, D.; Souleimanova, M.; et al. Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 12903–12908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho-Yen, C.M.; Green, A.R.; Rakha, E.A.; Brentnall, A.R.; Ellis, I.O.; Kermorgant, S.; Jones, J.L. C-Met in invasive breast cancer: Is there a relationship with the basal-like subtype? Cancer 2014, 120, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fafalios, A.; Ma, J.; Tan, X.; Stoops, J.; Luo, J.; Defrances, M.C.; Zarnegar, R. A hepatocyte growth factor receptor (Met)-insulin receptor hybrid governs hepatic glucose metabolism. Nat. Med. 2011, 17, 1577–1584. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic; Technical Report for a WHO Consulation (WHO Technical Report Series 894); WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Edge, S.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.; Trotti, A. AJCC Cancer Staging Handbook from the AJCC Cancer Stagnig Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Rakha, E.A.; Reis-Filho, J.S.; Baehner, F.; Dabbs, D.J.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Breast cancer prognostic classification in the molecular era: The role of histological grade. Breast Cancer Res. 2010, 12, 207. [Google Scholar] [CrossRef] [Green Version]
- Stark, A.; Stahl, M.S.; Kirchner, H.L.; Krum, S.; Prichard, J.; Evans, J. Body mass index at the time of diagnosis and the risk of advanced stages and poorly differentiated cancers of the breast: Findings from a case-series study. Int. J. Obes. 2010, 34, 1381–1386. [Google Scholar] [CrossRef] [Green Version]
- Spitale, A.; Mazzola, P.; Soldini, D.; Mazzucchelli, L.; Bordoni, A. Breast cancer classification according to immunohistochemical markers: Clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann. Oncol. 2009, 20, 628–635. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Ademuyiwa, F.O.; Groman, A.; O’Connor, T.; Ambrosone, C.; Watroba, N.; Edge, S.B. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer 2011, 117, 4132–4140. [Google Scholar] [CrossRef]
- Vona-Davis, L.; Rose, D.P.; Hazard, H.; Howard-McNatt, M.; Adkins, F.; Partin, J.; Hobbs, G. Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3319–3324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.H.; Gao, H.F.; Wang, Y.; Liu, F.; Tian, X.F.; Zhang, Y. Overexpression of Gli1 in cancer interstitial tissues predicts early relapse after radical operation of breast cancer. Chinese J. Cancer Res. = Chung-kuo yen cheng yen chiu 2012, 24, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Ten Haaf, A.; Bektas, N.; von Serenyi, S.; Losen, I.; Arweiler, E.C.; Hartmann, A.; Knuchel, R.; Dahl, E. Expression of the glioma-associated oncogene homolog (GLI) 1 in human breast cancer is associated with unfavourable overall survival. BMC Cancer 2009, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Qi, Y.; Chen, J.; Kaczorowski, D.; Di, W.; Wang, W.; Xia, P. Sphingosine kinase (SphK) 1 and SphK2 play equivalent roles in mediating insulin’s mitogenic action. Mol. Endocrinol. 2014, 28, 197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciacca, L.; Costantino, A.; Pandini, G.; Mineo, R.; Frasca, F.; Scalia, P.; Sbraccia, P.; Goldfine, I.D.; Vigneri, R.; Belfiore, A. Insulin receptor activation by IGF-II in breast cancers: Evidence for a new autocrine/paracrine mechanism. Oncogene 1999, 18, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Bjorner, S.; Rosendahl, A.H.; Simonsson, M.; Markkula, A.; Jirstrom, K.; Borgquist, S.; Rose, C.; Ingvar, C.; Jernstrom, H. Combined and individual tumor-specific expression of insulin-like growth factor-I receptor, insulin receptor and phospho-insulin-like growth factor-I receptor/insulin receptor in primary breast cancer: Implications for prognosis in different treatment groups. Oncotarget 2017, 8, 9093–9107. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Hong, L.Q. Insulin promotes proliferation and migration of breast cancer cells through the extracellular regulated kinase pathway. Asian Pac. J. Cancer Prev. 2014, 15, 6349–6352. [Google Scholar] [CrossRef] [Green Version]
- Fox, E.M.; Kuba, M.G.; Miller, T.W.; Davies, B.R.; Arteaga, C.L. Autocrine IGF-I/insulin receptor axis compensates for inhibition of AKT in ER-positive breast cancer cells with resistance to estrogen deprivation. Breast Cancer Res. 2013, 15, R55. [Google Scholar] [CrossRef] [Green Version]
- Belfiore, A.; Malaguarnera, R. Insulin receptor and cancer. Endocr. Relat. Cancer 2011, 18, R125–R147. [Google Scholar] [CrossRef] [Green Version]
- Cianfrocca, M.; Goldstein, L.J. Prognostic and predictive factors in early-stage breast cancer. Oncologist 2004, 9, 606–616. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, A.M.; O’Malley, F.P.; Ennis, M.; Fantus, I.G.; Goodwin, P.J. Insulin receptor is an independent predictor of a favorable outcome in early stage breast cancer. Breast Cancer Res. Treat. 2007, 106, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Aljada, A.; Saleh, A.M.; Al-Aqeel, S.M.; Shamsa, H.B.; Al-Bawab, A.; Al Dubayee, M.; Ahmed, A.A. Quantification of insulin receptor mRNA splice variants as a diagnostic tumor marker in breast cancer. Cancer Biomark.: Sect. Dis. Markers 2015, 15, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.R.; Tsao, M.S. c-MET as a potential therapeutic target and biomarker in cancer. Adv. Med Oncol. 2011, 3, S21–S35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, R.E.; Salgia, R. MET molecular mechanisms and therapies in lung cancer. Cell Adhes. Migr. 2010, 4, 146–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, H.N.; Liu, P. Targeting MET in cancer therapy. Chronic Dis. Transl. Med. 2017, 3, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Yang, X.; Tian, W.; Guo, S.; Huang, W.; Zhao, W. Increased Expression of c-Met is Associated with Chemotherapy-Resistant Breast Cancer and Poor Clinical Outcome. Med Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 8239–8249. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, M.; Jin, K.; Wang, S.; Wei, H.; Fan, C.; Wu, Y.; Li, X.; Li, X.; Li, G.; et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer 2018, 17, 45. [Google Scholar] [CrossRef]
- Ho-Yen, C.M.; Jones, J.L.; Kermorgant, S. The clinical and functional significance of c-Met in breast cancer: A review. Breast Cancer Res. 2015, 17, 52. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Liang, L.; Lei, X.; Multani, A.; Meric-Bernstam, F.; Tripathy, D.; Wu, Y.; Chen, H.; Zhang, H. Evaluation of cMET aberration by immunohistochemistry and fluorescence in situ hybridization (FISH) in triple negative breast cancers. Ann. Diagn. Pathol. 2018, 35, 69–76. [Google Scholar] [CrossRef]
- Lengyel, E.; Prechtel, D.; Resau, J.H.; Gauger, K.; Welk, A.; Lindemann, K.; Salanti, G.; Richter, T.; Knudsen, B.; Vande Woude, G.F.; et al. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int. J. Cancer 2005, 113, 678–682. [Google Scholar] [CrossRef]
- Nakopoulou, L.; Gakiopoulou, H.; Keramopoulos, A.; Giannopoulou, I.; Athanassiadou, P.; Mavrommatis, J.; Davaris, P.S. c-met tyrosine kinase receptor expression is associated with abnormal beta-catenin expression and favourable prognostic factors in invasive breast carcinoma. Histopathology 2000, 36, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Baccelli, I.; Stenzinger, A.; Vogel, V.; Pfitzner, B.M.; Klein, C.; Wallwiener, M.; Scharpff, M.; Saini, M.; Holland-Letz, T.; Sinn, H.P.; et al. Co-expression of MET and CD47 is a novel prognosticator for survival of luminal breast cancer patients. Oncotarget 2014, 5, 8147–8160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagouri, F.; Bago-Horvath, Z.; Rossler, F.; Brandstetter, A.; Bartsch, R.; Papadimitriou, C.A.; Dimitrakakis, C.; Tsigginou, A.; Papaspyrou, I.; Giannos, A.; et al. High MET expression is an adverse prognostic factor in patients with triple-negative breast cancer. Br. J. Cancer 2013, 108, 1100–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, S.; Jiao, X.; Zou, H.; Li, K. Prognostic significance of c-Met in breast cancer: A meta-analysis of 6010 cases. Diagn. Pathol. 2015, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Litzenburger, B.C.; Creighton, C.J.; Tsimelzon, A.; Chan, B.T.; Hilsenbeck, S.G.; Wang, T.; Carboni, J.M.; Gottardis, M.M.; Huang, F.; Chang, J.C.; et al. High IGF-IR activity in triple-negative breast cancer cell lines and tumorgrafts correlates with sensitivity to anti-IGF-IR therapy. Clin. Cancer Res. 2011, 17, 2314–2327. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.K.; Dickson, M.A.; LoRusso, P.M.; Sausville, E.A.; Maekawa, Y.; Watanabe, Y.; Kashima, N.; Nakashima, D.; Akinaga, S. Preclinical and first-in-human phase I studies of KW-2450, an oral tyrosine kinase inhibitor with insulin-like growth factor receptor-1/insulin receptor selectivity. Cancer Sci. 2016, 107, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Huang, F.; Macedo, L.F.; Harrington, S.C.; Reeves, K.A.; Greer, A.; Finckenstein, F.G.; Brodie, A.; Gottardis, M.M.; Carboni, J.M.; et al. Dual IGF-1R/InsR inhibitor BMS-754807 synergizes with hormonal agents in treatment of estrogen-dependent breast cancer. Cancer Res. 2011, 71, 7597–7607. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.Y.; Hackel, B.J.; Yee, D. Targeting Insulin Receptor in Breast Cancer Using Small Engineered Protein Scaffolds. Mol. Cancer Ther. 2017, 16, 1324–1334. [Google Scholar] [CrossRef] [Green Version]
- Chia, S.K.; Ellard, S.L.; Mates, M.; Welch, S.; Mihalcioiu, C.; Miller, W.H., Jr.; Gelmon, K.; Lohrisch, C.; Kumar, V.; Taylor, S.; et al. A phase-I study of lapatinib in combination with foretinib, a c-MET, AXL and vascular endothelial growth factor receptor inhibitor, in human epidermal growth factor receptor 2 (HER-2)-positive metastatic breast cancer. Breast Cancer Res. 2017, 19, 54. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Ziehr, D.R.; Guo, H.; Ng, M.R.; Barry, W.T.; Higgins, M.J.; Isakoff, S.J.; Brock, J.E.; Ivanova, E.V.; Paweletz, C.P.; et al. Phase II and Biomarker Study of Cabozantinib in Metastatic Triple-Negative Breast Cancer Patients. Oncologist 2017, 22, 25–32. [Google Scholar] [CrossRef] [Green Version]


| Characteristics | n (%) |
|---|---|
| Age, years | |
| <45 | 26 (36.6) |
| 45–60 | 33 (46.5) |
| >60 | 12 (16.9) |
| BMI, kg/m2 | |
| Normal | 16 (22.5) |
| Overweight | 25 (35.2) |
| Obese | 30 (42.3) |
| Marital status | |
| Single | 8 (11.3) |
| Married | 63 (88.7) |
| Menopausal status | |
| Premenopausal | 37 (52.1) |
| Postmenopausal | 30 (42.3) |
| Unknown | 4 (5.6) |
| Family history of breast cancer | |
| Absent | 49 (69.0) |
| Present | 14 (19.7) |
| Unknown | 8 (11.3) |
| Smoking | |
| Never smoker | 61 (85.9) |
| Past smoker | 3 (4.2) |
| Current smoker | 6 (8.5) |
| Unknown | 1 (1.4) |
| Alcohol intake | |
| Never | 66 (93.0) |
| Unknown | 5 (7.0) |
| HRT use | |
| No | 60 (84.5) |
| Yes | 1 (1.4) |
| Unknown | 10 (14.1) |
| Site of disease | |
| Right | 35 (49.3) |
| Left | 33 (46.5) |
| Bilateral | 3 (4.2) |
| Tumor size | |
| <2 cm | 6 (8.5) |
| ≥2 cm | 59 (83.1) |
| Unknown | 6 (8.5) |
| Lymph nodes | |
| Negative | 24 (33.8) |
| Positive | 39 (54.9) |
| Unknown | 8 (11.3) |
| Stage | |
| I | 3 (4.2) |
| II | 30 (42.3) |
| III | 24 (33.8) |
| IV | 11 (15.5) |
| Unknown | 3 (4.2) |
| Grade | |
| I | 5 (7.0) |
| II | 14 (19.7) |
| III | 50 (70.4) |
| Unknown | 2 (2.8) |
| Histologic type | |
| IDC | 58 (81.7) |
| ILC | 11 (15.5) |
| Other | 2 (2.8) |
| LVI | |
| Unidentified | 28 (39.4) |
| Identified | 37 (52.1) |
| Unknown | 6 (8.5) |
| ER | |
| Positive | 23 (32.4) |
| Negative | 48 (67.6) |
| PR | |
| Positive | 19 (26.8) |
| Negative | 52 (73.2) |
| HER2 | |
| Positive | 19 (26.8) |
| Negative | 52 (73.2) |
| Molecular subtype | |
| Luminal | 23 (32.4) |
| HER2–positive | 19 (26.8) |
| Triple–negative | 29 (40.8) |
| Surgery | |
| Mastectomy | 60 (84.5) |
| Wide local excision | 11 (15.5) |
| Adjuvant chemotherapy | |
| Yes | 59 (83.1) |
| No | 4 (5.6) |
| Unknown | 8 (11.3) |
| Parameter | IR [n (%)] | p-Value | |
|---|---|---|---|
| Low (n = 53) | High (n = 15) | ||
| Age, years | 0.341 | ||
| <50 | 28 (73.7%) | 10 (26.3%) | |
| ≥50 | 25 (83.3%) | 5 (16.7%) | |
| Menopausal status | 0.195 | ||
| Premenopausal | 26 (72.2%) | 10 (27.8%) | |
| Postmenopausal | 24 (85.7%) | 4 (14.3%) | |
| BMI, kg/m2 | 0.533 | ||
| Non-obese | 33 (80.5%) | 8 (19.5%) | |
| Obese | 20 (74.1%) | 7 (25.9%) | |
| Grade | 0.017 * | ||
| I/II | 11 (57.9%) | 8 (42.1%) | |
| III | 40 (85.1%) | 7 (14.9%) | |
| Stage | 0.635 | ||
| Early (I and II) | 26 (81.3%) | 6 (18.8%) | |
| Advanced (III and IV) | 26 (76.5%) | 8 (23.5%) | |
| Lymph nodes | 0.571 | ||
| Negative | 18 (75.0%) | 6 (25.0%) | |
| Positive | 30 (81.1%) | 7 (18.9%) | |
| ER | 0.015 * | ||
| Positive | 14 (60.9%) | 9 (39.1%) | |
| Negative | 39 (86.7%) | 6 (13.3%) | |
| PR | 0.067 | ||
| Positive | 12 (63.2%) | 7 (36.8%) | |
| Negative | 41 (83.7%) | 8 (16.3%) | |
| HER2 | 0.081 | ||
| Positive | 15 (93.8%) | 1 (6.3%) | |
| Negative | 39 (73.1%) | 14 (26.9%) | |
| LVI | 0.244 | ||
| Unidentified | 19 (70.4%) | 8 (29.6%) | |
| Identified | 29 (82.9%) | 6 (17.1%) | |
| Parameter | c-MET [n (%)] | p-Value | |
|---|---|---|---|
| Low (n = 19) | High (n = 52) | ||
| Age, years | 0.064 | ||
| <50 | 7 (17.9%) | 32 (82.1%) | |
| ≥50 | 12 (37.5%) | 20 (62.5%) | |
| Menopausal status | 0.057 | ||
| Premenopausal | 7 (18.9%) | 30 (81.1%) | |
| Postmenopausal | 12 (40.0%) | 18 (60.0%) | |
| Body mass index (BMI), kg/m2 | 0.271 | ||
| Non-obese | 13 (31.7%) | 28 (68.3%) | |
| Obese | 6 (20.0%) | 24 (80.0%) | |
| Grade | 0.979 | ||
| I/II | 5 (26.3%) | 14 (73.7%) | |
| III | 13 (26.0%) | 37 (74.0%) | |
| Stage | 0.327 | ||
| Early (I and II) | 10 (30.3%) | 23 (69.7%) | |
| Advanced (III and IV) | 7 (20.0%) | 28 (80.0%) | |
| Lymph nodes | 0.373 | ||
| Negative | 8 (33.3%) | 16 (66.7%) | |
| Positive | 9 (23.1%) | 30 (76.9%) | |
| ER | 0.929 | ||
| Positive | 6 (26.1%) | 17 (73.9%) | |
| Negative | 13 (27.1%) | 35 (72.9%) | |
| PR | 0.959 | ||
| Positive | 5 (26.3%) | 14 (73.7%) | |
| Negative | 14 (26.9%) | 38 (73.1%) | |
| HER2 | 0.511 | ||
| Positive | 4 (21.1%) | 15 (78.9%) | |
| Negative | 15 (28.8%) | 37 (71.2%) | |
| LVI | 0.700 | ||
| Unidentified | 8 (28.6%) | 20 (71.4%) | |
| Identified | 9 (24.3%) | 28 (75.7%) | |
| Parameter | IR Score | c-MET Score | ||
|---|---|---|---|---|
| rho | p-Value | rho | p-Value | |
| Age, years | 0.054 | 0.659 | −0.103 | 0.394 |
| BMI, kg/m2 | 0.155 | 0.207 | 0.001 | 0.991 |
| Tumor size, cm | 0.029 | 0.823 | 0.129 | 0.306 |
| Lymph nodes | 0.199 | 0.130 | 0.151 | 0.244 |
| IR score | ------ | ------ | 0.458 | <0.001 * |
| c-MET score | 0.458 | <0.001 * | ------ | ------ |
| Parameter | Premenopausal (n = 37) | Postmenopausal (n = 30) | ||
|---|---|---|---|---|
| IR Score | p-Value | IR Score | p-Value | |
| Median (IQR) | Median (IQR) | |||
| Grade | 0.025 * | 0.978 | ||
| I/II | 8.0 (4.0–12.0) | 3.0 (2.0–8.0) | ||
| III | 4.0 (2.0–6.0) | 3.5 (3.0–4.0) | ||
| Stage | 0.686 | 0.085 | ||
| Early (I/II) | 4.0 (2.0–8.0) | 3.0 (1.0–4.0) | ||
| Advanced (III/IV) | 4.0 (3.0–7.5) | 3.5 (3.0–5.0) | ||
| Lymph nodes | 0.944 | 0.085 | ||
| Negative | 4.0 (2.0–8.0) | 2.5 (2.0–3.75) | ||
| Positive | 4.0 (3.0–6.5) | 3.5 (3.0–4.0) | ||
| ER | 0.030 * | 0.376 | ||
| Positive | 4.0 (4.0–12.0) | 3.5 (2.75–8.0) | ||
| Negative | 4.0 (2.0–6.0) | 3.0 (2.0–4.00) | ||
| PR | 0.015 * | 0.404 | ||
| Positive | 6.0 (4.0–12.0) | 3.5 (3.0–7.0) | ||
| Negative | 3.5 (2.0–6.0) | 3.0 (2.0–4.0) | ||
| HER2 | 0.429 | 0.198 | ||
| Positive | 6.0 (2.5–9.0) | 4.0 (3.0–4.0) | ||
| Negative | 4.0 (2.0–8.0) | 3.0 (2.0–4.0) | ||
| LVI | 0.986 | 0.834 | ||
| Unidentified | 4.0 (1.0–12.0) | 3.0 (2.0–4.0) | ||
| Identified | 4.0 (3.0–6.0) | 3.0 (3.0–4.0) | ||
| Parameter | Premenopausal (n = 37) | Postmenopausal (n = 30) | ||
|---|---|---|---|---|
| c-MET Score | p-Value | c-MET Score | p-Value | |
| Median (IQR) | Median (IQR) | |||
| Grade | 0.404 | 0.780 | ||
| I/II | 8.0 (8.0–12.0) | 7.0 (3.75–9.0) | ||
| III | 8.0 (7.5–12.0) | 8.0 (3.75–8.0) | ||
| Stage | 0.911 | 0.143 | ||
| Early (I/II) | 8.0 (8.0–12.0) | 7.0 (3.0–8.0) | ||
| Advanced (III/IV) | 8.0 (8.0–11.0) | 8.0 (6.0–8.0) | ||
| Lymph nodes | 0.569 | 0.143 | ||
| Negative | 8.0 (8.0–12.0) | 8.0 (5.0–8.0) | ||
| Positive | 8.0 (8.0–9.0) | 5.0 (3.0–8.0) | ||
| ER | 0.007 * | 0.317 | ||
| Positive | 12.0 (8.0–12.0) | 5.0 (3.0–9.0) | ||
| Negative | 8.0 (6.0–8.0) | 8.0 (6.0–8.0) | ||
| PR | 0.024 * | 0.372 | ||
| Positive | 10.0 (8.0–12.0) | 5.0 (3.0–11.0) | ||
| Negative | 8.0 (6.0–8.0) | 8.0 (5.5–8.0) | ||
| HER2 | 0.117 | 0.083 | ||
| Positive | 7.0 (3.75–9.0) | 8.0 (8.0–8.0) | ||
| Negative | 8.0 (8.0–12.0) | 6.0 (3.0–8.0) | ||
| LVI | 0.956 | 0.238 | ||
| Unidentified | 8.0 (8.0–11.0) | 8.0 (3.0–8.0) | ||
| Identified | 8.0 (8.0–12.0) | 8.0 (4.0–8.0) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, N.M.; Yaghan, R.J.; Al-Mohtaseb, A.H.; Aldaoud, N.; Matalka, I.I.; Elhassan, M.E. Expression of Insulin Receptor and c-MET Is Associated with Clinicopathologic Characteristics and Molecular Subtypes in Premenopausal Breast Cancer Patients. Appl. Sci. 2020, 10, 1614. https://doi.org/10.3390/app10051614
Ayoub NM, Yaghan RJ, Al-Mohtaseb AH, Aldaoud N, Matalka II, Elhassan ME. Expression of Insulin Receptor and c-MET Is Associated with Clinicopathologic Characteristics and Molecular Subtypes in Premenopausal Breast Cancer Patients. Applied Sciences. 2020; 10(5):1614. https://doi.org/10.3390/app10051614
Chicago/Turabian StyleAyoub, Nehad M., Rami J. Yaghan, Alia H. Al-Mohtaseb, Najla Aldaoud, Ismail I. Matalka, and Muwada E. Elhassan. 2020. "Expression of Insulin Receptor and c-MET Is Associated with Clinicopathologic Characteristics and Molecular Subtypes in Premenopausal Breast Cancer Patients" Applied Sciences 10, no. 5: 1614. https://doi.org/10.3390/app10051614






