Review on an Advanced Combustion Technology: Supercritical Hydrothermal Combustion
Abstract
:1. Introduction
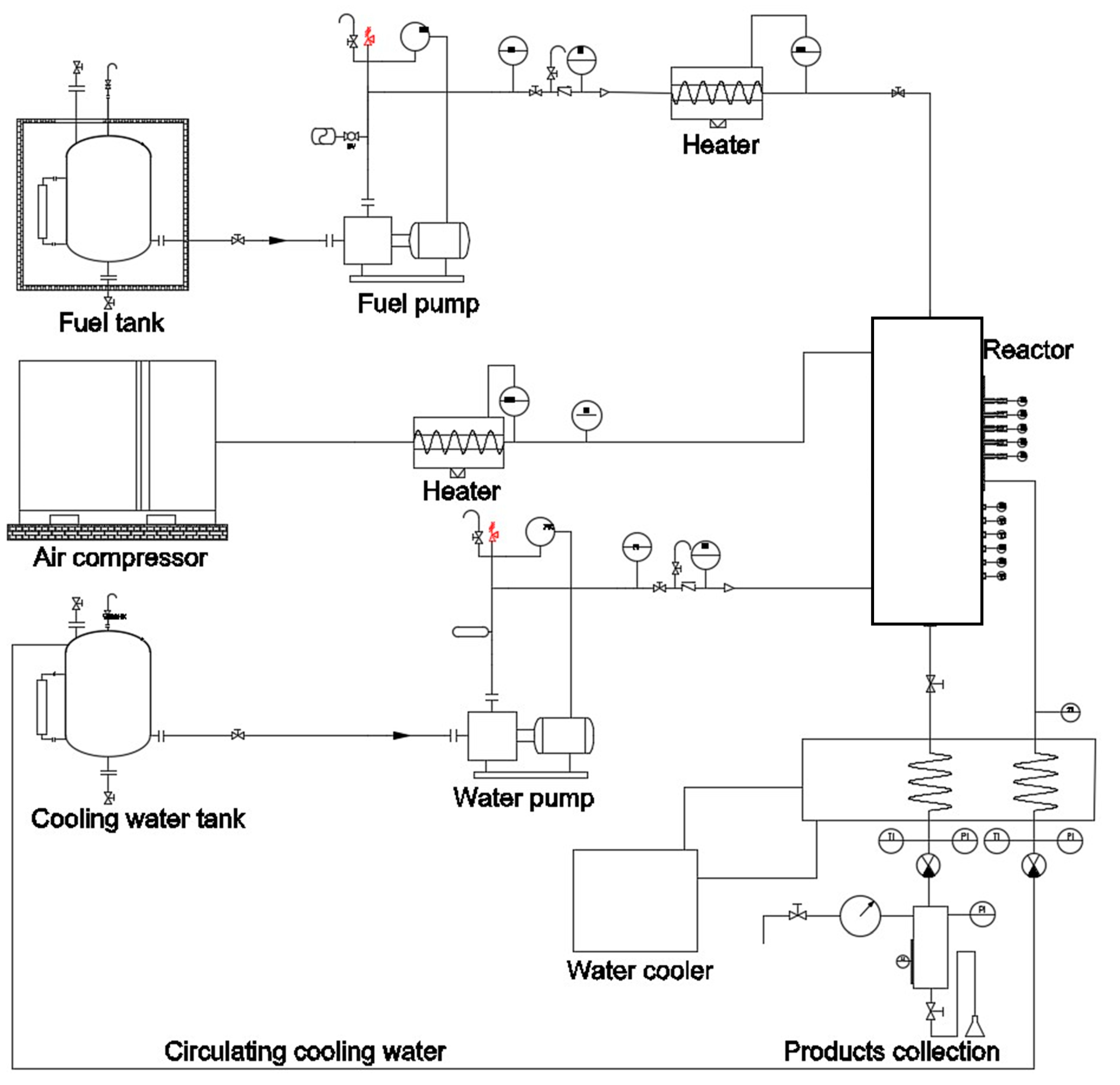
2. Characteristics and Improvements of Experimental Apparatus
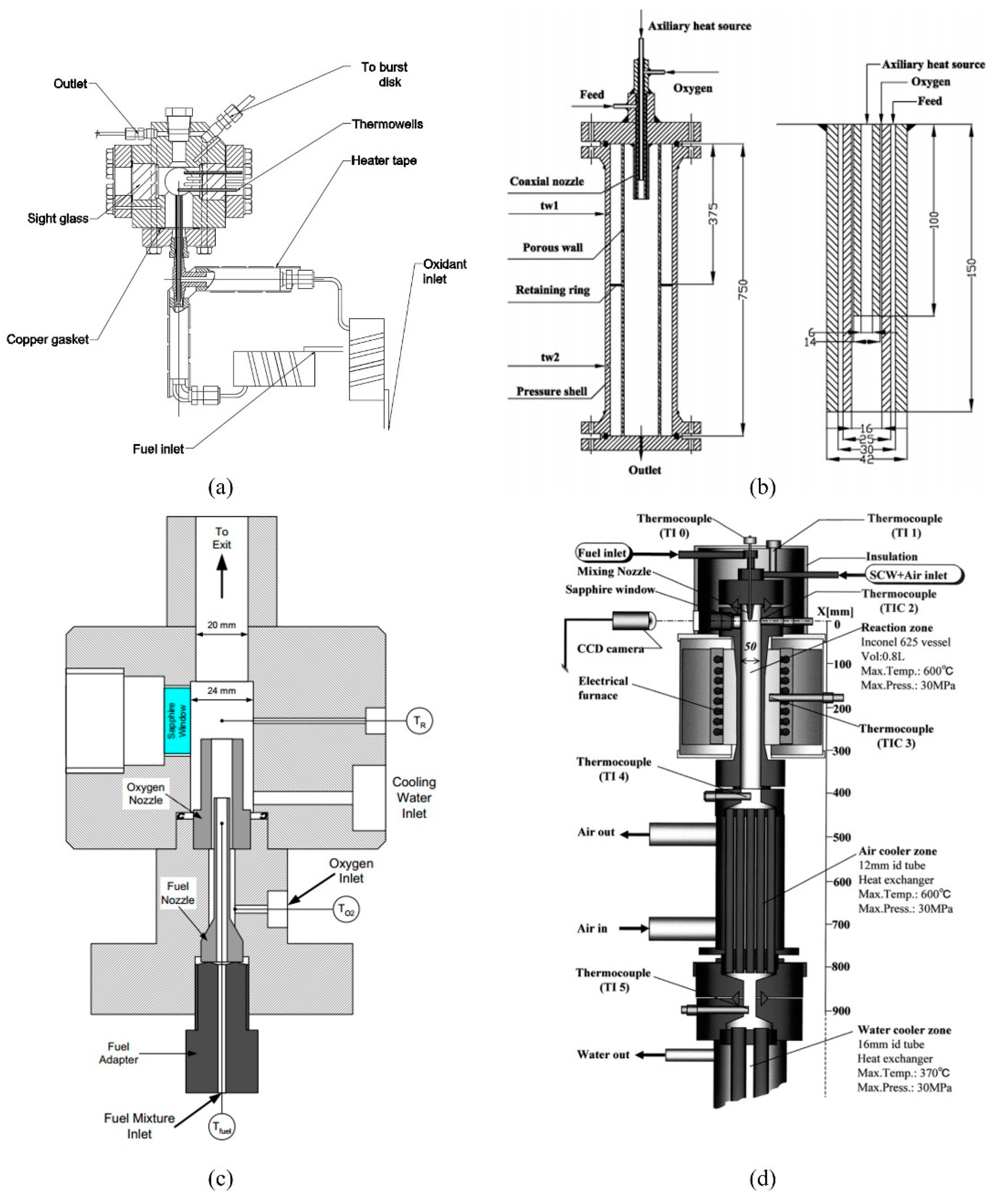
2.1. Semi-Batch Reactors
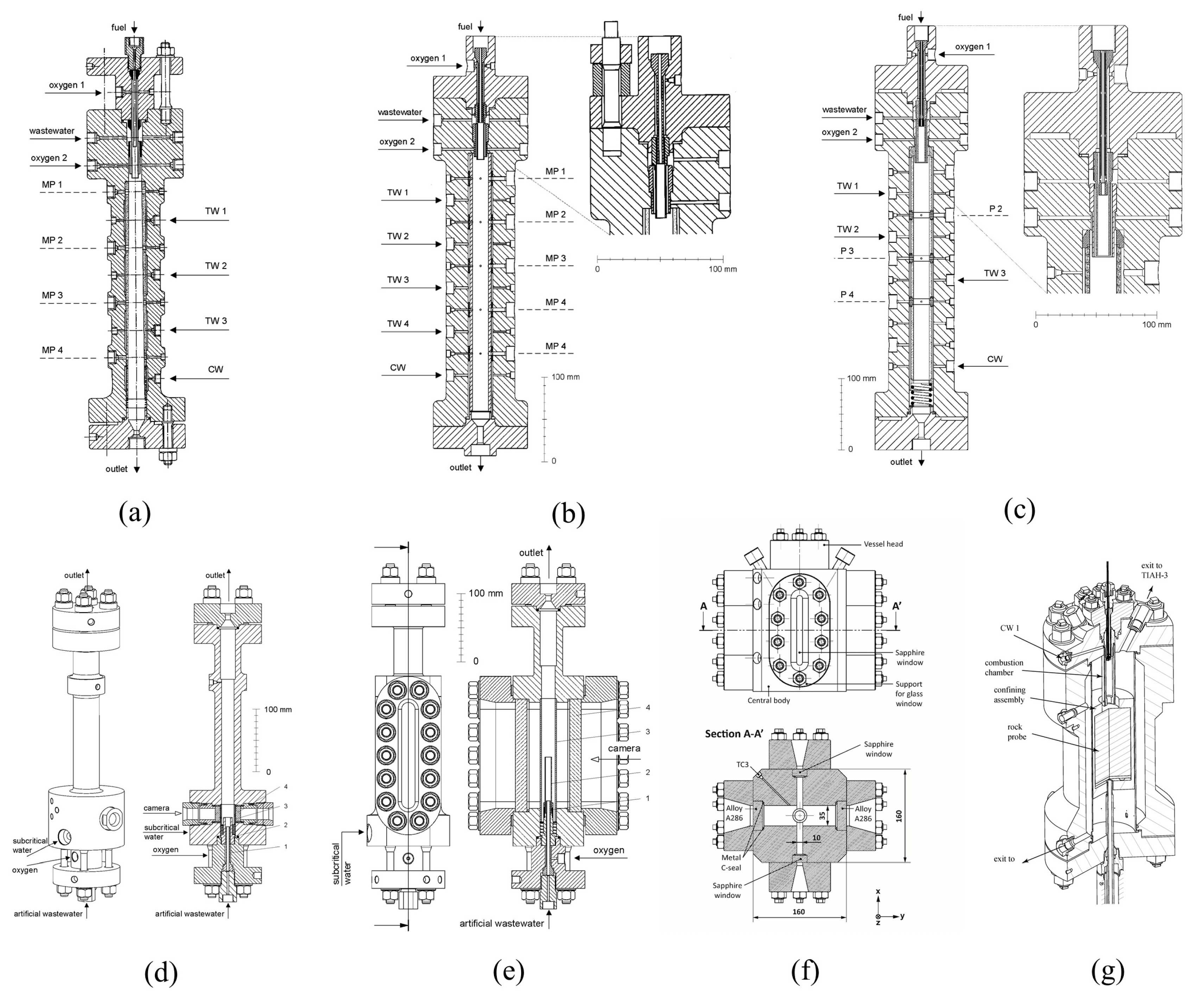
2.2. Continuous Reactors
2.2.1. ETH Zurich
2.2.2. University of Valladolid
2.2.3. Xi’an Jiaotong University
2.2.4. Others
3. Research Status of Numerical Simulation
3.1. Reaction Kinetics
3.2. Flow-Reaction
4. Characteristics of Flame and Its Combustion
4.1. Ignition Temperature
4.2. Extinction Temperature
4.3. Ignition Delay Time
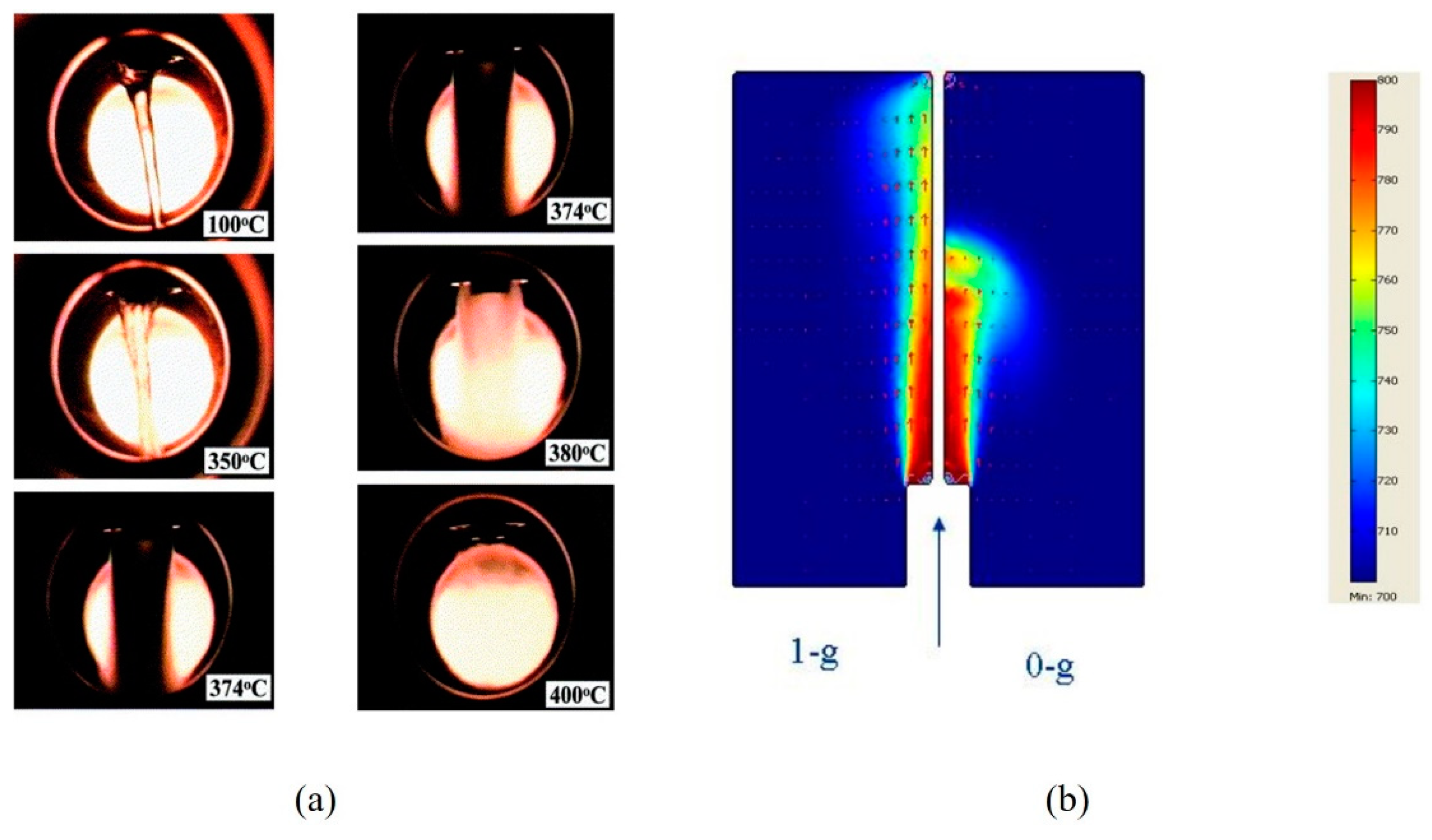
4.4. Flame Temperature Distribution
4.5. Flame Propagation Speed
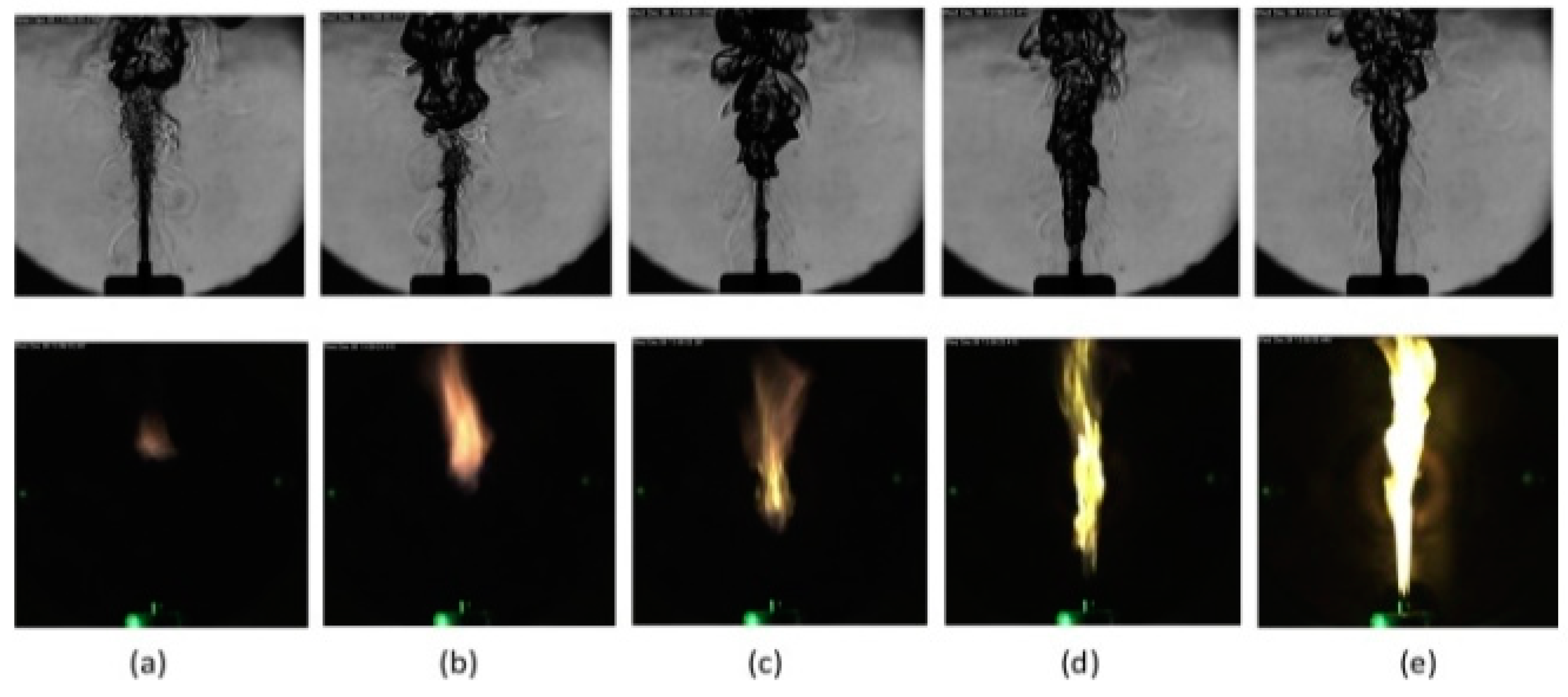
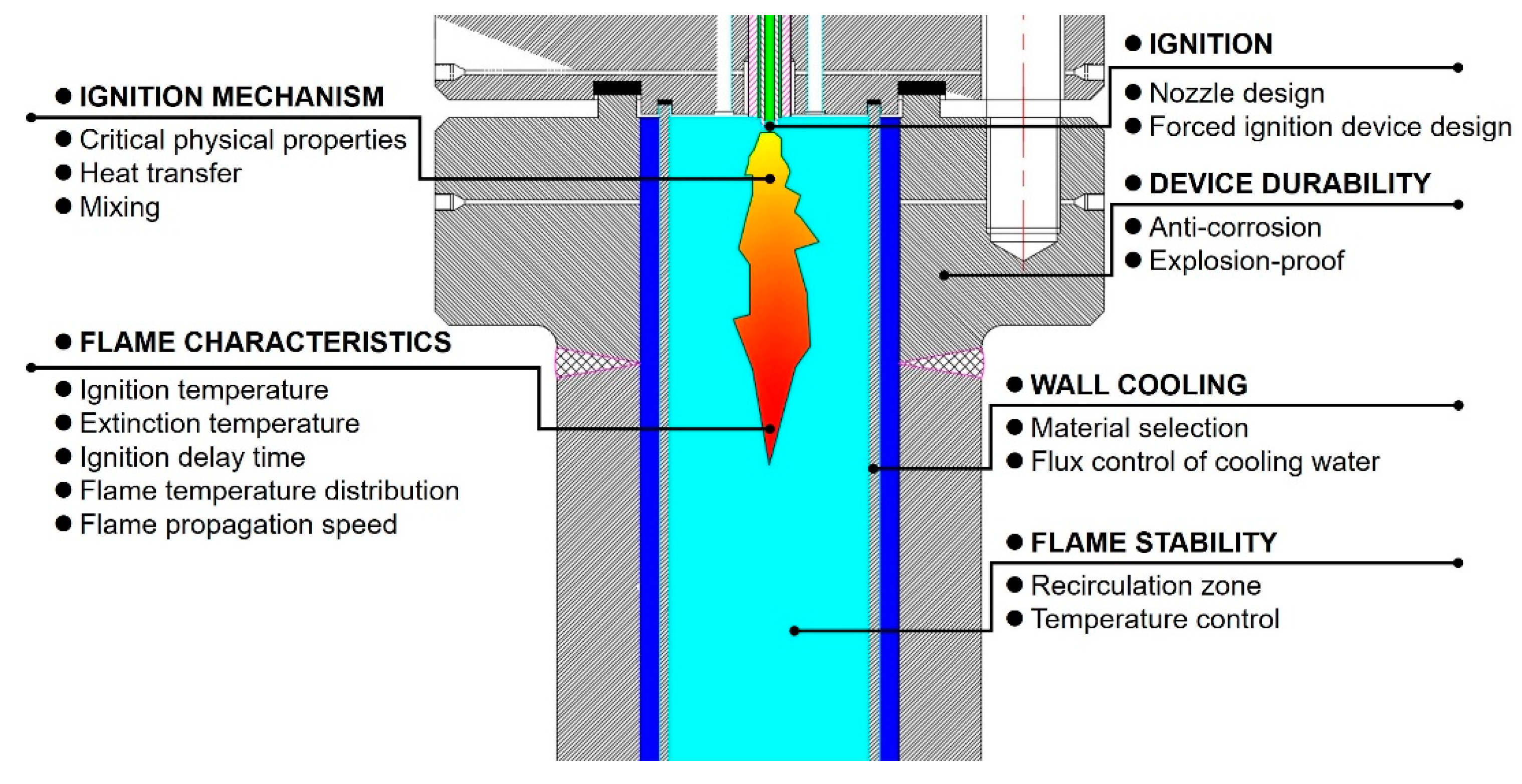
5. Ignition Process and Mechanisms
5.1. Ignition Process
5.2. Ignition Mechanism
5.2.1. Critical Physical Properties
5.2.2. Heat Transfer
5.2.3. Mixing
Buoyancy Mixing
Mechanical Mixing
6. Existing and Potential Industrial Applications
6.1. Hydrothermal Spallation Drilling
6.2. Thermal Recovery of Heavy Oil
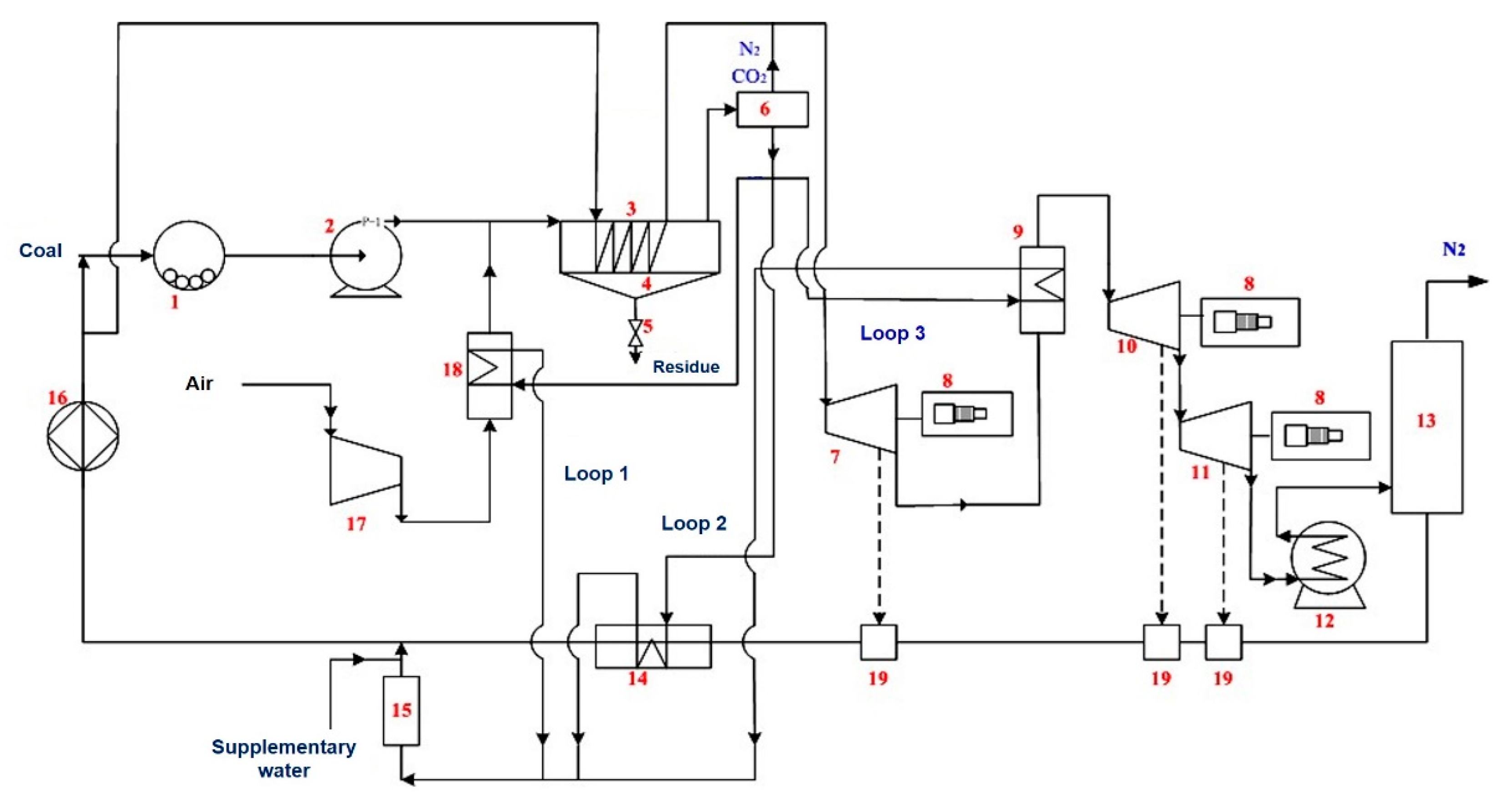
6.3. Clean Conversion and Utilization of Coal-Based Fuel
6.4. Harmless Treatment of Pollutants
7. Research Needs and Challenges
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, Y.; Zhang, J.; Ma, H.; Qian, L.; Li, Y. Oxidative Mechanisms and Kinetics of Organics in Supercritical Water. In Supercritical Water Processing Technologies for Environment, Energy and Nanomaterial Applications; Springer: Singapore, 2020; pp. 47–108. [Google Scholar]
- Qian, L.; Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, L. Treatment of municipal sewage sludge in supercritical water: A review. Water Res. 2016, 89, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Marrone, P.A. Supercritical water oxidation—Current status of full-scale commercial activity for waste destruction. J. Supercrit. Fluids 2013, 79, 283–288. [Google Scholar] [CrossRef]
- Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, Y.; Zhang, J.; Ma, H.; Qian, L.; Li, Y. Oxidative Mechanisms and Kinetics of Organics in Supercritical Water. In Supercritical Water Processing Technologies for Environment, Energy and Nanomaterial Applications; Springer: Singapore, 2020; pp. 327–352. [Google Scholar]
- Li, Y.; Wang, S. Supercritical water oxidation for environmentally friendly treatment of organic wastes. In Advanced Supercritical Fluids Technologies; Pioro, I.L., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Xu, D.; Huang, C.; Wang, S.; Lin, G.; Guo, Y. Salt deposition problems in supercritical water oxidation. Chem. Eng. J. 2015, 279, 1010–1022. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Song, W.; Yang, J.; Xu, T.; Li, J.; Yang, C.; Li, Y. Characteristics of sodium sulfate deposition in hydrogen production from supercritical water gasification: A review. Int. J. Hydrog. Energy 2019, 44, 29467–29482. [Google Scholar] [CrossRef]
- Marrone, P.A.; Hong, G.T. Corrosion control methods in supercritical water oxidation and gasification processes. J. Supercrit. Fluids 2009, 51, 83–103. [Google Scholar] [CrossRef]
- Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, Y.; Zhang, J.; Ma, H.; Qian, L.; Li, Y. Oxidative Mechanisms and Kinetics of Organics in Supercritical Water. In Supercritical Water Processing Technologies for Environment, Energy and Nanomaterial Applications; Springer: Singapore, 2020; pp. 149–259. [Google Scholar]
- Xu, T.; Wang, S.; Tang, X.; Li, Y.; Yang, J.; Li, J.; Zhang, Y. Corrosion mechanism of inconel 600 in oxidizing supercritical aqueous systems containing multiple salts. Ind. Eng. Chem. Res. 2019, 58, 23046–23056. [Google Scholar] [CrossRef]
- Bermejo, M.D.; ndez-Polanco, F.F.; Cocero, M.J. Modeling of a transpiring wall reactor for the supercritical water oxidation using simple flow patterns: Comparison to experimental results. Ind. Eng. Chem. Res. 2005, 44, 3835–3845. [Google Scholar] [CrossRef]
- Príkopský, K.; Wellig, B.; von Rohr, P.R. SCWO of salt containing artificial wastewater using a transpiring-wall reactor: Experimental results. J. Supercrit. Fluids 2007, 40, 246–257. [Google Scholar] [CrossRef]
- Li, Y.; Xu, T.; Wang, S.; Yang, J.; Li, J.; Xu, T.; Xu, D. Characterization of oxide scales formed on heating equipment in supercritical water gasification process for producing hydrogen. Int. J. Hydrogen Energy 2019, 44, 29508–29515. [Google Scholar] [CrossRef]
- Augustine, C.; Tester, J.W. Hydrothermal flames: From phenomenological experimental demonstrations to quantitative understanding. J. Supercrit. Fluids 2009, 47, 415–430. [Google Scholar] [CrossRef]
- Schilling, W.; Franck, E.U. Combustion and diffusion flames at high pressures to 2000 bar. Ber. Der Bunsenges. Für Phys. Chem. 1988, 92, 631–636. [Google Scholar] [CrossRef]
- Lavric, E.D.; Weyten, H.; De Ruyck, J.; Pleşu, V.; Lavric, V. Delocalized organic pollutant destruction through a self-sustaining supercritical water oxidation process. Energy Convers. Manag. 2005, 46, 1345–1364. [Google Scholar] [CrossRef]
- Cocero, M.J.; Alonso, E.; Sanz, M.T.; Fdz-Polanco, F. Supercritical water oxidation process under energetically self-sufficient operation. J. Supercrit. Fluids 2002, 24, 37–46. [Google Scholar] [CrossRef]
- Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, Y.; Zhang, J.; Ma, H.; Qian, L.; Li, Y. Oxidative Mechanisms and Kinetics of Organics in Supercritical Water. In Supercritical Water Processing Technologies for Environment, Energy and Nanomaterial Applications; Springer: Singapore, 2020; pp. 109–116. [Google Scholar]
- Mobley, P.D.; Pass, R.Z.; Edwards, C.F. Exergy analysis of coal energy conversion with carbon sequestration via combustion in supercritical saline aquifer water. In Proceedings of the ASME 2011 5th International Conference on Energy Sustainability, Washington, DC, USA, 7–10 August 2011. [Google Scholar]
- Ma, H. Fundamental Research on Power-Generating System Coupling of Gasification and Hydrothermal Combustion of Coal in Supercritical Water. Ph.D. Thesis, Xi’an Jiaotong University, Xi’an, China, 2013. [Google Scholar]
- Wang, L. Research on the Hydrothermal Combustion of Coal in Supercritical Water. Master’s Thesis, Xi’an Jiaotong University, Xi’an, China, 2007. [Google Scholar]
- Meier, T.; Schuler, M.J.; Stathopoulos, P.; Kramer, B.; Rudolf von Rohr, P. Hot surface ignition and monitoring of an internal oxygen–ethanol hydrothermal flame at 260 bar. J. Supercrit. Fluids 2017, 130, 230–238. [Google Scholar] [CrossRef]
- Meier, T.; Stathopoulos, P.; Rudolf von Rohr, P. Hot surface ignition of oxygen–ethanol hydrothermal flames. J. Supercrit. Fluids 2016, 107, 462–468. [Google Scholar] [CrossRef]
- Wellig, B.; Weber, M.; Lieball, K.; Príkopský, K.; Rudolf von Rohr, P. Hydrothermal methanol diffusion flame as internal heat source in a SCWO reactor. J. Supercrit. Fluids 2009, 49, 59–70. [Google Scholar] [CrossRef]
- Wellig, B.; Lieball, K.; Rudolf von Rohr, P. Operating characteristics of a transpiring-wall SCWO reactor with a hydrothermal flame as internal heat source. J. Supercrit. Fluids 2005, 34, 35–50. [Google Scholar] [CrossRef]
- Stathopoulos, P.; Ninck, K.; von Rohr, P.R. Heat transfer of supercritical mixtures of water, ethanol and nitrogen in a bluff body annular flow. J. Supercrit. Fluids 2012, 70, 112–118. [Google Scholar] [CrossRef]
- Príkopský, K. Characterization of Continuous Diffusion Flames in Supercritical Water. Ph.D. Thesis, ETH Zürich, Swiss, Switzerland, 2007. [Google Scholar]
- Phenix, B.D.; DiNaro, J.L.; Tester, J.W.; Howard, J.B.; Smith, K.A. The effects of mixing and oxidant choice on laboratory-scale measurements of supercritical water oxidation kinetics. Ind. Eng. Chem. Res. 2002 41, 624–631. [CrossRef]
- Sierra-Pallares, J.; Parra-Santos, M.T.; García-Serna, J.; Castro, F.; Cocero, M.J. Numerical modelling of hydrothermal flames. Micromixing effects over turbulent reaction rates. J. Supercrit. Fluids 2009, 50, 146–154. [Google Scholar]
- Bermejo, M.D.; Cabeza, P.; Bahr, M.; Fernández, R.; Ríos, V.; Jiménez, C.; Cocero, M.J. Experimental study of hydrothermal flames initiation using different static mixer configurations. J. Supercrit. Fluids 2009, 50, 240–249. [Google Scholar] [CrossRef]
- Bermejo, M.D.; Jiménez, C.; Cabeza, P.; Matías-Gago, A.; Cocero, M.J. Experimental study of hydrothermal flames formation using a tubular injector in a refrigerated reaction chamber. Influence of the operational and geometrical parameters. J. Supercrit. Fluids 2011, 59, 140–148. [Google Scholar] [CrossRef]
- Cabeza, P.; Bermejo, M.D.; Jimenez, C.; Cocero, M.J. Experimental study of the supercritical water oxidation of recalcitrant compounds under hydrothermal flames using tubular reactors. Water Res. 2011, 45, 2485–2495. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, M.D.; Cabeza, P.; Queiroz, J.P.S.; Jiménez, C.; Cocero, M.J. Analysis of the scale up of a transpiring wall reactor with a hydrothermal flame as a heat source for the supercritical water oxidation. J. Supercrit. Fluids 2011, 56, 21–32. [Google Scholar] [CrossRef]
- Queiroz, J.P.S.; Bermejo, M.D.; Cocero, M.J. Kinetic model for isopropanol oxidation in supercritical water in hydrothermal flame regime and analysis. J. Supercrit. Fluids 2013, 76, 41–47. [Google Scholar] [CrossRef]
- Queiroz, J.P.S.; Bermejo, M.D.; Cocero, M.J. Numerical study of the influence of geometrical and operational parameters in the behavior of a hydrothermal flame in vessel reactors. Chem. Eng. Sci. 2014, 112, 47–55. [Google Scholar] [CrossRef]
- Queiroz, J.P.S.; Bermejo, M.D.; Mato, F.; Cocero, M.J. Supercritical water oxidation with hydrothermal flame as internal heat source: Efficient and clean energy production from waste. J. Supercrit. Fluids 2015, 96, 103–113. [Google Scholar] [CrossRef]
- Cabeza, P.; Silva Queiroz, J.P.; Criado, M.; Jiménez, C.; Bermejo, M.D.; Mato, F.; Cocero, M.J. Supercritical water oxidation for energy production by hydrothermal flame as internal heat source. Experimental results and energetic study. Energy 2015, 90, 1584–1594. [Google Scholar]
- Zhang, J. Fundamental Study on Oxidation and Hydrothermal Combustion of High Concentration Textile Wastewater and Sludge in Supercritical Water. Ph.D. Thesis, Xi’an Jiaotong University, Xi’an, China, 2014. [Google Scholar]
- Zhang, J.; Wang, S.; Xu, D.; Guo, Y.; Ren, M.; Lu, J. Kinetics study on hydrothermal combustion of methanol in supercritical water. Chem. Eng. Res. Des. 2015, 98, 220–230. [Google Scholar] [CrossRef]
- Ren, M.; Wang, S.; Zhang, J.; Guo, Y.; Xu, D.; Wang, Y. Characteristics of methanol hydrothermal combustion: Detailed chemical kinetics coupled with simple flow modeling study. Ind. Eng. Chem. Res. 2017, 56, 5469–5478. [Google Scholar] [CrossRef]
- Ren, M.; Wang, S.; Yang, C.; Xu, H.; Guo, Y.; Roekaerts, D. Supercritical water oxidation of quinoline with moderate preheat temperature and initial concentration. Fuel 2019, 236, 1408–1414. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Ren, M.; Zhang, j.; Xu, D.; Qian, L.; Sun, P. Recent advances on research and application on supercritical hydrothermal combustion technology. Chem. Ind. Eng. Prog. 2016, 35, 1942–1955. [Google Scholar]
- Narayanan, C.; Frouzakis, C.; Boulouchos, K.; Príkopský, K.; Wellig, B.; Rudolf von Rohr, P. Numerical modelling of a supercritical water oxidation reactor containing a hydrothermal flame. J. Supercrit. Fluids 2008, 46, 149–155. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Kumar, P.; Hicks, M.C.; Hegde, U.G.; Kozinski, J.A. Impacts of oxidant characteristics on the ignition of n-propanol-air hydrothermal flames in supercritical water. Combust. Flame 2019, 203, 46–55. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Hegde, U.G.; Hicks, M.C.; Kozinski, J.A. Ignition of hydrothermal flames. RSC Adv. 2015, 5, 36404–36422. [Google Scholar] [CrossRef]
- Bermejo, M.; Rincón, D.; Vazquez, V.; Cocero, M. Supercritical water oxidation: Fundamentals and reactor modeling. Chem. Ind. Chem. Eng. Q. 2007, 13, 79–87. [Google Scholar] [CrossRef]
- Cocero, M.J. Supercritical water processes: Future prospects. J. Supercrit. Fluids 2018, 134, 124–132. [Google Scholar] [CrossRef]
- Steeper, R.R.; Rice, S.F.; Brown, M.S.; Johnston, S.C. Methane and methanol diffusion flames in supercritical water. J. Supercrit. Fluids 1992, 5, 262–268. [Google Scholar] [CrossRef]
- Serikawa, R.M.; Usui, T.; Nishimura, T.; Sato, H.; Hamada, S.; Sekino, H. Hydrothermal flames in supercritical water oxidation: Investigation in a pilot scale continuous reactor. Fuel 2002, 81, 1147–1159. [Google Scholar] [CrossRef]
- Sobhy, A.; Butler, I.S.; Kozinski, J.A. Selected profiles of high-pressure methanol–air flames in supercritical water. Proc. Combust. Inst. 2007, 31, 3369–3376. [Google Scholar] [CrossRef]
- Sobhy, A.; Guthrie, R.I.L.; Butler, I.S.; Kozinski, J.A. Naphthalene combustion in supercritical water flames. Proc. Combust. Inst. 2009, 32, 3231–3238. [Google Scholar] [CrossRef]
- Augustine, C.R. Hydrothermal Spallation Drilling and Advanced Energy Conversion Technologies for Engineered Geothermal Systems. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2009. [Google Scholar]
- Weber, M. Apparate Einer SCWO-Anlage Und Deren Leistungsfähigkeit. Ph.D. Thesis, ETH Zurich, Swiss, Switzerland, 1997. [Google Scholar]
- Weber, M.; Wellig, B.; Rudolf von Rohr, P. SCWO apparatus design—towards industrial availability. In Proceedings of the CORROSION 99, 25–30, San Antonio, TX, USA, 1 January 1999. [Google Scholar]
- Stathopoulos, P.; Ninck, K.; Rudolf von Rohr, P. Hot-wire ignition of ethanol–oxygen hydrothermal flames. Combust. Flame 2013, 160, 2386–2395. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, C.; Zhang, Y.; Chen, S.; Chen, G.; Ma, C. Experimental study on the operating characteristics of an inner preheating transpiring wall reactor for supercritical water oxidation: Temperature profiles and product properties. Energy 2014, 66, 577–587. [Google Scholar] [CrossRef]
- Hegde, U.; Gotti, D.; Hicks, M. The transition to turbulence of buoyant near-critical water jets. J. Supercrit. Fluids 2014, 95, 195–203. [Google Scholar] [CrossRef]
- Hicks, M.C.; Hegde, U.G.; Kojima, J.J. Spontaneous ignition of hydrothermal flames in supercritical ethanol water solutions. In Proceedings of the 10th U.S. National Combustion Meeting, College Park, MD, USA, 23–26 April 2017. [Google Scholar]
- Hicks, M.C.; Hegde, U.G.; Kojima, J.J. Hydrothermal ethanol flames in Co-flow jets. J. Supercrit. Fluids 2019, 145, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.N.; Nanda, S.; Hegde, U.G.; Hicks, M.C.; Kozinski, J.A. Ignition of n-propanol–air hydrothermal flames during supercritical water oxidation. Proc. Combust. Inst. 2017, 36, 2503–2511. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Lu, J.; Wang, B.; Chen, S. Numerical simulation for supercritical hydrothermal combustion reactor using methanol as auxiliary fuels. Mod. Chem. Ind. 2017, 37, 184–188. [Google Scholar]
- Ren, M.; Wang, S.; Roekaerts, D. Numerical study of the counterflow diffusion flames of methanol hydrothermal combustion: The real-fluid effects and flamelet analysis. J. Supercrit. Fluids 2019, 152. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, H.; Luo, K.; Song, C.; Zhao, C.; Xing, J.; Fan, J. Evaluation of real-fluid flamelet/progress variable model for laminar hydrothermal flames. J. Supercrit. Fluids 2019, 143, 232–241. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, H.; Song, C.; Luo, K.; Fan, J. Real-fluid effects on laminar diffusion and premixed hydrothermal flames. J. Supercrit. Fluids 2019, 153. [Google Scholar] [CrossRef]
- Song, C.; Luo, K.; Jin, T.; Wang, H.; Fan, J. Direct numerical simulation on auto-ignition characteristics of turbulent supercritical hydrothermal flames. Combust. Flame 2019, 200, 354–364. [Google Scholar] [CrossRef]
- Song, C.; Jin, T.; Wang, H.; Gao, Z.; Luo, K.; Fan, J. High-fidelity numerical analysis of non-premixed hydrothermal flames: Flame structure and stabilization mechanism. Fuel 2020, 259. [Google Scholar] [CrossRef]
- Hirth, T.; Franck, E.U. Oxidation and hydrothermolysis of hydrocarbons in supercritical water at high pressures. Phys. Chem. Chem. Phys. 1993, 97, 1091–1098. [Google Scholar] [CrossRef]
- Steeper, R.R. Methane and Methanol Oxidation in Supercritical Water: Chemical Kinetics and Hydrothermal Flame Studies. Ph.D. Thesis, University of California, Davis, CA, USA, 1996. [Google Scholar]
- Hicks, M.C.; Hegde, U.G.; Fisher, J.W. Investigation of supercritical water phenomena for space and extraterrestrial application. In Proceedings of the 10th International Symposium on Supercritical Fluids, San Francisco, CA, USA, 12 May 2012. [Google Scholar]
- Xu, D.; Wang, S.; Huang, C.; Tang, X.; Guo, Y. Transpiring wall reactor in supercritical water oxidation. Chem. Eng. Res. Des. 2014, 92, 2626–2639. [Google Scholar] [CrossRef]
- La Roche, L.; Weber, M.; Trepp, C. Design rules for the wallcooled hydrothermal burner (WHB). Chem. Eng. Technol. 1997, 20, 208–211. [Google Scholar] [CrossRef]
- Wellig, B. Transpiring wall reactor for supercritical water oxidation. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 2003. [Google Scholar]
- Bermejo, M.D.; Fdez-Polanco, F.; Cocero, M.J. Effect of the transpiring wall on the behavior of a supercritical water oxidation reactor: Modeling and experimental results. Ind. Eng. Chem. Res. 2006, 45, 3438–3446. [Google Scholar] [CrossRef]
- Bermejo, M.D.; Fdez-Polanco, F.; Cocero, M.J. Experimental study of the operational parameters of a transpiring wall reactor for supercritical water oxidation. J. Supercrit. Fluids 2006, 39, 70–79. [Google Scholar] [CrossRef]
- Queiroz, J.P.S. On the Development of Computational Tools for the Modeling and Simulation of SCWO pProcess Intensified by Hydrothermal Flames. Ph.D. Thesis, University of Valladolid, Valladolid, Spain, 2014. [Google Scholar]
- Cabeza, P.; Queiroz, J.P.S.; Arca, S.; Jiménez, C.; Gutiérrez, A.; Bermejo, M.D.; Cocero, M.J. Sludge destruction by means of a hydrothermal flame. Optimization of ammonia destruction conditions. Chem. Eng. J. 2013, 232, 1–9. [Google Scholar]
- García-Rodríguez, Y.; Mato, F.A.; Martín, A.; Bermejo, M.D.; Cocero, M.J. Energy recovery from effluents of supercritical water oxidation reactors. J. Supercrit. Fluids 2015, 104, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Wang, S.; Ren, M.; Wang, S. Co-oxidation effects and mechanisms between sludge and alcohols (methanol, ethanol and isopropanol) in supercritical water. Chem. Eng. J. 2019, 366, 223–234. [Google Scholar] [CrossRef]
- Ren, M.M.; Wang, S.Z.; Qian, L.L.; Li, Y.H. High-pressure direct-fired steam-gas generator (HDSG) for heavy oil recovery. App. Mech. Mater. 2014, 577, 523–526. [Google Scholar] [CrossRef]
- Schinner, R.M.; Eson, R.L. A direct-fired downhole steam generator-from design to field test. J. Pet. Technol. 1985, 37, 1903–1908. [Google Scholar]
- Vogel, F.; Blanchard, J.L.D.; Marrone, P.A.; Rice, S.F.; Webley, P.A.; Peters, W.A.; Smith, K.A.; Tester, J.W. Critical review of kinetic data for the oxidation of methanol in supercritical water. J. Supercrit. Fluids 2005, 34, 249–286. [Google Scholar] [CrossRef]
- Webley PA, T.J. Fundamental kinetics of methane oxidation in supercritical water. Energy Fuels 1991, 5, 411–419. [Google Scholar] [CrossRef]
- Holgate, H.R.; Tester, J.W. Oxidation of hydrogen and carbon-monoxide in subcritical and supercritical water—reaction-kinetics, pathways, and water-density effects. 2. Elementary reaction modeling. J. Phys. Chem. 1994, 98, 810–822. [Google Scholar]
- Dagaut, P.; Cathonnet, M.; Boettner, J.-C. Chemical kinetic modeling of the supercritical-water oxidation of methanol. J. Supercrit. Fluids 1996, 9, 33–42. [Google Scholar] [CrossRef]
- Alkam, M.K.; Pal, V.M.; Butler, P.B. Methanol and hydrogen oxidation kinetics in water at supercritical states. Combust. Flame 1996, 106, 110–130. [Google Scholar] [CrossRef]
- Brock, E.E.; Savage, P.E. Detailed chemical kinetics model for supercritical water oxidation of C1 compounds and H2. AIChE J. 1995, 41, 1874–1888. [Google Scholar] [CrossRef] [Green Version]
- Baulch, D.L.; Cobos, C.J.; Cox, R.A.; Esser, C.; Frank, P.; Just, T.; Kerr, J.A.; Pilling, M.J.; Troe, J.; Walker, R.W.; et al. Evaluated kinetic data for combustion modelling. J. Phys. Chem. Ref. Data 1992, 21, 411–734. [Google Scholar] [CrossRef] [Green Version]
- Baulch, D.L.; Cobos, C.J.; Cox, R.A.; Frank, P.; Hayman, G.; Just, T.; Kerr, J.A.; Murrells, T.; Pilling, M.J.; Troe, J.; et al. Evaluated kinetic data for combustion modeling. Supplement I. J. Phys. Chem. Ref. Data 1994, 23, 847–848. [Google Scholar] [CrossRef] [Green Version]
- Dinaro, J.L.; Howard, J.B.; Green, W.H.; Tester, J.W.; Bozzelli, J.W. Analysis of an elementary reaction mechanism for benzene oxidation in supercritical water. Proc. Combust. Inst. 2000, 28, 1529–1536. [Google Scholar]
- Li, J.; Zhao, Z.; Kazakov, A.; Chaos, M.; Dryer, F.L.; Scire, J.J. A comprehensive kinetic mechanism for CO, CH2O, and CH3OH combustion. Int. J. Chem. Kinet. 2007, 39, 109–136. [Google Scholar] [CrossRef]
- Koido, K.; Hirosaka, K.; Kubo, T.; Fukayama, M.; Ouryouji, K.; Hasegawa, T. Numerical study on premixed hydrothermal combustion in tube reactor. Combust. Theory Model. 2009, 13, 295–318. [Google Scholar] [CrossRef]
- Schanzenbächer J, T.J. Tester JW, Ethanol oxidation and hydrolysis rates in supercritical water. J. Supercrit. Fluids 2002, 22, 139–147. [Google Scholar] [CrossRef]
- Chung, T.-H.; Ajlan, M.; Lee, L.L.; Starling, K.E. Generalized multiparameter correlation for nonpolar and polar fluid transport properties. Ind. Eng. Chem. Res. 1988, 27, 671–679. [Google Scholar] [CrossRef]
- Wilke, C.R. A viscosity equation for gas mixtures. J. Chem. Phys. 1950, 18, 517–519. [Google Scholar] [CrossRef]
- Wilke, C.R.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Hjertager, L.K.; Hjertager, H.B.; Solberg, T. CFD modelling of fast chemical reactions in turbulent liquid flows. Comput. Chem. Eng. 2002, 26, 507–515. [Google Scholar] [CrossRef]
- Mathur, G.P.; Thodos, G. The self-diffusivity of substances in the gaseous and liquid state. AIChE J. 1965, 11, 613–616. [Google Scholar] [CrossRef]
- Baldyga, J. Turbulent mixer model with application to homogeneous, instantaneous chemical reactions. Chem. Eng. Sci. 1989, 44, 1175–1182. [Google Scholar] [CrossRef]
- Ren, M. Study on Supercritical Hydrothermal Combustion: Chemical Reaction Mechanism and the Flame Characteristics under Different Flow Patterns. Ph.D. Thesis, Xi’an Jiaotong University, Xi’an, China, 2019. [Google Scholar]
- Wang, Q. Numerical Simulation of CH4-LOX Non-Premixed Turbulent Combustion at Supercritical Pressure. Master’s Thesis, Zhejiang University, Hangzhou, China, 2016. [Google Scholar]
- Fujii, T.; Hayashi, R.; Kawasaki, S.-i.; Suzuki, A.; Oshima, Y. Water density effects on methanol oxidation in supercritical water at high pressure up to 100 MPa. J. Supercrit. Fluids 2011, 58, 142–149. [Google Scholar] [CrossRef]
- Henrikson, J.T.; Grice, C.R.; Savage, P.E. Effect of water density on methanol oxidation kinetics in supercritical water. J. Phys. Chem. A 2006, 110, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Richard Holgate, H.; Tester, J.W. Fundamental kinetics and mechanisms of hydrogen oxidation in supercritical water. Combust. Sci. Technol. 1993, 88, 369–397. [Google Scholar] [CrossRef]
- Gordon, P.V.; Gotti, D.J.; Hegde, U.G.; Hicks, M.C.; Kulis, M.J.; Sivashinsky, G.I. An elementary model for autoignition of laminar jets. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20150059. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; De, S.; Pandey, A.; Singh, A.P. Combustion for Power Generation and Transportation; Springer: Singapore, 2017; pp. 20–23. [Google Scholar]
- Stathopoulos, P.; Rothenfluh, T.; Schuler, M.; Brkic, D.; Rohr, P.R.V. Assisted ignition of hydrothermal flames in a hydrothermal spallation drilling pilot plant. In Proceedings of the Thirty-Seventh Workshop on Geothermal Reservoir Engineering, Stanford, CA, USA, 30 January 2012. [Google Scholar]
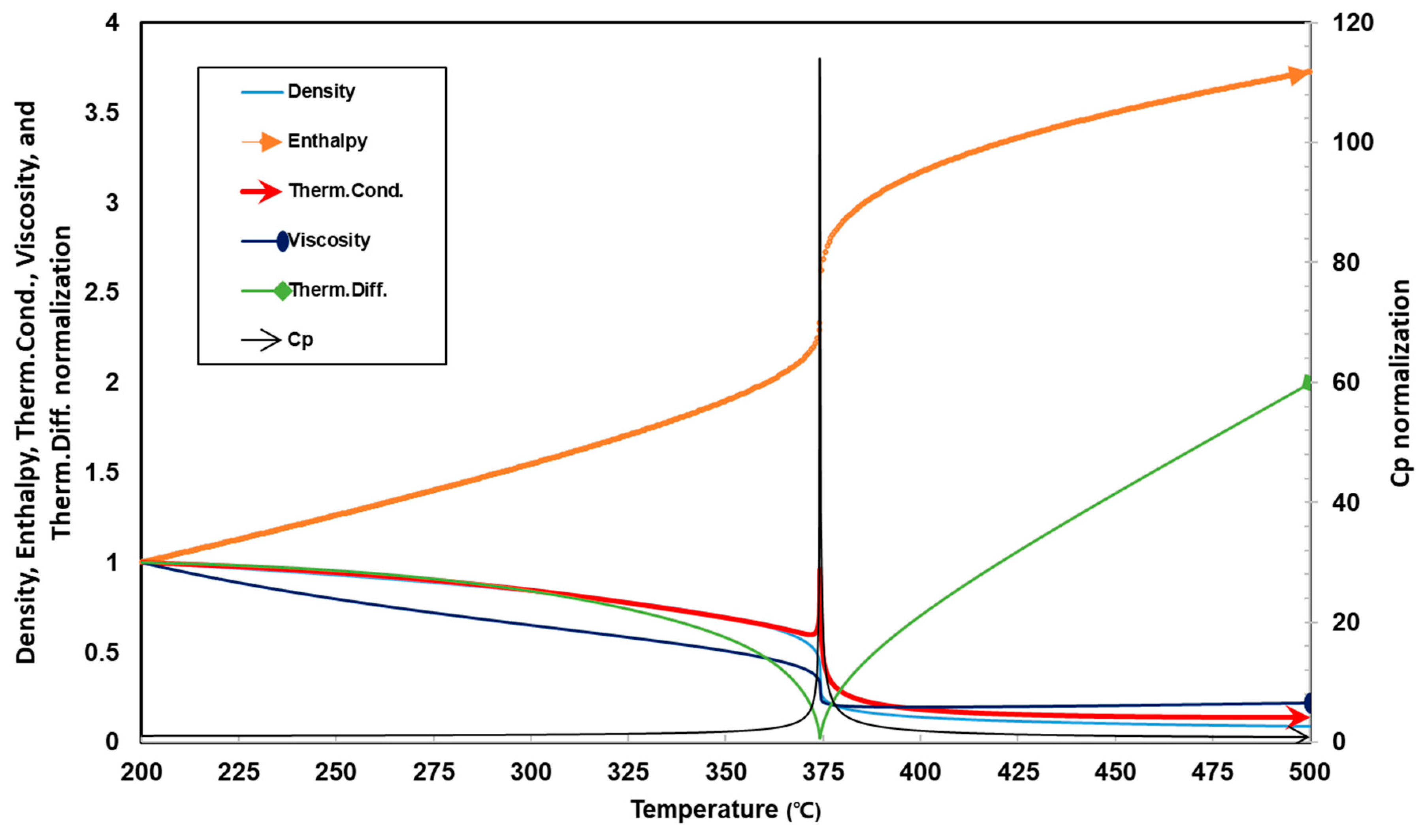
- Ma, D.; Zhou, T.; Feng, X.; Huang, Y. Study on critical region determination method of supercritical water. Chem. Bull. 2019, 82, 151–157. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill: New York, NY, USA, 2001; pp. 512–514. [Google Scholar]
- Ruan, B. Numerical Simulation of Heat Absorption and Convective Heat Transfer of n-Decane Cracking under Supercritical Pressure. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2013. [Google Scholar]
- Mohseni, M.; Bazargan, M. The effect of the low Reynolds number k-e turbulence models on simulation of the enhanced and deteriorated convective heat transfer to the supercritical fluid flows. Heat Mass Transf. 2010, 47, 609–619. [Google Scholar] [CrossRef]
- Hicks, M.C.; Lauver, R.W.; Hegde, U.G.; Sikora, T.J. Diffusion limited supercritical water oxidation (SCWO) in microgravity environments. In Proceedings of the SAE 2006 International Conference on Environmental Systems (ICES), Norfolk, VA, USA, 17 July 2006. [Google Scholar]
- Jelacic, A.J.; Renner, J.L. A perspective on the future of geothermal energy in the United States. In Proceedings of the Power and Energy Society General Meeting—Conversion and Delivery of Electrical Energy in the 21st Century, Pittsburgh, PA, USA, 20–24 July 2008. [Google Scholar]
- Schuler, M.J. Fundamental Investigations of Supercritical wWater Fflows for Hydrothermal Spallation Drilling; ETH Zurich: Zürich, Switzerland, 2014. [Google Scholar]
- Kant, M.A.; Rossi, E.; Duss, J.; Amann, F.; Saar, M.O.; Rudolf von Rohr, P. Demonstration of thermal borehole enlargement to facilitate controlled reservoir engineering for deep geothermal, oil or gas systems. Appl. Energy 2018, 212, 1501–1509. [Google Scholar] [CrossRef]
- Stathopoulos, P. Hydrothermal Spallation Drilling Experiments in a Novel High Ppressure Pilot Plant. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 2013. [Google Scholar]
- Stathopoulos, P.; Hofmann, F.; Rothenfluh, T.; von Rohr, P.R. Calibration of a gardon sensor in a high-temperature high heat flux stagnation facility. Exp. Heat Transf. 2012, 25, 222–237. [Google Scholar] [CrossRef]
- Kant, M.A.; Rudolf von Rohr, P. Minimal required boundary conditions for the thermal spallation process of granitic rocks. Int. J. Rock Mech. Min. Sci. 2016, 84, 177–186. [Google Scholar] [CrossRef]
- Kant, M.A.; Rudolf von Rohr, P. Determination of surface heat flux distributions by using surface temperature measurements and applying inverse techniques. Int. J. Heat Mass Transfer 2016, 99, 1–9. [Google Scholar] [CrossRef]
- Bermejo, M.D.; Cocero, M.J.; Fernández-Polanco, F. A process for generating power from the oxidation of coal in supercritical water. Fuel 2004, 83, 195–204. [Google Scholar] [CrossRef]




| Institute | Rector Type | Objective | Ref. |
|---|---|---|---|
| Karlsruhe Institute of Technology, Germany | Semi-batch rector | The visual observation and temperature measurement of hydrothermal flame | [15] |
| Sandia National Laboratories, USA | Semi-batch rector | Threshold concentrations for ignition and their relations with temperature | [48] |
| Ebara Research Company, Japan | Continuous reactors | SCWO strengthened by hydrothermal flame to deal with recalcitrant reactants | [49] |
| McGill University, Canada | Semi-batch rector | The observation, description and environmental evaluation of hydrothermal flame as a tool to destruct complex organic wastes | [50] |
| Semi-batch rector | The effects of hydrothermal flame on the destruction of naphthalene | [51] | |
| MIT, USA | Continuous reactors | Verified the generation and the stability of hydrothermal flame and, its application in thermal spallation drilling | [52] |
| ETH Zurich, Switzerland | Continuous reactors (WCHB-1) | SCWO strengthened by hydrothermal flame to deal with organic components | [53] |
| Continuous reactors (WCHB-2) | Evaluation on the newly-designed HFR reactor | [54] | |
| Continuous reactors (Transpiring wall) | The influence of mass flux and temperature of the transpiration on the reactor performance | [11] | |
| Continuous reactors (Transpiring wall) | The performance of the transpring wall reactor in anti-corrosion and anti-deposition | [12] | |
| Continuous reactors (WCHB-3) | Combined experiments with numerical simulation to investigate the ignition process | [43] | |
| Continuous reactors (Transpiring wall) | The ignition, extinction and stability of hydrothermal flame | [24] | |
| Continuous reactors (WCHB-4) | The forced ignition of methanol-oxygen and its influencing factors | [55] | |
| Continuous reactors (WCHB-4) | The forced ignition of turbulent diffusion flame and the drawing of its ignition map | [23] | |
| Continuous reactors (WCHB-4) | The new type of ignition device and the performance of different nozzles | [22] | |
| Shandong University, China | Continuous reactors (Transpiring wall) | The effects of operation parameters on the performance of the reactor and the determination of optimal parameters from the angles of energy consumption and investment cost | [56] |
| University of Valladolid, Spain | Continuous reactors (Transpiring wall) | The status of SCWO with hydrothermal flame in different mixers | [30] |
| Continuous reactors (Tubular reactor) | The formation and the stability of premixed hydrothermal flame and its influencing factors, including feed rate, temperature and concentration | [33] | |
| Continuous reactors (Tubular reactor) | SCWO strengthened by hydrothermal flame to deal with recalcitrant compounds | [32] | |
| Continuous reactors (Transpiring wall) | The impact of operation parameters including flow rate, temperature and concentration on the characteristics of hydrothermal flame | [31] | |
| Continuous reactors (Transpiring wall) | Analysis on the new reactor and its energy recovery performance by test and numerical simulation | [37] | |
| NASA Glenn Research Center, USA | Continuous reactors | Transition to the turbulence of buoyant near-critical water jets and the role of buoyancy | [57] |
| The characteristics of laminar and turbulent diffusion flame and the formation of soot | [58] | ||
| The auto-ignition and stability of ethanol hydrothermal flame | [59] | ||
| Indian Institute of Technology Roorkee, India | Semi-batch rector | The impact of the temperature of semi-batch reactor and oxidant on the ignition of hydrothermal flame | [60] |
| The impact of oxidant characteristics like flow rate on the ignition of the hydrothermal flame at near-critical temperature and supercritical temperature | [44] | ||
| Xi’an Jiaotong University, China | No (numerical simulation) | Built and validated the detailed chemical kinetics model for methanol SCHC | [39] |
| No (numerical simulation) | Investigated the effects of operation parameters like methanol concentration on the characteristics of the hydrothermal flame | [61] | |
| No (numerical simulation) | Built and validated the detailed chemical kinetics model coupled with a simple flow model for methanol SCHC | [40] | |
| Semi-batch rector | Verified that quinoline solutions could be ignited without co-fuels like methanol and isopropanol | [41] | |
| No (numerical simulation) | Used counterflow diffusion flames to deeply understand hydrothermal flames and developed a Flamelet Generated Manifold table for advanced turbulent flame simulations | [62] | |
| Zhejiang University, China | No (numerical simulation) | Developed a real-fluid flameless/progress variable model for laminar hydrothermal flames | [63] |
| No (numerical simulation) | Investigated the real-fluid effects on both laminar diffusion and premixed laminar hydrothermal flames | [64] | |
| No (numerical simulation) | Directly used numerical simulation to investigate auto-ignition characteristics of turbulent diffusion hydrothermal flames | [65] | |
| No (numerical simulation) | Use high-fidelity numerical simulation and homogeneous auto-ignition to study the flame structure and stabilization mechanism of laminar diffusion hydrothermal flames | [66] |
| Reactants | Operation Parameters | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Fuel | Oxidants | Fuel Conc. | Fuel Temp. (°C) | Fuel Flow Rate | Oxidant Temp. (°C) | Oxidant Flow Rate | |
| Methane | Oxygen | 30 mol% | / | / | / | 1.6 mL/s | [15] |
| Methanol, methane | Oxygen | 1–50 mol% | / | / | / | 16.7-50 mL/s | [48] |
| Isopropanol | Air | 2–6 vol% | 470 | 3 mL/min | 377 | 1.3–2.4 fold of stoichiometric | [49] |
| Methanol | Air | 18.3–45.9 wt% | / | / | / | 0.5–1.5ml/s | [50] |
| Methanol, Hydrogen | Oxygen | Methanol 25 wt%; Hydrogen 3–6 wt% | Methanol 500–550; Hydrogen 600–300 | Methanol 0.2–0.5 g/s; Hydrogen 0.04–0.06 g/s | 400–450 | 1.5 fold of stoichiometric | [52] |
| Methanol | Oxygen | 4–25 wt% | / | 2.1 g/s | / | 1.1 g/s | [53] |
| Methanol | Oxygen | 16.5–30 wt% | / | 3.2 g/s | / | 1.5 g/s | [54] |
| Methanol | Oxygen | 6–28 wt% | / | 1.5–1.6 g/s | / | 1.2 fold of stoichiometric | [72] |
| Methanol | Oxygen | 16–22 wt% | 200–350 | 1.5 g/s | 300–400 | 1.2 fold of stoichiometric | [11] |
| Methanol | Oxygen | 16–22 wt% | 345–370 | 1.6 g/s | 395–430 | 1.2 fold of stoichiometric | [12] |
| Methanol | Oxygen | 12–16 wt% | 698–675 | 1.8–2.0 g/s | 675,656 | 0.64–0.62 g/s | [43] |
| Methanol | Oxygen | 6–28 wt% | Varied | 1.5 g/s | 400 | 1.2 fold of stoichiometric | [24] |
| Ethanol | Oxygen | 7.5–20 wt% | Ethanol 370–420; Ignitor 450–800 | 20 and 30 kg/h | 390 | 45–119 Nl/min | [55] |
| Ethanol | Oxygen | 20–32.5 wt% | Ethanol 23–83; Ignitor 360 ± 40 | / | / | / | [23] |
| Ethanol | Oxygen | 32.5 wt% | 300–400 | 20 and 50 kg/h | / | 0.8,1.0,1.2 fold of stoichiometric | [22] |
| Methanol | Oxygen | 35 wt% | Water 400–600 | 2–5 kg/h | / | 1.4–1.8 fold of stoichiometric | [56] |
| Isopropanol | Air | 4 and 5 wt% | >400 | 5.6–18 kg/h | >400 | 1.0–1.1 fold of stoichiometric | [30] |
| Isopropanol | Air | 8–9.5 wt% | >400 | 13, 20 and 25 kg/h | >400 | / | [33] |
| Isopropanol | Oxygen | Isopropanol 1–4.5wt%; Ammonia 2–8% | >400 | 9 kg/h | >400 | 1.0–1.1 fold of stoichiometric | [32] |
| Isopropanol | Oxygen | 8 wt% | >400 | 20 and 40 kg/h | >400 | 1.0–1.1 fold of stoichiometric | [31] |
| Isopropanol | Air | Isopropanol 13.5 wt%; Ammonia 25 wt% | 200–25 | / | 20 | 1.0 fold of stoichiometric | [37] |
| Ethanol | Air | 2.3 wt% | 425 | Start at 1.0 mL/min and increase by 0.1 mL/min at a time, until reach 2.0 mL/min | 425 | 1.0–3.0 mL/min | [58] |
| Ethanol | Air | 30–50 vol% | Water 425 | 2.0 mL/min | Close to reactor temperature | 1.0 and 7.0 mL/min | [59] |
| N-Propanol | Oxygen | 1.2–6 wt% | 380 and 420 | / | 450 and 400 | 1.5 mL/s | [60] |
| N-Propanol | Oxygen | 2.4 wt% | 380 and 400 | / | 400 °C and 450 °C | 0.5–3 mL/s | [44] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, C.; Li, Y.; Wang, S.; Ren, M.; Yang, C.; Jiang, Z.; Zhang, J. Review on an Advanced Combustion Technology: Supercritical Hydrothermal Combustion. Appl. Sci. 2020, 10, 1645. https://doi.org/10.3390/app10051645
Cui C, Li Y, Wang S, Ren M, Yang C, Jiang Z, Zhang J. Review on an Advanced Combustion Technology: Supercritical Hydrothermal Combustion. Applied Sciences. 2020; 10(5):1645. https://doi.org/10.3390/app10051645
Chicago/Turabian StyleCui, Chengchao, Yanhui Li, Shuzhong Wang, Mengmeng Ren, Chuang Yang, Zhuohang Jiang, and Jie Zhang. 2020. "Review on an Advanced Combustion Technology: Supercritical Hydrothermal Combustion" Applied Sciences 10, no. 5: 1645. https://doi.org/10.3390/app10051645





