Future Applications
(1) Matching Lung Ultrasonography (LUS) patterns and high-resolution chest CT scan (HRCT) grades of peripheral fibrotic alterations in order to create an “acoustic map”. (2) Follow up fibrotic peripheral changes and optimize the timing of subsequent chest CT scans. (3) Differentiate ultrasonographic features in case of GGOs and large honeycomb cysts. (4) Characterization of pleural line in order to better assess specific alteration of peripheral airspace geometry of the lungs resulting in specific acoustic traps. Preliminary data of this work have been presented as a poster to the ERS congress, Madrid 2019.
Abstract
Lung ultrasonography (LUS) provides an estimation of peripheral airspace (PAS) geometry of the lung. Altered PAS produces sonographic interstitial syndrome (SIS). Idiopathic pulmonary fibrosis (IPF) involves peripheral lung with altered PAS. The aim of the study is to correlate echographic patterns with peripheral fibrotic changes on high-resolution Chest CT scan (HRCT). Patients underwent LUS and HRCT on the same date. Four LUS patterns were described: (1) near normal; (2) SIS with predominance of reverberant artifacts; (3) SIS with vertical predominance; (4) white lung. Four HRCT grades of peripheral fibrotic infiltrates were reported: grade 1 mild; grade 2 moderate; grade 3 severe; grade 4 massive or honeycomb. LUS pattern 1 was indicative of mild to moderate fibrotic alterations in 100% of cases. LUS pattern 2 matched with HRCT grade 2 in 24 out of 30 cases (77%). Huge discordance in four cases because of large honeycomb cysts. LUS pattern 3 was indicative of severe to massive alterations in 100% of cases. LUS pattern 4 showed a heterogeneous distribution of HRCT grades, severe changes, and ground glass opacities (GGO). This preliminary work demonstrates some level of agreement between LUS patterns and HRCT grades. Limitations and methodological issues have been shown to support subsequent studies of agreement.
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common and severe form of idiopathic interstitial pneumonia with an extremely poor prognosis. It is defined as a specific form of chronic, progressive, irreversible fibrosing interstitial pneumonia of unknown cause with an unpredictable and variable clinical course [1,2,3].
Early diagnosis of IPF is crucial for predicting prognosis and optimizing management, including the initiation of therapies [4]. For instance, an ever-growing interest for early diagnostic approaches is developing in order to get the correct diagnosis in a very early stage. To hone and improve classic semiotic maneuvers with the aid of modern technologies could be the answer. The earlier is the suspected diagnosis, the earlier IPF patients can be followed with the most correct management process.
As far as chest auscultation is concerned, velcro crackles have been considered the key for early diagnosis of IPF [5]. Velcro crackles can be distinguished in terms of frequency and coefficient of transmission from crackles generated by both flogistic and cardiogenic causes [6].
Quantitative digital analysis of lung sounds could be useful for early diagnosis and to intercept the progression of the disease [7,8].
As far as chest percussion is concerned, the natural evolution can be considered chest ultrasonography. Similarities with medical percussion are evident. Ultrasound technology in medicine uses acoustic energy to assess internal body parts. Physicians interpret interactions between ultrasonic waves and body tissues to get signs leading to diagnostic hypothesis [9].
The interactions of ultrasound beams with peripheral lung in a pre-consolidated state, which exhibits altered peripheral airspace geometry, results in the so-called “sonographic interstitial syndrome” (SIS) [10,11,12].
Some features of SIS have been reported that might help physicians to differentiate cardiogenic from pneumogenic causes [11,13].
In case of features suspected for diffuse pneumogenic SIS with predominance in basal regions, and respiratory symptoms with subacute-chronic onset, interstitial lung diseases (ILDs) evolving in pulmonary fibrosis is the most likely diagnosis.
Actually, some fibrotic interstitial lung diseases, like idiopathic pulmonary fibrosis, involve peripheral lung with dramatic structural changes and altered peripheral airspace geometry [14].
Alterations of the pleural line, which can appear irregular, cobbled and coarse, have been reported in literature for pulmonary diseases with evolution in peripheral fibrosis [15,16,17].
As for what concerns an ultrasonographic assessment of pulmonary fibrosis, it could be possible to distinguish areas with only light alterations of the pleural line from areas of more severe peripheral airspace distribution alteration and fibrosis.
The aim of this pilot study is to correlate echographic patterns with peripheral parenchymal fibrotic changes evaluated at high resolution computed tomography of the chest (HRCT), in order to create a kind of “acoustic map” of fibrotic peripheral alterations. This study was conducted in patients affected by idiopathic pulmonary fibrosis. A secondary end-point is to highlight and discuss cases of discordance between sonography and HRCT, in order to draw subsequent studies with the aim to better understand the phenomena at stake, and potentially to improve the use of LUS. Finally, this study can be useful to discuss the limitations and methodological issues that can influence the comparison of CT scans with echographic findings, with the aim to reduce and limit biases in subsequent studies.
2. Materials and Methods
This was an observational pilot study ruling in patients with a multidisciplinary diagnosis of IPF from June 2018 to December 2018. All the subjects included in this study had been already discussed and diagnosed as “Idiopathic pulmonary Fibrosis” (IPF) by a multidisciplinary team. Written informed consent was obtained for each patient. Fourteen patients affected by IPF (9 Males), who underwent routine HRCT, have been enrolled. On the same date, patients underwent lung ultrasonography (LUS) prior to HRCT. Other fibrosing ILDs have been excluded.
2.1. Ultrasonography
Patients were assessed in supine position with the arms extended above the head. The position is the same in which the Chest CT scan was performed. Chest ultrasonography was performed immediately before the Chest CT scan. Ultrasonographic assessment was performed using a convex probe 1–8 MHz. An Esaote™ (Genoa, Italy) MyLabAlpha® echographic machine was used.
Six echographic scans were performed, three for each hemithorax:
- (1)
- Basal landmark: Mid-Posterior axillary line, basal-lateral area;
- (2)
- Anterior landmark: Anterior axillary line, near nipple line;
- (3)
- Apical landmark: Hemiclavear line, area below clavicle.
These evaluations have been performed in order to obtain 6 echographic scans for each patient. Ten seconds Videos were recorded and stored in each landmark.
Standardized parameters have been used: an imaging depth of 10 cm (for convex probe), power 40% (MI: 0.9–1), gain 40%, focus on the pleural line, no harmonic imaging, no cosmetic filters enabled. The imaging center-frequency was 5 MHz.
Metal cutaneous landmarks have been positioned and left during the CT scans, indicating the areas of ultrasonographic assessment. This method supports a more accurate comparison between echographic patterns and CT scans peripheral lung findings.
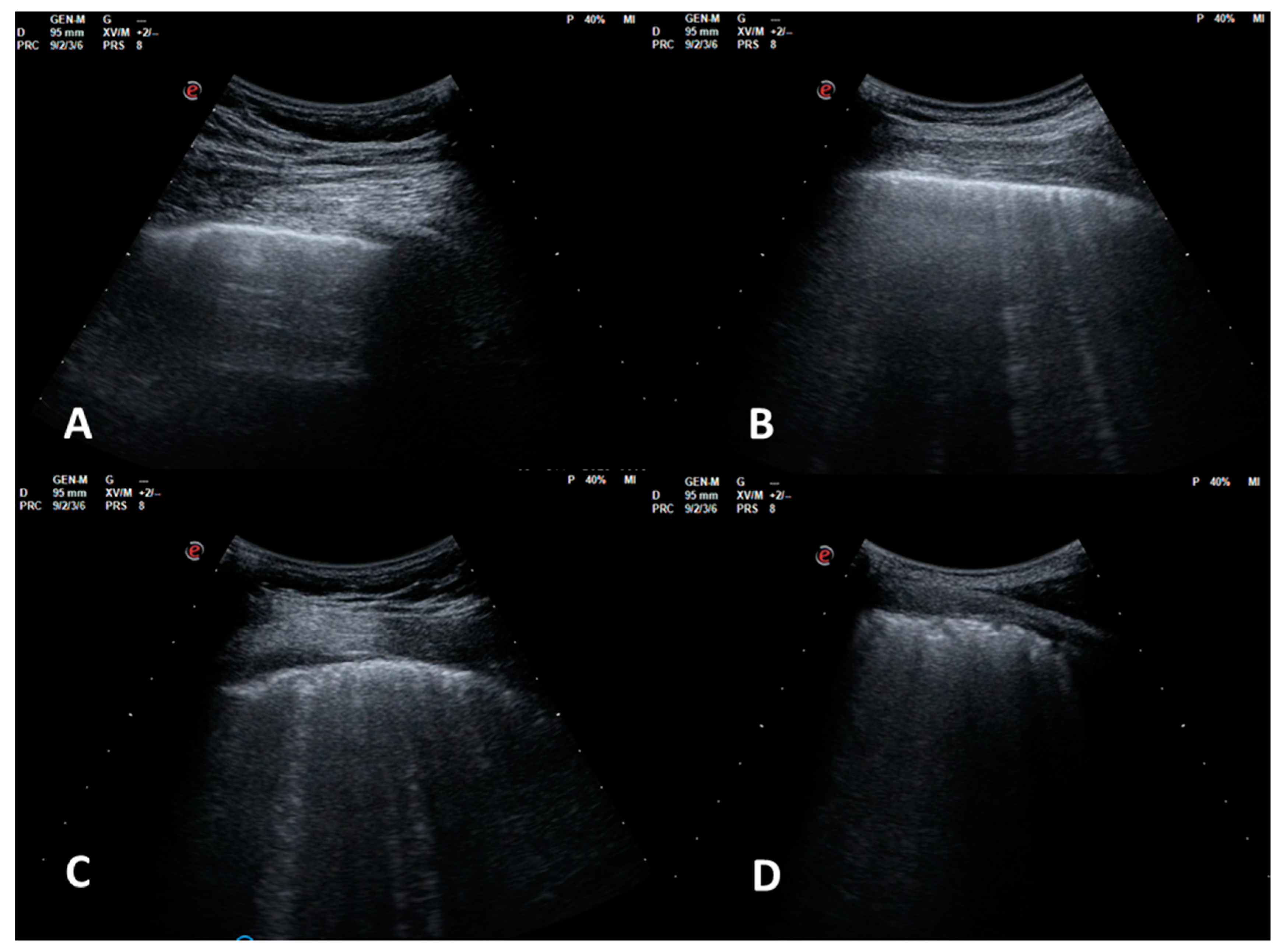
Four echographic patterns were described corresponding to worsening fibrotic changes of the peripheral lung: (1) normal or near normal; (2) SIS with predominance of reverberant horizontal artifacts; (3) SIS with vertical predominance and altered pleural line; (4) white lung with altered pleural line (Figure 1).
Figure 1.
LUS patterns. (A): pattern 1, near normal; (B): pattern 2, SIS with predominance of reverberant artifacts; (C): pattern 3, SIS with vertical predominance and altered pleural line; (D): pattern 4, white lung with altered pleural line.
Throughout a review of the evaluation process, the echographic pattern assigned to each video (each ten seconds long) was the worst observed. This video evaluation process has been performed blind to HRCT reports, and contemporaneously by 2 pneumologists (AS and RI) with expertise in lung ultrasonography. A concordant score has been provided.
2.2. Chest HRCT Scans
The HRCT scans of the chest were performed, for every patient, on the same day of the ultrasonographic assessment.
The HRCT scans were obtained using a 128-slice multidetector CT scanner (Somatom Definition Flash, Siemens, Erlangen, Germany). All scans were reconstructed using a high spatial frequency, B60 kernel (Siemens, Munich, Germany). All HRCT scans were acquired with the patients lying in the supine position at full inspiration. The technical parameters used were: 1.0-mm section thicknesses, peak voltage of 120 kV, and tube current modulation (Quality ref mAs 150). Images were viewed at window settings optimized for the assessment of the lung parenchyma (width 1600 H.U.; level −600 H.U.).
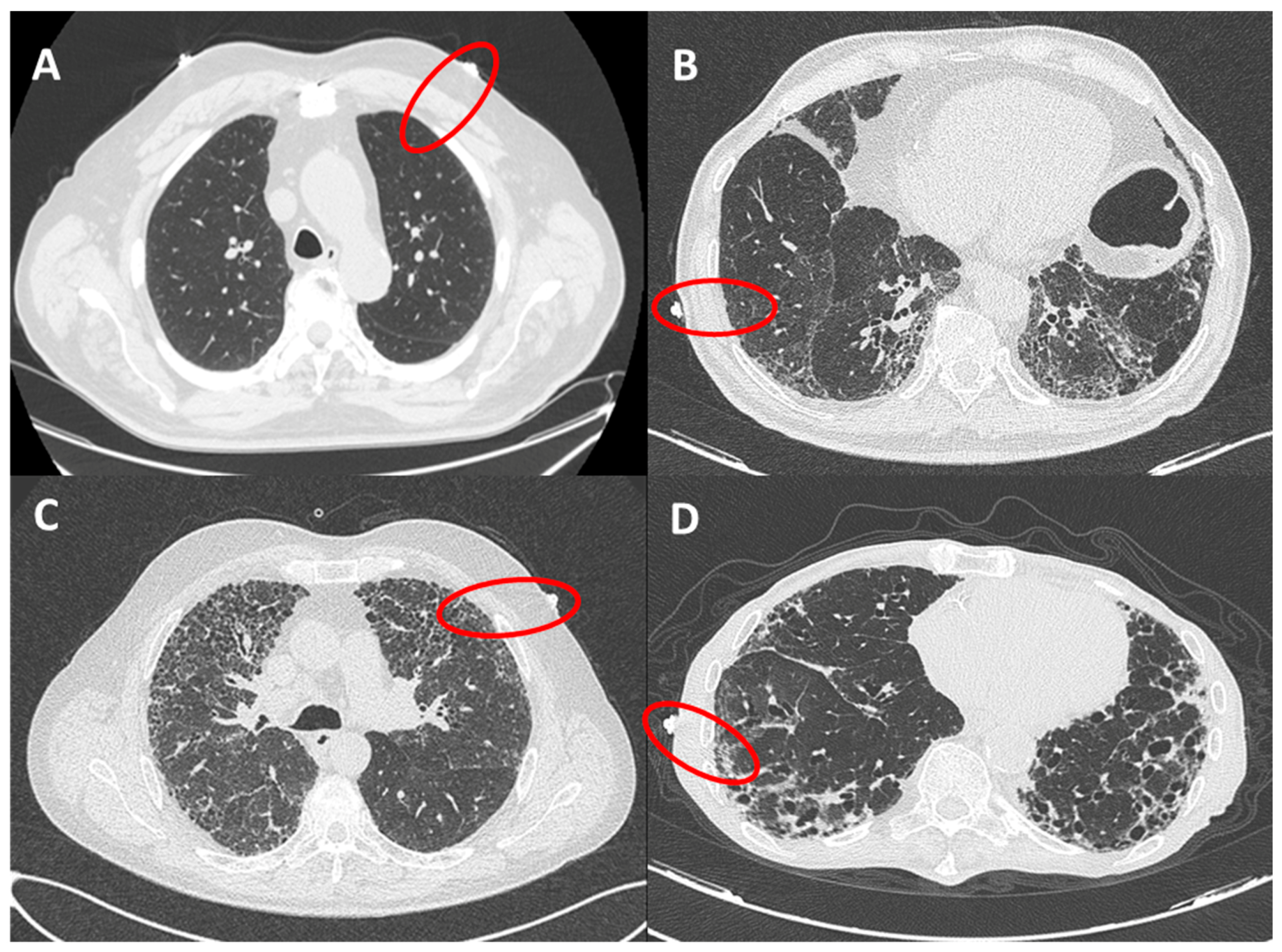
The presence of fibrotic infiltrates on HRCT was evaluated and contemporaneously graded by 2 radiologists (ARL and LC), with expertise in fibrotic interstitial diseases. The analysis was performed only on peripheral lung (within 2 cm in depth from visceral pleura and corresponding to dimension of secondary lobule) and reported as grade 1 (from 0% to 10% of fibrotic changes, mild alterations), grade 2 (10%–50%, moderate), grade 3 (50%–90% severe) and grade 4 (90%–100%, massive fibrotic subversion or honeycomb) (Figure 2). A concordant HRCT score was provided.
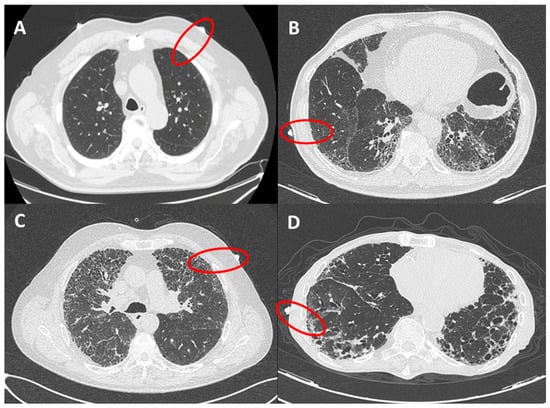
Figure 2.
HRCT grades related to red circles showing landmarks of ultrasound assessment. (A): grade 1 (from 0 to 10% of fibrotic changes, mild alterations); (B): grade 2 (10–50%, moderate); (C): grade 3 (50–90% severe); (D): grade 4 (90–100%, massive fibrotic subversion or honeycomb).
2.3. Statistical Analysis
A descriptive analysis of the distribution of LUS patterns and HRCT scan grades was reported. Agreement between LUS patterns and HRCT grades was determined by Cohen’s kappa-coefficient statistic. Inter-rater agreement magnitude has been considered based on Landis and Koch proposal: Cohen’s kappa values <0 as indicating no agreement; 0–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1 as almost perfect agreement. Simple percentage agreement was also reported.
3. Results
Each patient enrolled received 6 echographic scans in the anatomic landmarks previously described. Eighty-four echographic reports were generated.
LUS pattern 1 was reported in 25 scans out of 84: 21 cases corresponded to grade 1 HRCT and four cases to grade 2 HRCT. LUS pattern 1 was thus in agreement with HRCT grade 1 in 84% of cases. However, in 100% of cases LUS pattern 1 was indicative of fibrotic alterations <50% (mild to moderate).
LUS pattern 2 was reported in 30 scans out of 84: in 24 cases (77%) was associated with HRCT grade 2; in 2 cases with HRCT grade 1; in four cases with HRCT grade 4, thus showing a huge discordance between sonography and HRCT. These cases will be carefully addressed in the discussion section.
LUS pattern 3 was reported in 23 scans out of 84: in 19 cases was associated with HRCT grade 3, with an agreement of 83%. Four cases presented HRCT grade 4. However, in 100% of cases LUS pattern 3 was indicative of fibrotic alterations >50% (severe to massive).
LUS pattern 4 was reported in six scans out of 84. For this pattern, the corresponding HRCT grades presented a heterogeneous distribution: in two cases the HRCT grade was concordantly 4. In three cases, the HRCT grade was 3, and in one case the HRCT grade was 1. This case will also be further analyzed in the discussion section. Table 1 shows distribution of LUS patterns and HRCT grades.

Table 1.
Distribution of LUS patterns and HRCT grades.
As far as inter-rater agreement between LUS patterns and HRCT grades is concerned, the analysis of the K Cohen’s coefficient shows close to perfect agreement for LUS patterns 1, 2, and 3 when compared to HRCT grades 1, 2, and 3, respectively. On the contrary, the agreement between LUS pattern 4 and HRCT grade 4 is weaker. The low number of cases studied could have influenced this result.
Table 2, Table 3, Table 4 and Table 5 show inter-rater agreement computation by Cohen’s k coefficient between patterns and grades.

Table 2.
Agreement between LUS pattern 1 and HRCT grade 1.

Table 3.
Agreement between LUS pattern 2 and HRCT grade 2.

Table 4.
Agreement between LUS pattern 3 and HRCT grade 3.

Table 5.
Agreement between LUS pattern 4 and HRCT grade 4.
4. Discussion
Lung ultrasound is able to intercept peripheral subversion of airspace geometry (PAS) which can occur in fibrotic interstitial lung diseases. Some of them, especially idiopathic pulmonary fibrosis (IPF), can be a good target for ultrasound because of its peculiar propensity to involve peripheral lung parenchyma.
Irregular, unpredictable, disordered acoustic traps at the pleural plane are able to generate pneumogenic sonographic interstitial syndrome (SIS). The more advanced and distributed is SIS, the greater will be the number and the distribution of acoustic traps. Consequently, vertical artifacts tend to prevail on horizontal reverberations accordingly with number and distribution of acoustic traps [10,11,18,19].
PAS geometry is typically subverted for structural matters. The architecture of the lung is deeply altered, especially on its periphery. On the contrary, in the case of early cardiogenic pulmonary edema, the structure of the lung remains unchanged, and acoustic traps are generated by thickened interlobular and intralobular septa. These kinds of acoustic traps are more regular, ordered, and predictable. The size, shape, regularity, as well as the nature of the content of acoustic traps, are able to influence the generation of different vertical artifacts. A fibrotic pulmonary disease, as IPF, alters the PAS geometry by producing heterogeneous and irregular shaped acoustic traps with fibrotic content. The corresponding pneumogenic sonographic interstitial syndrome can thus become a specific pattern with a thickened pleural line and more attenuated vertical artifacts, often without a clear periodic structure [20,21].
In this study four LUS patterns were described. The choice of this classification deserves to be motivated. LUS pattern 1 corresponds to a normal or near normal lung surface, which can be seen as formed by closely packed air-filled alveoli. Consequently, due to the high acoustic impedance mismatch between air and the intercostal tissue, the lung surface behaves essentially as a specular reflector to ultrasound waves. Ultrasound penetration is thus hindered beyond the pleura line, and the typical reverberant A-line artifacts are displayed by standard imaging systems. With the progression of a pathology such as fibrosis, for which there is a replacement or the lung volume originally occupied by air in favor of tissue, the lung surface becomes progressively permeable to ultrasound, resulting in the increasing appearance of vertical B-line artifacts associated to irregularities of pleural line, and ultimately leading to the visualization of images displaying White Lung (LUS pattern 4) [21].
This preliminary work was able to demonstrate some level of agreement between LUS patterns, as previously described, and HRCT grades of fibrotic alterations.
First of all, LUS pattern 1 in 100% of cases was indicative of mild to moderate fibrotic alterations. In particular in 21 out of 25 cases (84%) HRCT scans showed properly normal or near normal conditions with poor fibrotic changes (grade 1, <10% of fibrotic changes).
On the contrary, LUS pattern 3 in 100% of cases was indicative of severe to massive fibrotic alterations.
An acoustic map of the peripheral lung is already possible based on these considerations. Echographic scans with a pattern of SIS displaying vertical artifacts prevailing on horizontal phenomena and altered pleural line is in fact indicative of important fibrotic alterations in the peripheral parenchyma [22,23]. Moreover, if, in correspondence of a specific landmark, a LUS pattern 1 changes towards a LUS pattern 3 in a subsequent evaluation over time, this could be indicative of a progression of the diseases in that specific point, and guide the timing of HRCT scan re-evaluation. This was not the aim of this work, but it might be extremely interesting to evaluate in further studies.
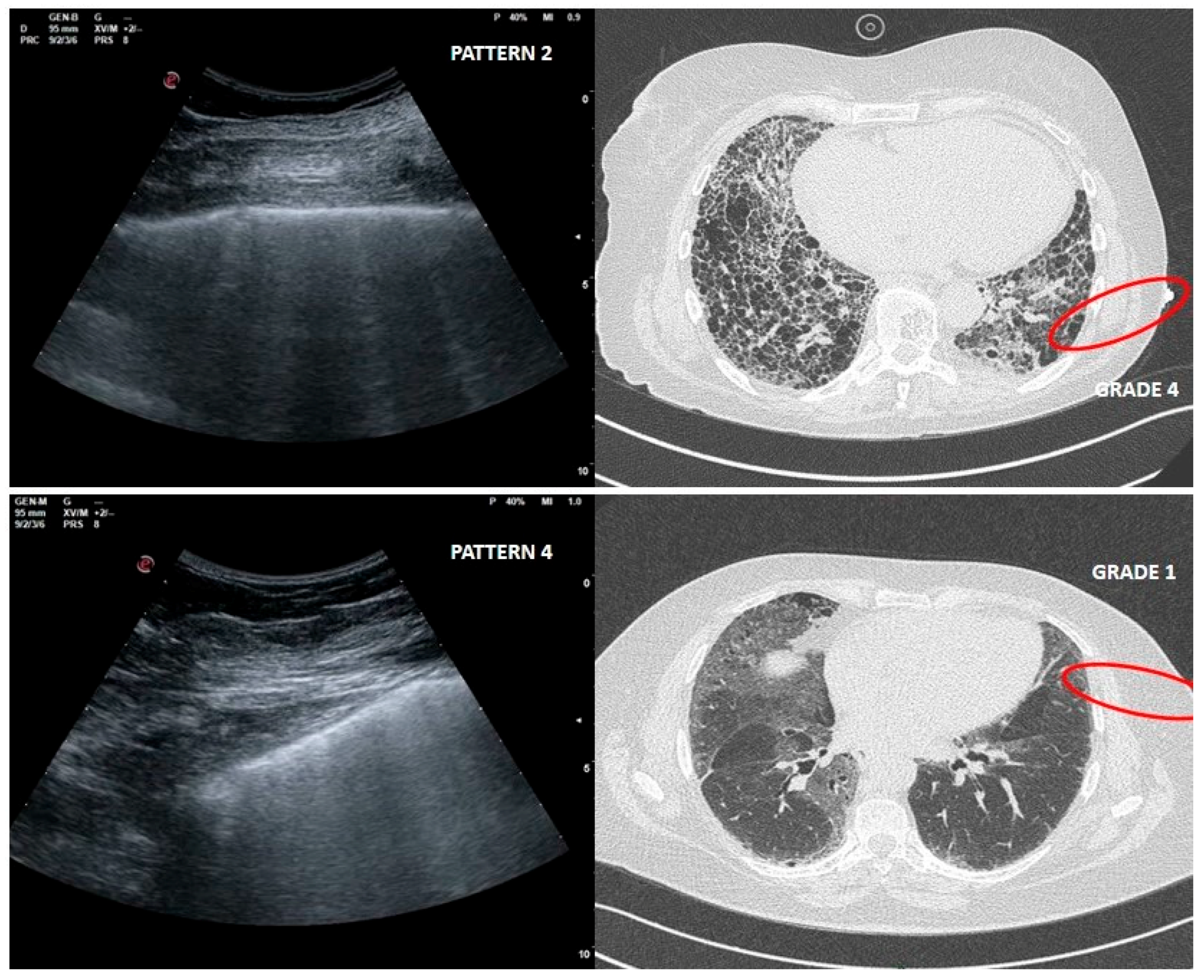
As far as LUS pattern 2 is concerned, agreement with HRCT grade 2 has been reported in 24 out of 30 cases (77%). It is really important to focus on the 4 cases in which a LUS pattern 2 was associated with HRCT grade 4 related to peripheral large honeycomb cysts (massive fibrosis subversion) (Figure 3).
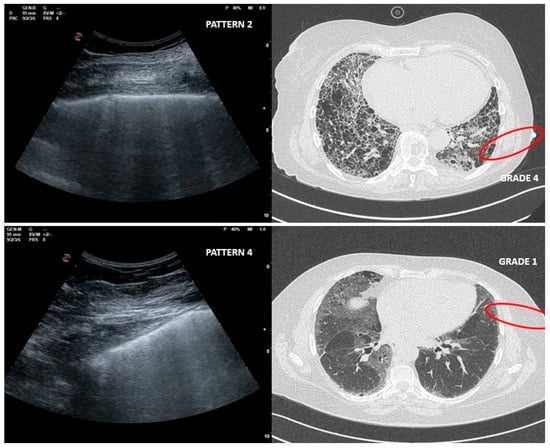
Figure 3.
Upper right and left: large cysts honeycombing and severe fibrotic changes (red circle) with corresponding LUS pattern 2. Bottom right and left: ground glass opacities and moderate fibrotic alterations (red circle) with corresponding LUS pattern 4.
Large cysts honeycomb could represent a limit for LUS, as in this case the air content is greater than in a healthy condition [24].
Standard ultrasound imaging evaluation could thus underestimate the degree of alteration in this context. The development of dedicated ultrasound imaging solutions designed around the properties of lungs may play an important role in mitigating these effects. In particular, the characterization of the response of bubbly aggregates when exposed to ultrasound waves centered at different insonating frequencies has already proven to be able to discriminate between bubbly aggregates formed by bubbles of different diameters [19,25].
Further studies deserve to focus specifically with ultrasound beam behavior in presence of honeycomb.
LUS pattern 4 presented a heterogeneous distribution of corresponding HRCT grades. These could be affected by the low number of cases studied. In five cases out of six, HRCT pattern was however indicative of severe to massive fibrotic changes. Instead, in one case, LUS pattern 4 corresponded to a HRCT grade 1.
This misleading case, in which fibrotic changes were moderate but ground glass opacity (GGO) was reported, corresponded to an early phase of acute exacerbation of IPF (Figure 3).
The physical origin of white lung artefact (LUS pattern 4) is not yet completely known. It would be very interesting to demonstrate in further studies how GGOs can influence ultrasound assessment and why. Ground glass opacities in CT scans are indicative of areas of increased lung density with poor air content, which are however not consolidating and not obscuring the visibility of the underlying vasculature. This can be indicative of inflammatory changes, pulmonary edema, pulmonary hemorrhage, fine fibrotic alterations, etc. [26].
The hypothesis is that fibrotic GGOs can be caused by the presence of multiple layers of randomly distributed small-sized airspaces. In this case, it is reasonable to expect the visualization of a granular white texture of speckle-like appearances (i.e., white lung), when investigating such an area with standard ultrasound imaging [21].
Finally, it is worth addressing the limits and methodological issues of this preliminary work.
The first limitation is the impossibility to provide information about basal posterior areas, usually the more involved and more informative in case of IPF patients. Echographic assessment of these areas needs the patients to be seated. Thus, the cutaneous landmark positioned would indicate pleural plane in a point very different from that evaluated by ultrasound, when the patient assumes a supine position for HRCT scans. It is known that changing posture, the position of the diaphragm, motility and lung volumes dramatically change as well [27,28,29,30]. Also, the position of arms affects diaphragm height, and consequently the position of the lung [31].
Patient position must be necessarily the same during both LUS evaluation and HRCT scan. Changing position would be an unacceptable bias in a work in which the match between the lung surface assessed by ultrasound and HRCT scan is crucial.
Methodological issues constitute the second point that needs to be discussed. Echographic assessment is a real time imaging technique. While recording videos, the patient is normally breathing. This implies that the imaging plane intercepts over time different areas of the pleura plane. During breathing, in fact, LUS pattern can change. In this study, the LUS pattern reported was the worst (the greater) observed during the evaluation of the ultrasound data. Thus, inter-observer variability can be an important limitation in this scoring system. Echographic acquisitions during breath holding at the end of a deep inspiration would make the condition closest to that of HRCT scans. Two blind observers would allow a better definition of the pattern by computing inter-observer agreement.
Finally, in our work apical landmarks seem not to have a corresponding lung surface right below their location, by observation of the HRCT scans in the axial plane in three patients out of 14. Free hand echographic evaluation allows to scan the patient also in unconventional planes moving from coronal to axial. For these patients, the HRCT grade was provided guessing the ultrasound scan plane, but a better evaluation would be possible in future studies by confining the ultrasound evaluation to the axial plane as much as possible.
It is extremely important to discuss the limitation and the methodological issues also with respect to previous studies, which already tried to report on the correspondence between LUS images and HRCT findings for fibrotic lung diseases [32,33,34]. When performing a comparison between LUS artifactual images and chest CT scans it is crucial to respect the same position and the same conditions in both techniques. Otherwise the obvious risk is that the comparison can be misleading. This is especially true for IPF, which is characterized by an extreme heterogeneity of peripheral changes. In this case series, this is an important methodological aspect which has been addressed for the first time.
Moreover, the origins of vertical artifacts are only now beginning to be understood. As showed here, honeycomb and GGOs can result in misleading findings for LUS.
On the other hand, weaknesses of this paper compared with previous works [18,23,32,33,34] are the lack of information about basal posterior areas, usually the more involved and more informative in the case of IPF patients, the lack of data about respiratory function tests [32], and the lack of ultrasound evaluations of the pleural line performed with linear probes.
Finally, we do not agree with the possibility to distinguish lung interstitial patterns (probable UIP, definite UIP, NSIP etc.) with LUS, as reported in previous work [33]. The definition of these patterns is based on HRCT scan, which is able to provide morphological and panoramic views of these pathologies. In these contexts, ultrasound interact only with the pleural surface and peripheral airspace geometry. To differentiate radiological interstitial patterns, which is even a difficult task for a morphologic HRCT scan, is not the aim of artifactual sonography, especially when the underlying physical phenomena have not yet been completely understood. Counting vertical artifacts is likewise not the better approach to quantify these phenomena and explain their significance [34,35].
Chest ultrasonography, instead, might serve as a useful tool to early intercept fibrotic peripheral changes during medical examination in order to widen medical semiotic [9,36]. Moreover, perhaps LUS could have a role in the follow up of peripheral changes and optimize the timing of subsequent chest CT scans.
5. Conclusions
Detecting SIS with integrated clinical data, highly indicative for fibrotic ILDs during the first medical examination, would allow physicians to identify patients who deserve to directly undergo HRCT, sparing chest X-ray or low-resolution chest CT scans, and speeding up the diagnostic process. This preliminary work was able to demonstrate some level of agreement between LUS patterns and HRCT grades of peripheral fibrotic alterations. Potentially, LUS could play a role also in the follow up study of these changes, and optimize the timing of subsequent chest CT scans. Further studies are needed to differentiate ultrasonographic features in case of GGOs and large honeycomb cysts. The characterization of the pleural line as performed with different ultrasound probes and imaging frequencies could be crucial to integrate the data obtained by convex probe evaluation, and to better assess specific alterations of the peripheral airspace geometry of lungs.
Finally, the limitations and methodological issues previously described when comparing CT scans and echographic acquisitions are extremely important to support subsequent studies, limiting methodological biases.
Author Contributions
Conceptualization and rationale A.S., G.S.; methodology, A.S., R.I., L.C., L.D.; validation, A.R.L., L.R., G.S.; formal analysis, A.S.; investigation, A.S., R.I.; resources, F.L., A.C., M.S.; data curation A.S., F.L., A.C., M.S.; writing—original draft preparation, A.S.; writing—review and editing, L.C., L.D., A.R.L.; visualization, L.R.; supervision, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Chisholm, A.; Collard, H.R.; Flaherty, K.R.; Myers, J.; Raghu, G.; Walsh, S.L.; White, E.S.; Richeldi, L. The diagnosis of idiopathic pulmonary fibrosis: Current and future approaches. Lancet Respir. Med. 2017, 5, 61–71. [Google Scholar] [CrossRef]
- Cottin, V.; Cordier, J.F. Velcro crackles: The key for early diagnosis of idiopathic pulmonary fibrosis? Eur. Respir. J. 2012, 40, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Vyshedskiy, A.; Bezares, F.; Paciej, R.; Ebril, M.; Shane, J.; Murphy, R. Transmission of crackles in patients with interstitial pulmonary fibrosis, congestive heart failure, and pneumonia. Chest 2005, 128, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Sgalla, G.; Larici, A.R.; Sverzellati, N.; Bartholmai, B.; Walsh, S.L.F.; Nikolic, D.; Barney, A.; Fletcher, S.; Jones, M.; Davies, D.D.; et al. Quantitative analysis of lung sounds for monitoring idiopathic pulmonary fibrosis: A prospective pilot study. Eur. Respir. J. 2019, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Cottin, V.; Würtemberger, G.; Kreuter, M.; Calvello, M.; Sgalla, G. Digital Lung Auscultation: Will Early Diagnosis of Fibrotic Interstitial Lung Disease Become a Reality? Am. J. Respir. Crit. Care Med. 2019, 200, 261–263. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Mariani, A.A.; Inchingolo, R. Novel aspects in diagnostic approach to respiratory patients: Is it the time for a new semiotics? Multidiscip. Respir. Med. 2017, 27, 12–15. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Sher, S.; Nenna, R.; Valente, S.; Inchingolo, C.D.; Corbo, G.M. Lung ultrasonography may provide an indirect estimation of lung porosity and airspace geometry. Respiration 2014, 88, 458–468. [Google Scholar] [CrossRef]
- Soldati, G.; Demi, M.; Smargiassi, A.; Inchingolo, R.; Demi, L. The role of ultrasound lung artifacts in the diagnosis of respiratory diseases. Expert. Rev. Respir. Med. 2019, 13, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Demi, M.; Inchingolo, R.; Smargiassi, A.; Demi, L. On the physical basis of pulmonary sonographic interstitial syndrome. J. Ultrasound Med. 2016, 35, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Smargiassi, A.; Inchingolo, R.; Soldati, G.; Copetti, R.; Marchetti, G.; Zanforlin, A.; Giannuzzi, R.; Testa, A.; Nardini, S.; Valente, S. The role of chest ultrasonography in the management of respiratory diseases: Document II. Multidiscip. Respir. Med. 2013, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Muller, N.L.; Colby, T.V. Idiopathic interstitial pneumonias: High-resolution CT and histologic findings. RadioGraphics 1997, 17, 1016–1022. [Google Scholar] [CrossRef]
- Sperandeo, M.; De Cata, A.; Molinaro, F.; Trovato, F.M.; Catalano, D.; Simeone, A.; Varriale, A.; Martines, G.F.; Trovato, G. Ultrasound signs of pulmonary fibrosis in systemic sclerosis as timely indicators for chest computed tomography. Scand. J. Rheumatol. 2015, 44, 389–398. [Google Scholar] [CrossRef]
- Tardella, M.; Gutierrez, M.; Salaffi, F.; Carotti, M.; Ariani, A.; Bertolazzi, C.; Filippucci, E.; Grassi, W. Ultrasound in the assessment of pulmonary fibrosis in connective tissue disorders: Correlation with high-resolution computed tomography. J. Rheumatol. 2012, 39, 1641–1647. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Pallisa-Nuñez, E.; Selva-O’Callaghan, A.; Castella-Fierro, E.; Simeon-Aznar, C.P.; Fonollosa-Pla, V.; Vilardell-Tarres, M. Pleural irregularity, a new ultrasound sign for the study of interstitial lung disease in systemic sclerosis and antisynthetase syndrome. Clin. Exp. Rheumatol. 2015, 33, S136–S141. [Google Scholar]
- Buda, N.; Piskunowicz, M.; Porzezińska, M.; Kosiak, W.; Zdrojewski, Z. Lung ultrasonography in the evaluation of interstitial lung disease in systemic connective tissue diseases: Criteria and severity of pulmonary fibrosis—analysis of 52 patients. Ultraschall Med. 2016, 37, 379–385. [Google Scholar] [CrossRef]
- Demi, L.; Demi, M.; Smargiassi, A.; Inchingolo, R.; Faita, F.; Soldati, G. Ultrasonography in lung pathologies: New perspectives. Multidiscip. Respir. Med. 2014, 9, 9. [Google Scholar] [CrossRef]
- Soldati, G.; Demi, M. The use of lung ultrasound images for the differential diagnosis of pulmonary and cardiac interstitial pathology. J. Ultrasound. 2017, 20, 91–96. [Google Scholar] [CrossRef]
- Demi, M.; Prediletto, R.; Soldati, G.; Demi, L. Physical mechanisms providing clinical information from ultrasound lung images: Hypotheses and early confirmations. In IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control; IEEE: Piscataway, NJ, USA, 2019. [Google Scholar]
- Tardella, M.; Di Carlo, M.; Carotti, M.; Filippucci, E.; Grassi, W.; Salaffi, F. Ultrasound B-lines in the evaluation of interstitial lung disease in patients with systemic sclerosis: Cut-off point definition for the presence of significant pulmonary fibrosis. Medicine 2018, 97, e0566. [Google Scholar] [CrossRef]
- Wang, Y.; Gargani, L.; Barskova, T.; Furst, D.E.; Cerinic, M.M. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: A literature review. Arthritis Res. Ther. 2017, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, M.; Varriale, A.; Sperandeo, G.; Filabozzi, P.; Piattelli, M.L.; Carnevale, V.; Decuzzi, M.; Vendemiale, G. Transthoracic ultrasound in the evaluation of pulmonary fibrosis: Our experience. Ultrasound Med. Biol. 2009, 35, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Demi, L.; van Hoeve, W.; van Sloun, R.J.G.; Soldati, G.; Demi, M. Determination of a potential quantitative measure of the state of the lung using lung ultrasound spectroscopy. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gotway, M.B.; Reddy, G.P.; Webb, W.R.; Elicker, B.M.; Leung, J.W. High-resolution CT of the lung: Patterns of disease and differential diagnoses. Radiol. Clin. N. Am. 2005, 43, 513–542. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Arish, N.; Rokach, A.; Zaltzman, Y.; Marcus, E.L. The effect of body position on pulmonary function: A systematic review. BMC Pulm. Med. 2018, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; Tseng, S.C.; Mitchell, K.; Roddey, T. Body Position Affects Ultrasonographic Measurement of Diaphragm Contractility. Cardiopulm. Phys. Ther. J. 2018, 29, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Druz, W.S.; Sharp, J.T. Activity of respiratory muscles in upright and recumbent humans. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 1552–1561. [Google Scholar] [CrossRef]
- Barnas, G.M.; Green, M.D.; Mackenzie, C.F.; Fletcher, S.J.; Campbell, D.N.; Runcie, C.; Broderick, G.E. Effect of posture on lung and regional chest wall mechanics. Anesthesiology 1993, 78, 251–259. [Google Scholar] [CrossRef]
- Onozawa, S.; Murata, S.; Kimura, T.; Ueda, T.; Sugihara, F.; Yasui, D.; Tajima, H. Diaphragm height varies with arm position: Comparison between angiography and CT. Jpn J. Radiol. 2016, 34, 724–729. [Google Scholar] [CrossRef]
- Manolescu, D.; Oancea, C.; Timar, B.; Traila, D.; Malita, D.; Birsasteanu, F.; Tudorache, V.; Clin Respir, J. Ultrasound mapping of lung changes in idiopathic pulmonary fibrosis. Clin. Respir. J. 2020, 14, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Manolescu, D.; Davidescu, L.; Traila, D.; Oancea, C.; Tudorache, V. The reliability of lung ultrasound in assessment of idiopathic pulmonary fibrosis. Clin. Interv. Aging 2018, 13, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Mansour, O.F.; Agha, M.A.; Al-Asdody, A.A.; Mehana, N.S.; Habib, R.M. Sonographic features of idiopathic pulmonary fibrosis. Egypt. J. Chest Dis. Tuberc. 2018, 67, 50–55. [Google Scholar]
- Zanforlin, A.; Smargiassi, A.; Inchingolo, R.; Sher, S.; Ramazzina, E.; Corbo, G.M.; Soldati, G. B-lines: To count or not to count? JACC Cardiovasc. Imaging 2014, 7, 635–636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Narula, J.; Chandrashekhar, Y.; Braunwald, E. Time to Add a Fifth Pillar to Bedside Physical Examination: Inspection, Palpation, Percussion, Auscultation, and Insonation. JAMA Cardiol. 2018, 3, 346–350. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).