Sensitivity of EGFR/HER-2 Positive Cells Isolated from Ascitic Fluid of Advanced Ovarian Cancer Patients to EGFR/HER-2 Inhibitors
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Isolation of Cells from Ascitic Fluids
2.3. Establishment of Cell Clusters of Ovarian Cancer Cell Lines and Ascitic Fluid-Derived Cells
2.4. Measurement of Cellular Viability Using Alamar Blue Dye Assay
2.5. Immunofluorescence of Cell Clusters
2.6. Immunoblotting Analysis
2.7. Statistical Analysis
3. Results
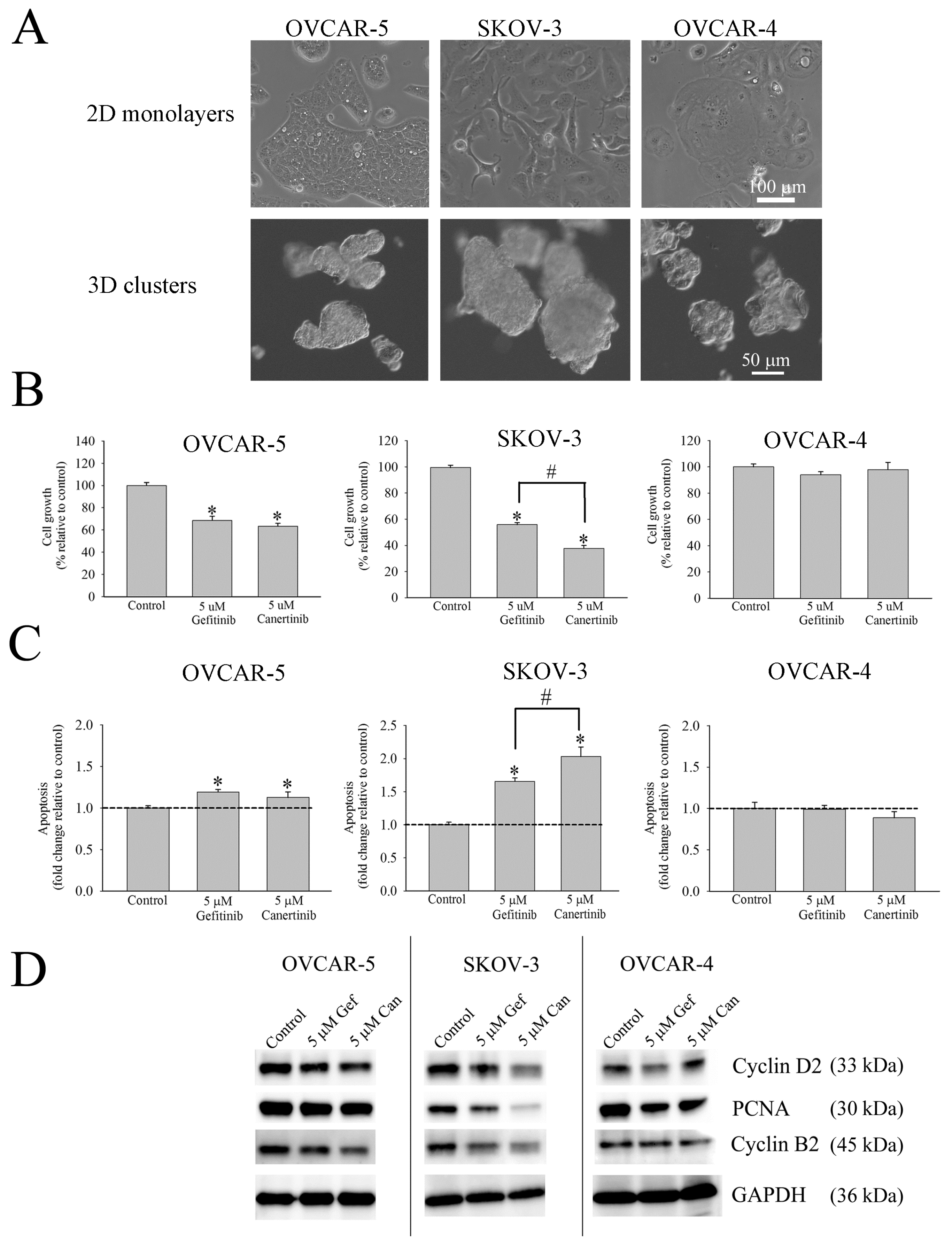
3.1. Morphology, Cell Growth and Apoptosis of Cell Clusters of Ovarian Cancer Cell Lines
3.2. Responsiveness of Ovarian Cancer Cell Line to Gefitinib and Canertinib Is Receptor Dependent
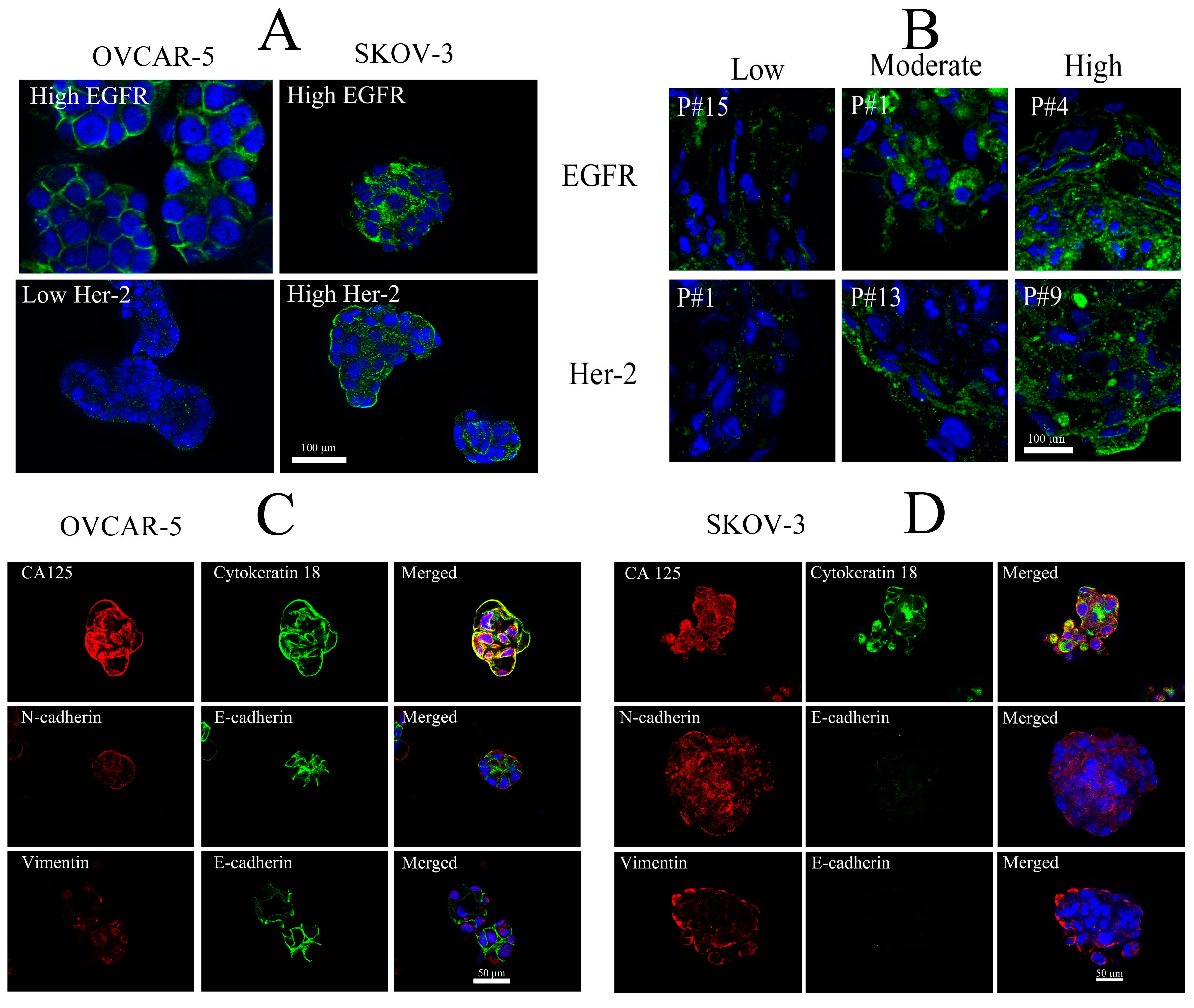
3.3. Characterisation of Cells from Ascitic Fluids
3.4. Identification of EGFR and HER-2 in Cells Isolated from Ascitic Fluid
3.5. Immunostaining of Selective Protein Markers for Ovarian Cancer
3.6. Effects of Gefitinib and Canertinib on Cellular Viability Using Alamar Blue Dye Assay
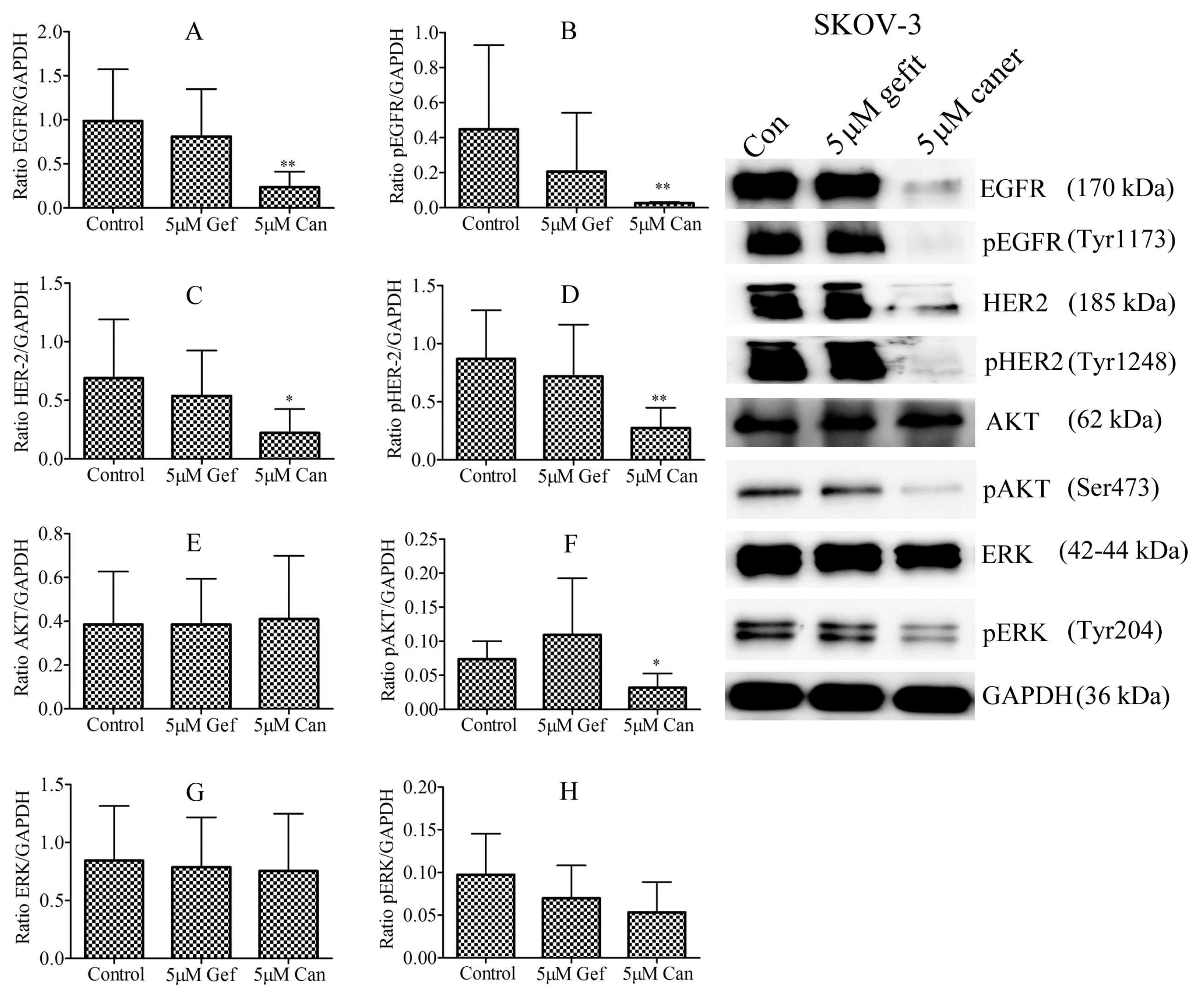
3.7. Effect of Gefitinib and Canertinib on Total Expression and Activation of EGFR and HER-2 Proteins
3.8. Effect of Gefitinib and Canertinib on PCNA, Caspase-3, pERK and pAKT
4. Discussion
4.1. Expression of Protein Markers in Ascitic Fluid-Derived Ovarian Cancer Cells
4.2. Expression of EGFR and HER-2 in Ascitic Fluid-Derived Ovarian Cancer Cells
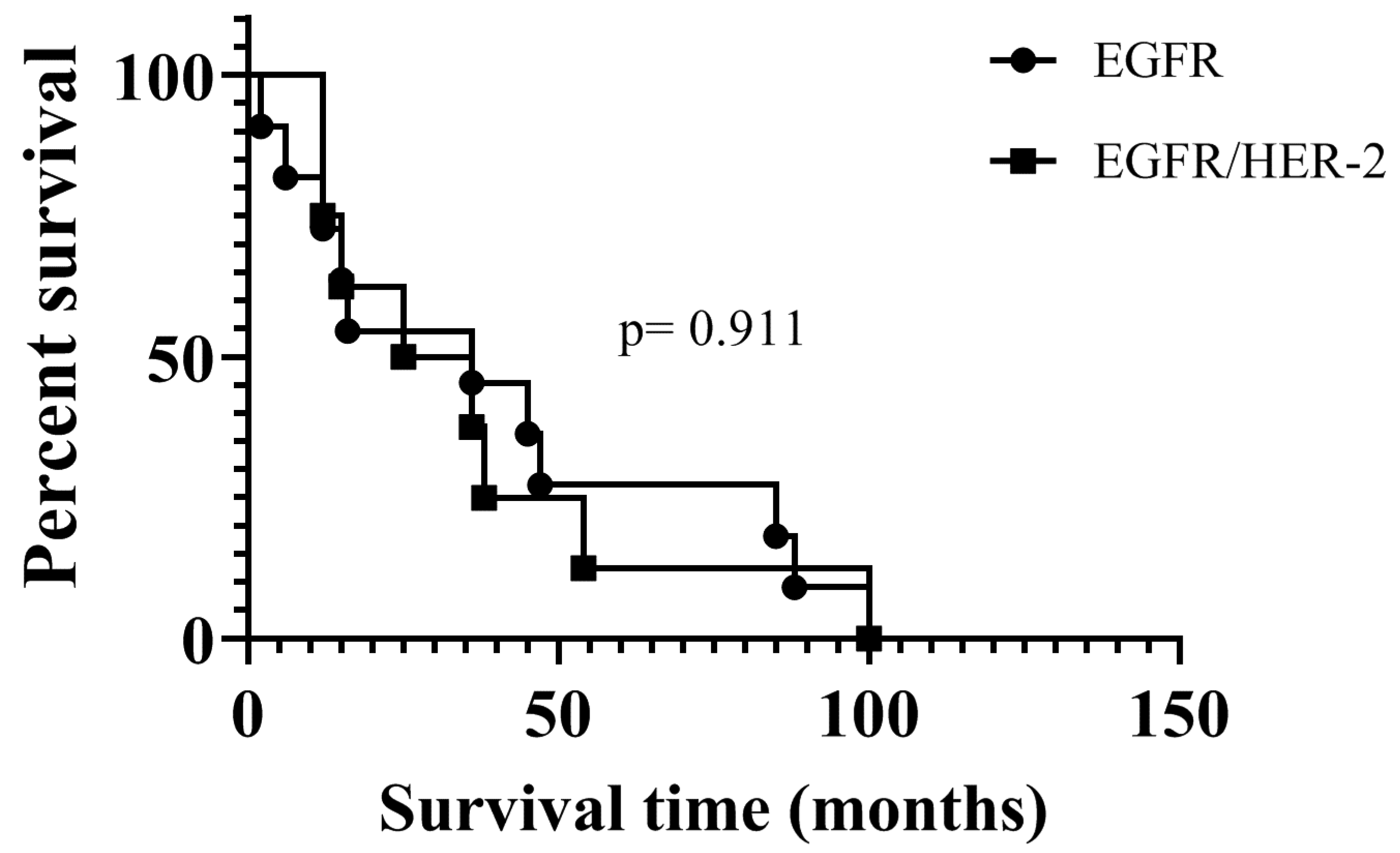
4.3. Sensitivity of EGFR and HER-2 Positive Cells to Gefitinib and Canertinib
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayantunde, A.A.; Parsons, S.L. Pattern and prognostic factors in patients with malignant ascites: A retrospective study. Ann. Oncol. 2007, 18, 945–949. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.W.; Chung, H.H.; Park, N.H.; Song, Y.S.; Kang, S.B. Disease confined within the ovary and smaller amount of ascites are good prognostic factors for survival of patients with squamous cell carcinoma arising from mature cystic teratoma of the ovary: A case series in Korea and review of the published reports. J. Obs. Gynaecol. Res. 2009, 35, 99–105. [Google Scholar] [CrossRef]
- Ferriss, J.S.; Java, J.J.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Walker, J.L.; Homesley, H.D.; Fowler, J.; Greer, B.E.; Boente, M.P.; et al. Ascites predicts treatment benefit of bevacizumab in front-line therapy of advanced epithelial ovarian, fallopian tube and peritoneal cancers: An NRG Oncology/GOG study. Gynecol. Oncol. 2015, 139, 17–22. [Google Scholar] [CrossRef]
- Kim, S.; Kim, B.; Song, Y.S. Ascites modulates cancer cell behavior, contributing to tumor heterogeneity in ovarian cancer. Cancer Sci. 2016, 107, 1173–1178. [Google Scholar] [CrossRef]
- Trachana, S.; Pilalis, E.; Gavalas, N.G.; Tzannis, K.; Papadodima, O.; Liontos, M.; Rodolakis, A.; Vlachos, G.; Thomakos, N.; Haidopoulos, D.; et al. The Development of an Angiogenic Protein “Signature” in Ovarian Cancer Ascites as a Tool for Biologic and Prognostic Profiling. PLoS ONE 2016, 11, e0156403. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Interleukin 6 Present in Inflammatory Ascites from Advanced Epithelial Ovarian Cancer Patients Promotes Tumor Necrosis Factor Receptor 2-Expressing Regulatory T Cells. Front Immunol. 2017, 8, 1482. [Google Scholar] [CrossRef]
- Worzfeld, T.; Strandmann, E.P.; Huber, M.; Adhikary, T.; Wagner, U.; Reinartz, S.; Müller, R. The Unique Molecular and Cellular Microenvironment of Ovarian Cancer. Front Oncol. 2017, 7, 24. [Google Scholar] [CrossRef]
- Burleson, K.M.; Boente, M.P.; Pambuccian, S.E.; Skubitz, A.P.N. Disaggregation and invasion of ovarian carcinoma ascites spheroids. J. Transl. Med. 2006, 4, 6. [Google Scholar] [CrossRef]
- Shepherd, T.G.; Thériault, B.L.; Campbell, E.J.; Nachtigal, M.W. Primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients. Nat. Protoc. 2006, 1, 2643–2649. [Google Scholar] [CrossRef]
- Alvero, A.B.; Chen, R.; Fu, H.; Montagna, M.; Schwartz, P.E.; Rutherford, T.; Silasi, D.; Steffensen, K.D.; Waldstrom, M.; Visintin, I.; et al. Molecular phenotyping of human ovarian cancer stem cells unravel the mechanisms for repair and chemo-resistance. Cell Cycle 2009, 8, 158–166. [Google Scholar] [CrossRef]
- Bapat, S.A.; Mali, A.M.; Koppikar, C.B.; Kurrey, N.K. Stem and Progenitor-Like Cells Contribute to the Aggressive Behavior of Human Epithelial Ovarian Cancer. Cancer Res. 2005, 65, 3025–3029. [Google Scholar] [CrossRef]
- Alper, O.; Bergmann-Leitner, E.S.; Bennett, T.A.; Hacker, N.F.; Stromberg, K.; Stetler-Stevenson, W.G. Epidermal growth factor receptor signaling and the invasive phenotype of ovarian carcinoma cells. J. Natl. Cancer Inst. 2001, 93, 1375–1384. [Google Scholar] [CrossRef]
- Berchuck, A.; Kamel, A.; Whitaker, R.; Kerns, B.; Olt, G.; Kinney, R.; Soper, J.T.; Dodge, R.; Clarke-Pearson, D.L.; Marks, P. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990, 50, 4087–4091. [Google Scholar]
- Steffensen, K.D.; Waldstrom, M.; Andersen, R.F.; Olsen, D.A.; Jeppesen, U.; Knudsen, H.J.; Brandslund, I.; Jakobsen, A. Protein levels and gene expressions of the epidermal growth factor receptors, HER1, HER2, HER3 and HER4 in benign and malignant ovarian tumors. Int. J. Oncol. 2008, 33, 195–204. [Google Scholar] [CrossRef]
- Miller, V.A. EGFR mutations and EGFR tyrosine kinase inhibitor in non-small cell lung cancer. Semin. Oncol. Nurs. 2008, 24, 27–33. [Google Scholar] [CrossRef]
- Scartozzi, M.; Bearzi, I.; Berardi, R.; Mandolesi, A.; Pierantoni, C.; Cascinu, S. Epidermal growth factor receptor (EGFR) downstream signalling pathway in primary colorectal tumours and related metastatic sites: Optimising EGFR-targeted treatment options. Br. J. Cancer 2007, 97, 92–97. [Google Scholar] [CrossRef][Green Version]
- Prenzel, N.; Zwick, E.; Leserer, M.; Ullrich, A. Tyrosine kinase signalling in breast cancer Epidermal growth factor receptor: Convergence point for signal integration and diversification. Breast Cancer Res. 2000, 2, 184–190. [Google Scholar] [CrossRef]
- Hassan, W.; Chitcholtan, K.; Sykes, P.H.; Garrill, A. A Combination of Two Receptor Tyrosine Kinase Inhibitors, Canertinib and PHA665752 Compromises Ovarian Cancer Cell Growth in 3D Cell Models. Oncol. Ther. 2016, 4, 257. [Google Scholar] [CrossRef]
- Hassan, W.; Chitcholtan, K.; Sykes, P.H.; Garrill, A. Ascitic fluid from advanced ovarian cancer patients compromises the activity of receptor tyrosine kinase inhibitors in 3D cell clusters of ovarian cancer cells. Cancer Lett. 2018, 420, 168–181. [Google Scholar] [CrossRef]
- Montero, J.C.; García-Alonso, S.; Ocaña, A.; Pandiella, A. Identification of therapeutic targets in ovarian cancer through active tyrosine kinase profiling. Oncotarget 2015, 6, 30057–30071. [Google Scholar] [CrossRef]
- Matsudaa, N.; Lima, B.; Wanga, X.; Uenoa, N.T. Early clinical development of epidermal growth factor receptor targeted therapy in breast cancer. Expert Opin. Investig. Drugs 2017, 26, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, C.; Lupia, M.; Tsafou, K.; Villa, A.; Kowalczyk, K.; Jersie-Christensen, R.R.; Bertalot, G.; Confalonieri, S.; Brunak, S.; Jensen, L.J.; et al. Phosphoproteomics of Primary Cells Reveals Druggable Kinase Signatures in Ovarian Cancer. Cell Rep. 2017, 18, 3242–3256. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Espina, V.; Steinberg, S.; Flørenes, V.A.; Liotta, L.A.; Kristensen, G.B.; GTropé, C.; Berner, A.; Kohn, E.C. Proteomic Analysis ofMalignant Ovarian Cancer Effusions as a Tool for Biologic and Prognostic Profiling. Clin. Cancer Res. 2006, 12, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.; Majem, B.; Devis, L.; Lapyckyj, L.; Besso, M.J.; Llaurado, M.; Abascal, M.F.; Matos, M.L.; Lanau, L.; Castellví, J.; et al. E-cadherin: A determinant molecule associated with ovarian cancer progression, dissemination and aggressiveness. PLoS ONE 2017, 12, e0184439. [Google Scholar] [CrossRef] [PubMed]
- Latifi, A.; Luwor, R.B.; Bilandzic, M.; Nazaretian, S.; Stenvers, K.; Pyman, J.; Zhu, H.; Thompson, E.W.; Quinn, M.A.; Findlay, J.K.; et al. Isolation and Characterization of Tumor Cells from the Ascites of Ovarian Cancer Patients: Molecular Phenotype of Chemoresistant Ovarian Tumors. PLoS ONE 2012, 7, e46858. [Google Scholar] [CrossRef]
- Cai, Q.; Yan, L.; Xu, Y. Anoikis resistance is a critical feature of highly aggressive ovarian cancer cells. Oncogene 2015, 34, 3315–3324. [Google Scholar] [CrossRef]
- Ho, C.; Chang, S.; Hsiao, C.; Chien, T.; Shih, D.T. Isolation and characterization of stromal progenitor cells from ascites of patients with epithelial ovarian adenocarcinoma. J. Biomed. Sci. 2012, 19, 23. [Google Scholar] [CrossRef]
- Ó Donnell, R.L.; McCormick, A.; Mukhopadhyay, A.; Woodhouse, L.C.; Moat, M.; Grundy, A.; Dixon, M.; Kaufman, A.; Soohoo, S.; Elattar, A.; et al. The Use of Ovarian Cancer Cells from Patients Undergoing Surgery to Generate Primary Cultures Capable of Undergoing Functional Analysis. PLoS ONE 2014, 9, e90604. [Google Scholar]
- Strauss, R.; Li, Z.; Liu, Y.; Beyer, I.; Persson, J.; Sova, P.; Möller, T.; Pesonen, S.; Hemminki, A.; Hamerlik, P.; et al. Analysis of Epithelial and Mesenchymal Markers in Ovarian Cancer Reveals Phenotypic Heterogeneity and Plasticity. PLoS ONE 2011, 6, e16186. [Google Scholar] [CrossRef]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int. J. Cancer 2009, 124, 2060–2070. [Google Scholar] [CrossRef]
- Suster, N.K.; Smrkolj, S.; Virant-Klun, I. Putative stem cells and epithelialmesenchymal transition revealed in sections of ovarian tumor in patients with serous ovarian carcinoma using immunohistochemistry for vimentin and pluripotency-related markers. J. Ovarian Res. 2017, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Graeff, P.; Crijns, A.P.G.; Hoor, K.A.; Klip, H.G.; Hollema, H.; Oien, K.; Bartlett, J.M.; Wisman, G.B.A.; Bock, G.H.; Vries, E.G.E.; et al. The ErbB signalling pathway: Protein expression and prognostic value in epithelial ovarian cancer. Br. J. Cancer 2008, 99, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Lanitis, E.; Dangaj, D.; Hagemann, I.S.; Song, D.-G.; Best, A.; Sandaltzopoulos, R.; Coukos, G.; Powell, D.J. Primary Human Ovarian Epithelial Cancer Cells Broadly Express HER2 at Immunologically-Detectable Levels. PLoS ONE 2012, 7, e49829. [Google Scholar] [CrossRef] [PubMed]
- Stadlmann, S.; Gueth, U.; Reiser, U.; Diener, P.; Zeimet, A.G.; Wight, E.; Mirlacher, M.; Sauter, G.; Mihatsch, M.J.; Singer, G. Epithelial growth factor receptor status in primary and recurrent ovarian cancer. Mod. Pathol. 2006, 19, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, J.; Teugels, E.; Bourgain, C.; Xiangming, J.; in’t Veld, P.; Ghislain, V.; Neyns, B.; De Greve, J. Genomic activation of the EGFR and HER2-neu genes in a significant proportion of invasive epithelial ovarian cancers. BMC Cancer 2008, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Tuefferd, M.; Couturier, J.; Penault-Llorca, F.; Vincent-Salomon, A.; Broë, P.; Guastalla, J.; Allouache, D.; Combe, M.; Weber, B.; Pujade-Lauraine, E.; et al. HER2 Status in Ovarian Carcinomas: A Multicenter GINECO Study of 320 Patients. PLoS ONE 2007, 2, e1138. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, J.; Zhang, L.; Wu, D.; Yu, D.; Tian, X.; Liu, J.; Jiang, X.; Shen, Y.; Zhang, L.; et al. Expressions of fatty acid synthase and HER2 are correlated with poor prognosis of ovarian cance. Med. Oncol. 2015, 32, 391. [Google Scholar] [CrossRef]
- Siegel-Lakhai, W.S.; Beijnen, J.H.; Schellensa, J.H.M. Current Knowledge and Future Directions of the Selective Epidermal Growth Factor Receptor Inhibitors Erlotinib (Tarceva®) and Gefitinib (Iressa®). Oncologist 2005, 10, 579–589. [Google Scholar] [CrossRef]
- Leslie, K.K.; Sill, M.W.; Fischer, E.; Darcy, K.M.; Mannel, R.S.; Tewari, K.S.; Hanjani, P.; Wilken, J.A.; Baron, A.T.; Godwin, A.K.; et al. A Phase II Evaluation of Gefitinib in the Treatment of Persistent or Recurrent Endometrial Cancer: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2013, 129, 486–494. [Google Scholar] [CrossRef]
- Posadas, E.M.; Liel, M.S.; Kwitkowski, V.; Minasian, L.; Godwin, A.K.; Hussain, M.M.; Espina, V.; Wood, B.J.; Steinberg, S.M.; Kohn, E.C. A phase II and pharmacodynamic study of gefitinib in patients with refractory or recurrent epithelial ovarian cancer. Cancer 2007, 109, 1323–1330. [Google Scholar] [CrossRef]
- Argiris, A.; Ghebremichael, M.; Gilbert, J.; Lee, J.; Sachidanandam, K.; Kolesar, J.M.; Burtness, B.; Forastiere, A.A. Phase III Randomized, Placebo-Controlled Trial of Docetaxel With or Without Gefitinib in Recurrent or Metastatic Head and Neck Cancer: An Eastern Cooperative Oncology Group Trial. J. Clin. Oncol. 2013, 31, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kim, K.B.; Papadopoulos, N.E.; Hwu, W.; Hwu, P.; Prieto, V.G.; Bar-Eli, M.; Zigler, M.; Dobroff, A.; Bronstein, Y.; et al. A Phase II Study of Gefitinib in Patients with Metastatic Melanoma. Melanoma Res. 2011, 21, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.N.; Zweidler-McKay, P.A.; Roy, N.V.; Speleman, F.; Treviño, J.; Zage, P.E.; Hughes, D.P.M. Signaling of ERBB Receptor Tyrosine Kinases Promotes Neuroblastoma Growth in vitro and in vivo. Cancer 2010, 116, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.E.; Nelson, L.D.; Santiago-O’Farrill, J.M.; Knouse, P.D.; Miller, C.P.; Palla, S.L.; Siwak, D.R.; Mills, G.B.; Estrov, Z.; Li, S.; et al. Small Molecule ErbB Inhibitors Decrease Proliferative Signaling and Promote Apoptosis in Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia. PLoS ONE 2013, 8, e70608. [Google Scholar] [CrossRef]
- Dilworth, J.T.; Wojtkowiak, J.W.; Mathieu, P.; Tainsky, M.A.; Reiners, J.J., Jr.; Mattingly, R.R.; Hancock, C.N. Suppression of proliferation of two independent NF1 malignant peripheral nerve sheath tumor cell lines by the pan-ErbB inhibitor CI-1033. Can Biol. Ther. 2008, 7, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ambrogio, L.; Shimamura, T.; Kubo5, S.; Takahashi, M.; Chirieac, L.R.; Padera, R.F.; Shapiro, G.I.; Baum, A.; Himmelsbach, F.; et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008, 27, 4702–4711. [Google Scholar] [CrossRef]
- Calvo, E.; Tolcher, A.W.; Hammond, L.A.; Patnaik, A.; de Bono, J.S.; Eiseman, I.A.; Olson, S.C.; Lenehan, P.F.; McCreery, H.; LoRusso, P.; et al. Administration of CI-1033, an Irreversible Pan-erbB Tyrosine Kinase Inhibitor, Is Feasible on a 7-Day On, 7-Day Off Schedule: A Phase I Pharmacokinetic and Food Effect Study. Clin. Cancer Res. 2004, 10, 7112–7120. [Google Scholar] [CrossRef]
- Zinner, R.G.; Nemunaitis, J.; Eiseman, I.; Shin, H.C.; Olson, S.C.; Christensen, J.; Huang, X.; Lenehan, P.F.; Donato, N.J.; Shin, D.M. Phase I Clinical and Pharmacodynamic Evaluation of Oral CI-1033 in Patientswith Refractory Cancer. Clin. Cancer Res. 2007, 13, 3006–3014. [Google Scholar] [CrossRef][Green Version]
- Campos, S.; Hamid, O.; Seiden, M.V.; Oza, A.; Plante, M.; Potkul, R.K.; Lenehan, P.F.; Kaldjian, E.P.; Varterasian, M.L.; Jordan, C.; et al. Multicenter, Randomized Phase II Trial of Oral CI-1033 for Previously Treated Advanced Ovarian Cancer. J. Clin. Oncol. 2005, 23, 5597–5604. [Google Scholar] [CrossRef]
- Correa, R.J.M.; Valdes, Y.R.; Peart, T.M.; Fazio, E.N.; Bertrand, M.; McGee, J.; Préfontaine, M.; Sugimoto, A.; DiMattia, G.E.; Shepherd, T.G. Combination of AKT inhibition with autophagy blockade effectively reduces ascites-derived ovarian cancer cell viability. Carcinogenesis 2014, 35, 1951–1961. [Google Scholar] [CrossRef]
- Despierre, E.; Vergote, I.; Anderson, R.; Coens, C.; Katsaros, D.; Hirsch, F.R.; Boeckx, B.; Varella-Garcia, M.; Ferrero, A.; Ray-Coquard, I.; et al. Epidermal Growth Factor Receptor (EGFR) Pathway Biomarkers in the Randomized Phase III Trial of Erlotinib Versus Observation in Ovarian Cancer Patients with No Evidence of Disease Progression after First-Line Platinum-Based Chemotherapy. Target. Oncol. 2015, 10, 583–596. [Google Scholar] [CrossRef] [PubMed]

| Patient ID | Ages | Subtypes | Stages | Treatment |
|---|---|---|---|---|
| 1 | 59 | HGS | IIIC | Car/Pac |
| 2 | 74 | HGS | IIC | N-C |
| 3 | 70 | HGS | IIIC | Car/Pac |
| 4 | 67 | HGS | IV | Car/Pac/Gem |
| 5 | 74 | HGS | IIIC | N-C |
| 6 | 73 | HGS | IIIC | N-C |
| 7 | 50 | HGS | IV | N-C |
| 8 | 75 | LGS | IIC | N-C |
| 9 | 61 | HGS | IIIC | N-C |
| 10 | 58 | HGS | IIIA | N-C |
| 11 | 74 | HGS | IIC | Car/Pac/Gem |
| 12 | 50 | HGS | IIIC | N-C |
| 13 | 81 | HGS | IIIC | Car/Pac/Gem |
| 14 | 59 | HGS | IIIC | Car/Pac |
| 15 | 63 | HGS | IIIC | Car/Pac |
| 16 | 53 | HGS | IIIC | N-C |
| 17 | 56 | HGS | IIIC | Car/Taxol |
| 18 | 59 | HGS | IC | N-C |
| 19 | 67 | HGS | IIIC | N-C |
| 20 | 67 | HGS | IIIC | N-C |
| Characteristic | Number | (%) |
|---|---|---|
| Ovarian cancer | 20 | |
| Patients | ||
| Cell morphology | ||
| Cell monolayer | ||
| Compact colony | 4 | 20 |
| Loose colony | 16 | 80 |
| Floating condition | ||
| Compact aggregates | 12 | 60 |
| Small cluster | 7 | 35 |
| Single cells | 1 | 5 |
| Protein expression | ||
| CA125 | 20 | 100 |
| Cytokeratin-18 | 19 | 95 |
| E-cadherin | 5 | 25 |
| N-cadherin | 20 | 100 |
| Vimentin | 19 | 95 |
| High EGFR only | 9 | 45 |
| High HER-2 only | 0 | |
| High EGFR/HER-2 | 8 | 40 |
| Low EGFR/HER-2 | 3 | 15 |
| (A) (+) EGFR | |||
|---|---|---|---|
| Patient ID | Control | Cellular Viability (Relative to Control %) | |
| 5 µM Gef | 5 µM Can | ||
| OVCAR-5 | 100 | 99.9 ± 1.3 | 102 ± 1.2 |
| 1 | 100 | 74.6 ± 2.7 * | 87.8 ± 17.6 |
| 3 | 100 | 98.9 ± 6.7 | 104.5 ± 4.6 |
| 4 | 100 | 84.6 ± 0.8 * | 74.9 ± 4.3 *# |
| 6 | 100 | 95.5 ± 3.1 | 91.7 ± 1.9 * |
| 8 | 100 | 93.6 ± 3.1 | 92.5 ± 3.9 |
| 10 | 100 | 102.8 ± 5.5 | 95.4 ± 2.7 |
| 17 | 100 | 86.3 ± 1.1 * | 89.8 ± 1.3 * |
| 19 | 100 | 86.9 ± 2.0 * | 78.4 ± 4.1 * |
| 20 | 100 | 102. 8± 5.5 | 95.4 ± 2.7 |
| (B) (+) EGFR/HER-2 | |||
| Patient ID | Control | Cellular Viability (Relative to Control %) | |
| 5 µM Gef | 5 µM Can | ||
| SKOV-3 | 100 | 95.2 ± 2.7 | 82.9 ± 2.8 * |
| 7 | 100 | 105.1 ± 2.1 | 117.1 ± 4.5 |
| 9 | 100 | 91.7 ± 30 | 81.4 ± 33.3 * |
| 11 | 100 | 87.8 ± 6.5 | 82.8 ± 7.4 * |
| 12 | 100 | 86.5 ± 6.8 | 90.3 ± 4.6 |
| 13 | 100 | 94.3 ± 4.4 | 92.7 ± 4.3 |
| 15 | 100 | 95.4 ± 3.1 | 93.9 ± 2.1 |
| 16 | 100 | 95.6 ± 6.2 | 88.2 ± 1.9 * |
| 18 | 100 | 99.8 ± 4.9 | 102.7 ± 2.5 |
| (C) (-) EGFR/HER-2 | |||
| Patient ID | Control | Cellular Viability (Relative to Control %) | |
| 5 µM Gef | 5 µM Can | ||
| 2 | 100 | 68.3 ± 0.11 * | 65.3 ± 11.2 * |
| 5 | 100 | 95.3 ± 058 | 97.6 ± 0.78 |
| 14 | 100 | 96.45 ± 1.4 | 96.56 ± 0.12 |
| (A) (+) EGFR | |||||
|---|---|---|---|---|---|
| Patient ID | Control | EGFR (% Relative Expression to Control) | pEGFR (% Relative Expression to Control) | ||
| 5 µM Gef | 5 µM Can | 5 µM Gef | 5 µM Can | ||
| 1 | 100 | 65.93 ± 6.6 * | 72.59 ± 1.5 * | 76.04 ± 3.2 * | 67.36 ± 3.0 * |
| 3 | 100 | 86.51 ± 4.3 | 58.8 ± 10.4 * | 82.31 ± 4.37 * | 68.25 ± 3.6 * |
| 4 | 100 | 47.82 ± 4.3 * | 32.85 ± 3.9 * | 83.97 ± 6.2 | 54.52 ± 2.8 * |
| 6 | 100 | 71.55 ± 3.9 * | 60.25 ± 2.4 * | 69.27 ± 6.8 * | 85.94 ± 4.2 |
| 8 | 100 | 144.8 ± 5.9 | 108.3 ± 6.9 | 98.97 ± 2.8 | 84.83 ± 2.8 |
| 10 | 100 | 57.64 ± 2.5 * | 97.92 ± 4.6 | 61.19 ± 5.9 * | 131.43 ± 4.3 |
| 17 | 100 | 83.99 ± 18.3 | 124.1 ± 5.3 | 66.37 ± 2.7 * | 68.51 ± 2.1 * |
| 19 | 100 | 122.3 ± 6.9 | 142.2 ± 5.5 | 99.18 ± 2.5 | 111 ± 6.7 |
| 20 | 100 | 72.68 ± 9.8 * | 79.63 ± 4.1 * | 73.53 ± 2.5 * | 75.8 ± 3.7 * |
| (B) (+) EGFR/HER-2 | |||||
| Patient ID | Control | EGFR (% Relative Expression to Control) | pEGFR (% Relative Expression to Control) | ||
| 5 µM Gef | 5 µM Can | 5 µM Gef | 5 µM Can | ||
| 7 | 100 | 141.2 ± 10.5 | 132.4 ± 8.14 | 145.1 ± 13.7 | 74.63 ± 5.9 * |
| 9 | 100 | 62.4 ± 10.87 * | 52.2 ± 6.58 * | 69.6 ± 2.1 * | 72.4 ± 12.2 * |
| 11 | 100 | 87.3 ± 5.5 | 66.9 ± 3.8 * | 86.3 ± 9.1 | 77.7 ± 2.0 * |
| 12 | 100 | 75.3 ± 5.4 | 62.4 ± 2.2 * | 126.4 ± 6.3 | 116.1 ± 5.5 |
| 13 | 100 | 66.5 ± 5.4 * | 67.3 ± 4.6 * | 93.6 ± 3.5 | 92.1 ± 3.6 |
| 15 | 100 | 83.4 ± 8.6 | 93.3 ± 5.6 | 90.7 ± 2.6 | 78.4 ± 2.7 |
| 16 | 100 | 96.1 ± 7.1 | 119.8 ± 9.5 | 84.3 ± 1.7 | 91 ± 4.4 |
| 18 | 100 | 74.3 ± 8.1 | 66.0 ± 6.9 * | 91.5 ± 2.4 | 95.9 ± 4.6 |
| (C) (+) EGFR/HER-2 | |||||
| Patient ID | Control | Her-2 (% Relative Expression to Control) | pHer-2 (% Relative Expression to Control) | ||
| 5 µM Gef | 5 µM Can | 5 µM Gef | 5 µM Can | ||
| 7 | 100 | 100.3 ± 11.4 | 123.4 ± 9.5 | 78.7 ± 3.7 | 95.0 ± 8.3 |
| 9 | 100 | 106.2 ± 6.12 | 64.6 ± 1.3 * | 110 ± 6.11 | 62.5 ± 6.1 * |
| 11 | 100 | 115.7 ± 7.2 | 81.9 ± 7.1 | 87.3 ± 5.7 | 92.3 ± 6.3 |
| 12 | 100 | 128.7 ± 6.3 | 128.8 ± 2.3 | 117.6 ± 6.7 | 107.8 ± 3 |
| 13 | 100 | 78.9 ± 5.2 | 99 ± 8.4 | 94.7 ± 7.4 | 114.6 ± 4.4 |
| 15 | 100 | 86.2 ± 5.6 | 62.8 ± 3.1 * | 104.1 ± 7.6 | 77.6 ± 2.5 * |
| 16 | 100 | 78.5 ± 2.3 | 103.8 ± 3.5 | 100.8 ± 7.6 | 109.4 ± 5.2 |
| 18 | 100 | 72.22 ± 9.4 | 43.7 ± 6.3 * | 115.8 ± 3.7 | 100.8 ± 7.2 |
| (A) EGFR positive cells | |||||
|---|---|---|---|---|---|
| Patient ID | Control | PCNA (% Relative Expression to Control) | Cleaved Caspase-3 (% Relative Expression to Control) | ||
| 5 µM Gef | 5 µM Can | 5 µM Gef | 5 µM Can | ||
| 3 | 100 | 81.8 ± 5.1 * | 90.7 ± 3.3 | 73.7 ± 3.6 | 88.0 ± 6.2 |
| 4 | 100 | 100 ± 7.2 | 88.5 ± 5.2 | 96.3 ± 11.7 | 81.4 ± 7.7 |
| 6 | 100 | 93.4 ± 4.1 | 87.7 ± 2.3 | 91.2 ± 6.3 | 88.9 ± 3.2 |
| 8 | 100 | 108.9 ± 6.2 | 121.4 ± 3.2 | 90.0 ± 3.7 | 100 ± 2.1 |
| 10 | 100 | 93.9 ± 3.12 | 99.2 ± 3.6 | 300 ± 32 * | 357.7 ± 37.2 * |
| 17 | 100 | 84.6 ± 3.1 | 90.5 ± 3.8 | 83.7 ± 2.8 | 76.4 ± 2.7 |
| 19 | 100 | 119.5 ± 3.8 | 62.8 ± 4.8 * | 130.4 ± 27 | 54.6 ± 4.5 |
| 20 | 100 | 63.6 ± 3.0 * | 56.7 ± 4.3 * | 165.7 ± 11.9 * | 128.9 ± 14.2 |
| (B) | |||||
| Patient ID | Control | pErk (% Relative Expression to Control) | pAkt (% Relative Expression to Control) | ||
| 5 µM Gef | 5 µM Can | 5 µM Gef | 5 µM Can | ||
| 3 | 100 | 86.9 ± 4 * | 116.4 ± 5.3 | 101.6 ± 4.3 | 111.2 ± 3.5 |
| 4 | 100 | 65.2 ± 2.2 * | 67.4 ± 3.7 * | 46.2 ± 5.0 * | 50.6 ± 6.4 * |
| 6 | 100 | 95.7 ± 4.6 | 77.8 ± 2.6 * | 88.4 ± 2.6 * | 90.1 ± 5.3 |
| 8 | 100 | 120.6 ± 3.4 | 79.7 ± 3.4 * | 95.6 ± 2.3 | 73.2 ± 3.8 * |
| 10 | 100 | 96.6 ± 3.7 | 104.7 ± 2.3 | 70.9 ± 7.8 * | 92.0 ± 3.8 |
| 17 | 100 | 96.4 ± 3.0 | 94.3 ± 5.6 | 81 ± 3.8 | 103.4 ± 7.2 |
| 19 | 100 | 65.2 ± 1.5 * | 58.6 ± 1.3 * | 82.1 ± 2.1 * | 76.9 ± 2.4 * |
| 20 | 100 | 62.1 ± 3.2 * | 49.4 ± 2.9* | 82.5 ± 2.6 * | 68.9 ± 3.5 * |
| (A) EGFR/HER-2 positive cells | |||||
|---|---|---|---|---|---|
| Patient ID | Control | PCNA (% Relative Expression to Control) | Cleaved Caspase-3 (% Relative Expression to Control) | ||
| 5 µM Gef | 5 µM Can | 5 µM Gef | 5 µM Can | ||
| 7 | 100 | 117.7 ± 5 | 187.9 ± 20.2 | 346.9 ± 37.4 * | 426.3 ± 17.8 * |
| 9 | 100 | 63.2 ± 4.2 * | 77.6 ± 11.6 | 98.5 ± 16.7 | 121.5 ± 17.8 |
| 11 | 100 | 84.2 ± 8.4 | 87.1 ± 4.8 | 86 ± 8.4 | 75.1 ± 3.6 |
| 12 | 100 | 116.3 ± 4.37 | 123.7 ± 6.23 | 89.1 ± 2.67 | 79.7 ± 2.66 |
| 13 | 100 | 137 ± 5.2 | 101 ± 4.1 | 113.3 ± 3.2 * | 117.6 ± 9.4 |
| 15 | 100 | 92.8 ± 2.7 | 120.6 ± 6.6 | 97.6 ± 5.2 | 92.6 ± 2.8 |
| 16 | 100 | 83.7 ± 6.9 | 87.9 ± 4.5 | 76.8 ± 3.2 | 92.3 ± 2.9 |
| 18 | 100 | 113.2 ± 10.4 | 99.5 ± 4.9 | 94.3 ± 4.9 | 93 ± 4.4 |
| (B) | |||||
| Patient ID | Control | pErk (% Relative Expression to Control) | pAkt (% Relative Expression to Control) | ||
| 5 µM Gef | 5 µM Can | 5 µM Gef | 5 µM Can | ||
| 7 | 100 | 99.4 ± 3.6 | 70.5 ± 4.9 * | 104 ± 4.1 | 75.3 ± 4.8 * |
| 9 | 100 | 138.5 ± 10.9 | 65.43 ± 3.4 * | 120.4 ± 12.95 | 69.5 ± 4.3 * |
| 11 | 100 | 93.2 ± 3.9 | 84.3 ± 2.9 * | 96.4 ± 7.1 | 83.6 ± 3.1 * |
| 12 | 100 | 95.1 ± 5.3 | 88.9 ± 5.4 | 119.3 ± 12.8 | 87.8 ± 4 |
| 13 | 100 | 89.8 ± 5.3 | 57.4 ± 1.3 * | 76.9 ± 3.1 * | 55.4 ± 1.9 * |
| 15 | 100 | 120.4 ± 3.9 | 104.4 ± 3.7 | 95.3 ± 3.6 | 92.6 ± 2.7 |
| 16 | 100 | 78.3 ± 4.7 * | 80.1 ± 2.3 * | 106 ± 4.5 | 96.3 ± 3.6 |
| 18 | 100 | 196.5 ± 9.7 | 157.5 ± 17.2 | 127.2 ± 2.5 | 115.8 ± 7.5 |
| (A) (+) EGFR | |||
|---|---|---|---|
| Inhibitors | Response | No Response | |
| Gefitinib | 24 | 26 | |
| Canertinib | 24 | 26 | |
| B (+) EGFR/HER-2 | |||
| Inhibitors | Response | No Response | |
| Gefitinib | 6 | 58 | |
| Canertinib | 23 | 41 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitcholtan, K.; Harker, D.; Simcock, B.; Sykes, P. Sensitivity of EGFR/HER-2 Positive Cells Isolated from Ascitic Fluid of Advanced Ovarian Cancer Patients to EGFR/HER-2 Inhibitors. Appl. Sci. 2020, 10, 2343. https://doi.org/10.3390/app10072343
Chitcholtan K, Harker D, Simcock B, Sykes P. Sensitivity of EGFR/HER-2 Positive Cells Isolated from Ascitic Fluid of Advanced Ovarian Cancer Patients to EGFR/HER-2 Inhibitors. Applied Sciences. 2020; 10(7):2343. https://doi.org/10.3390/app10072343
Chicago/Turabian StyleChitcholtan, Kenny, Dianne Harker, Bryony Simcock, and Peter Sykes. 2020. "Sensitivity of EGFR/HER-2 Positive Cells Isolated from Ascitic Fluid of Advanced Ovarian Cancer Patients to EGFR/HER-2 Inhibitors" Applied Sciences 10, no. 7: 2343. https://doi.org/10.3390/app10072343
APA StyleChitcholtan, K., Harker, D., Simcock, B., & Sykes, P. (2020). Sensitivity of EGFR/HER-2 Positive Cells Isolated from Ascitic Fluid of Advanced Ovarian Cancer Patients to EGFR/HER-2 Inhibitors. Applied Sciences, 10(7), 2343. https://doi.org/10.3390/app10072343





